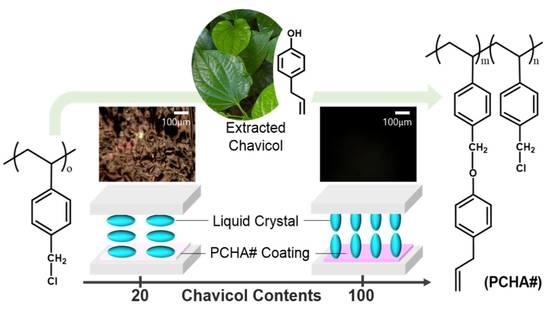
Vertical Alignment of Liquid Crystals on Comb-Like Renewable Chavicol-Modified Polystyrene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
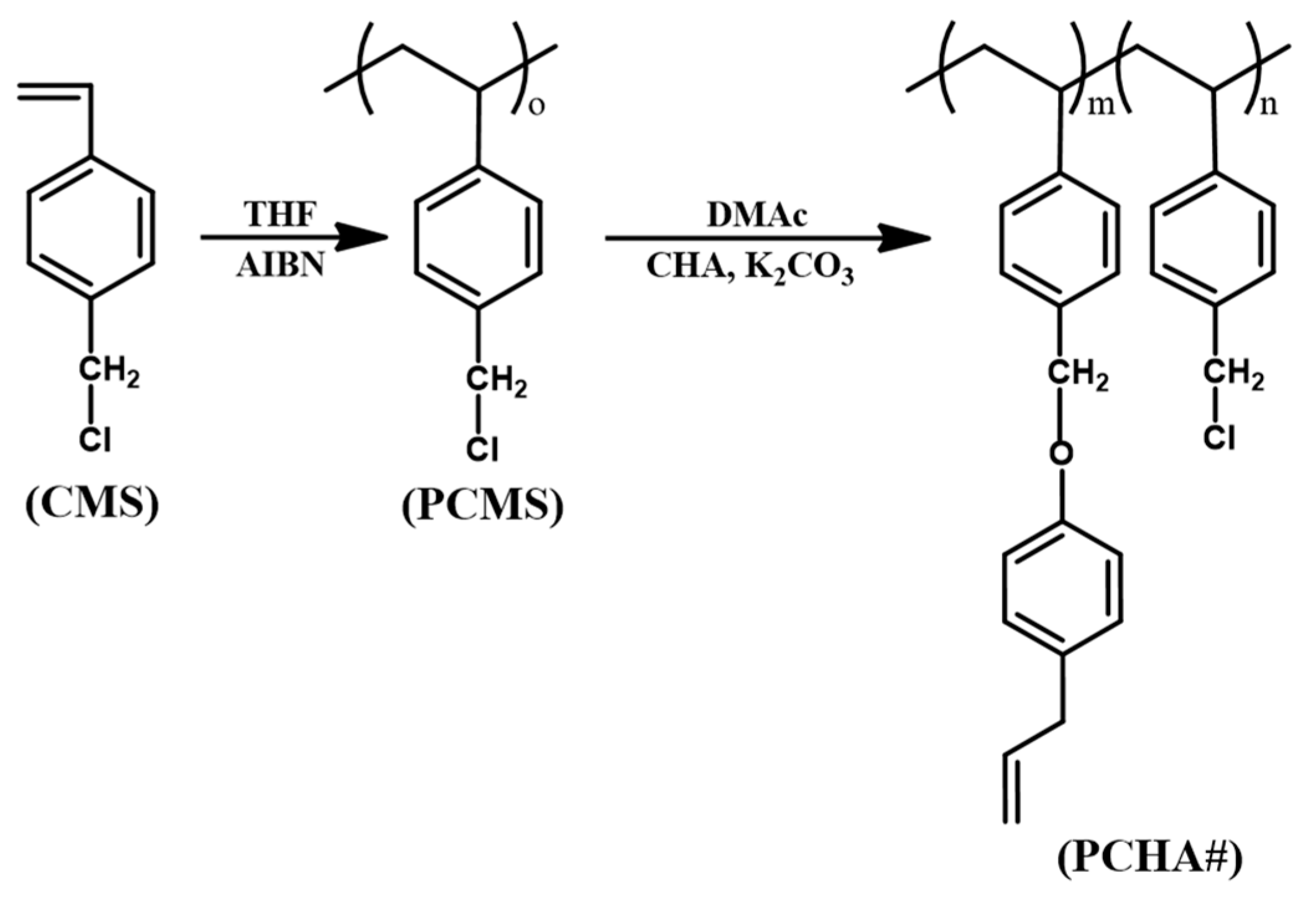
2.2. Synthesis of Chavicol-Modified Polystyrene
2.3. Film Preparation and LC Cell Assembly
2.4. Instrumentation
3. Results
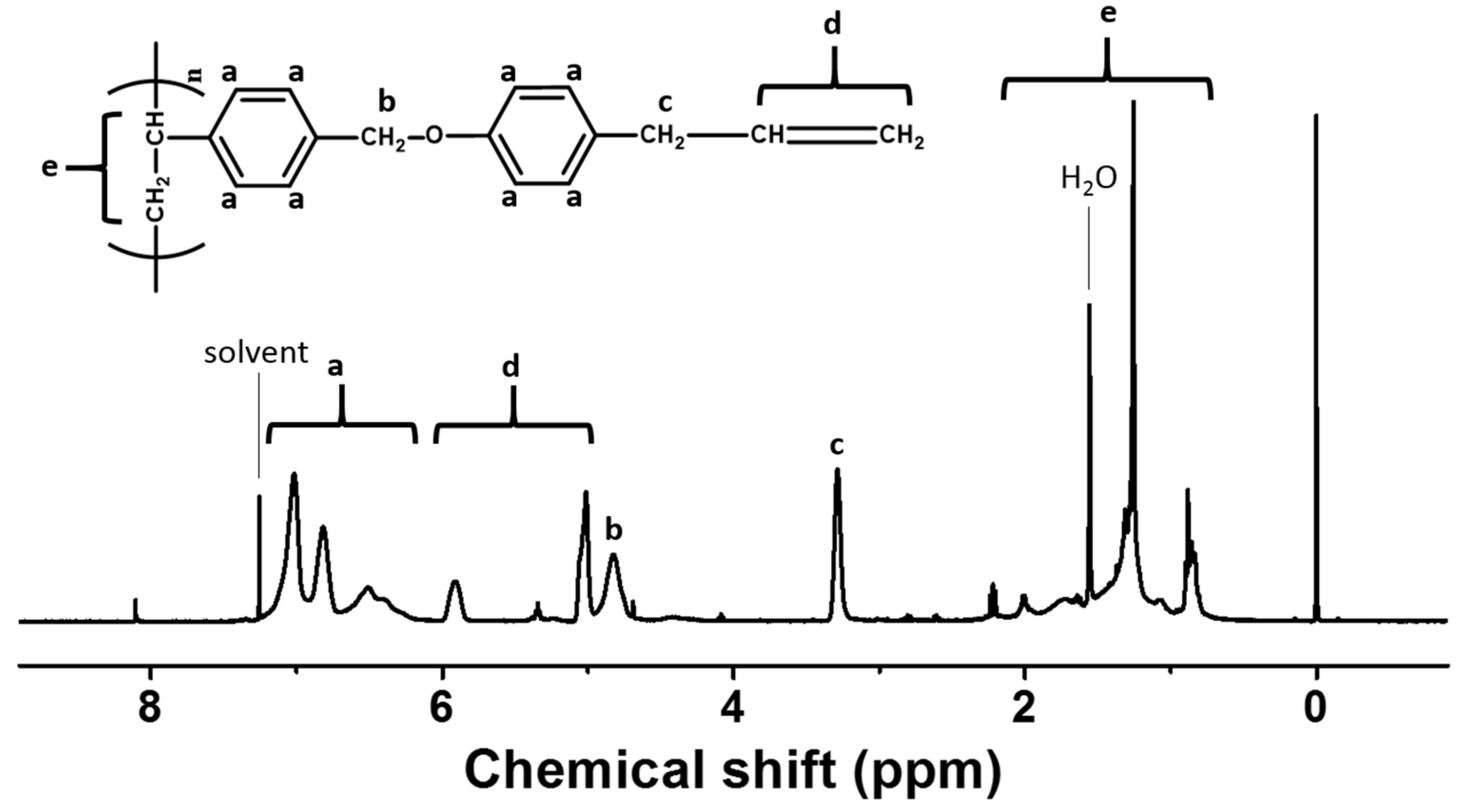
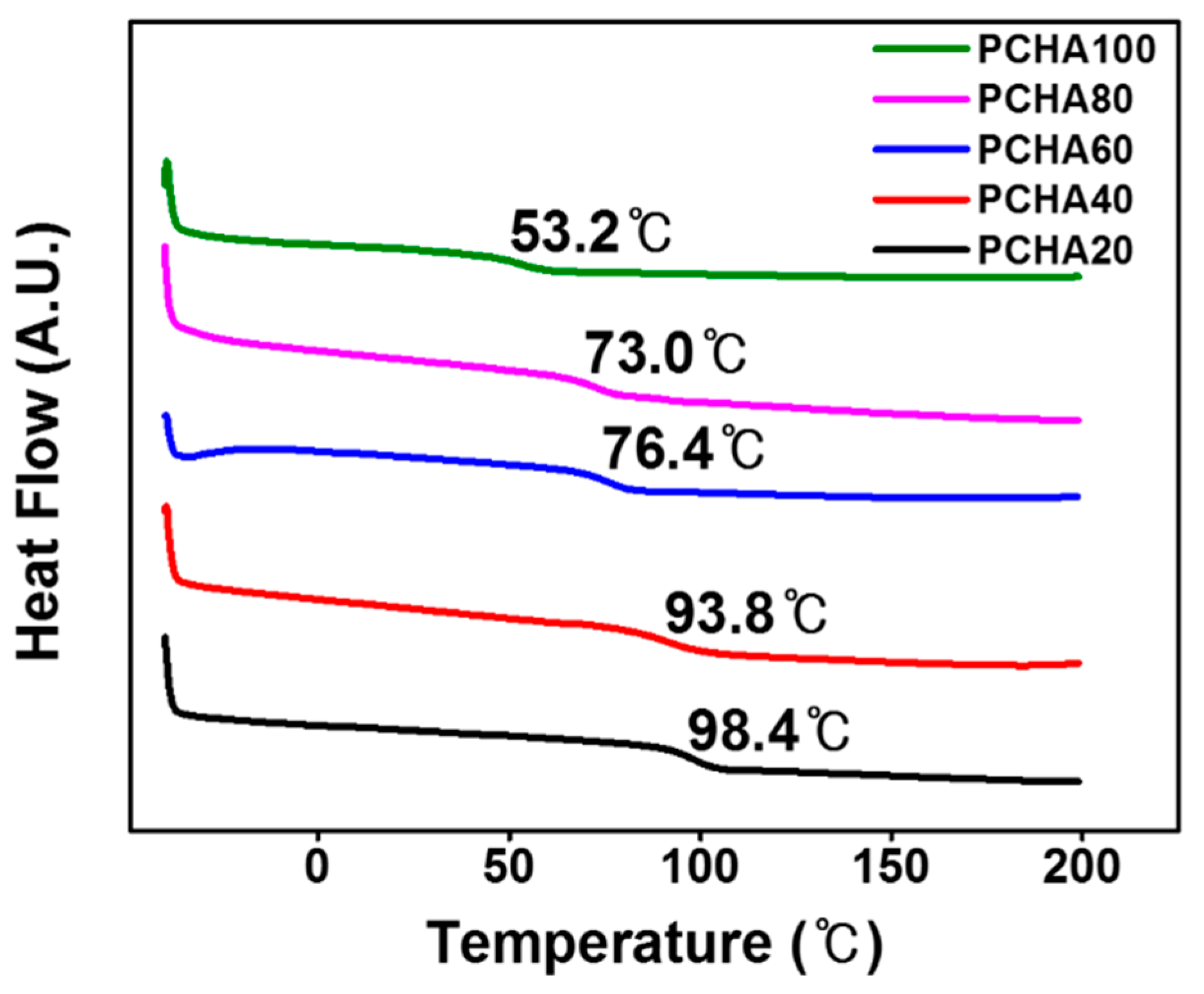
3.1. Synthesis and Characterization of Chavicol-Modified Plystyrene
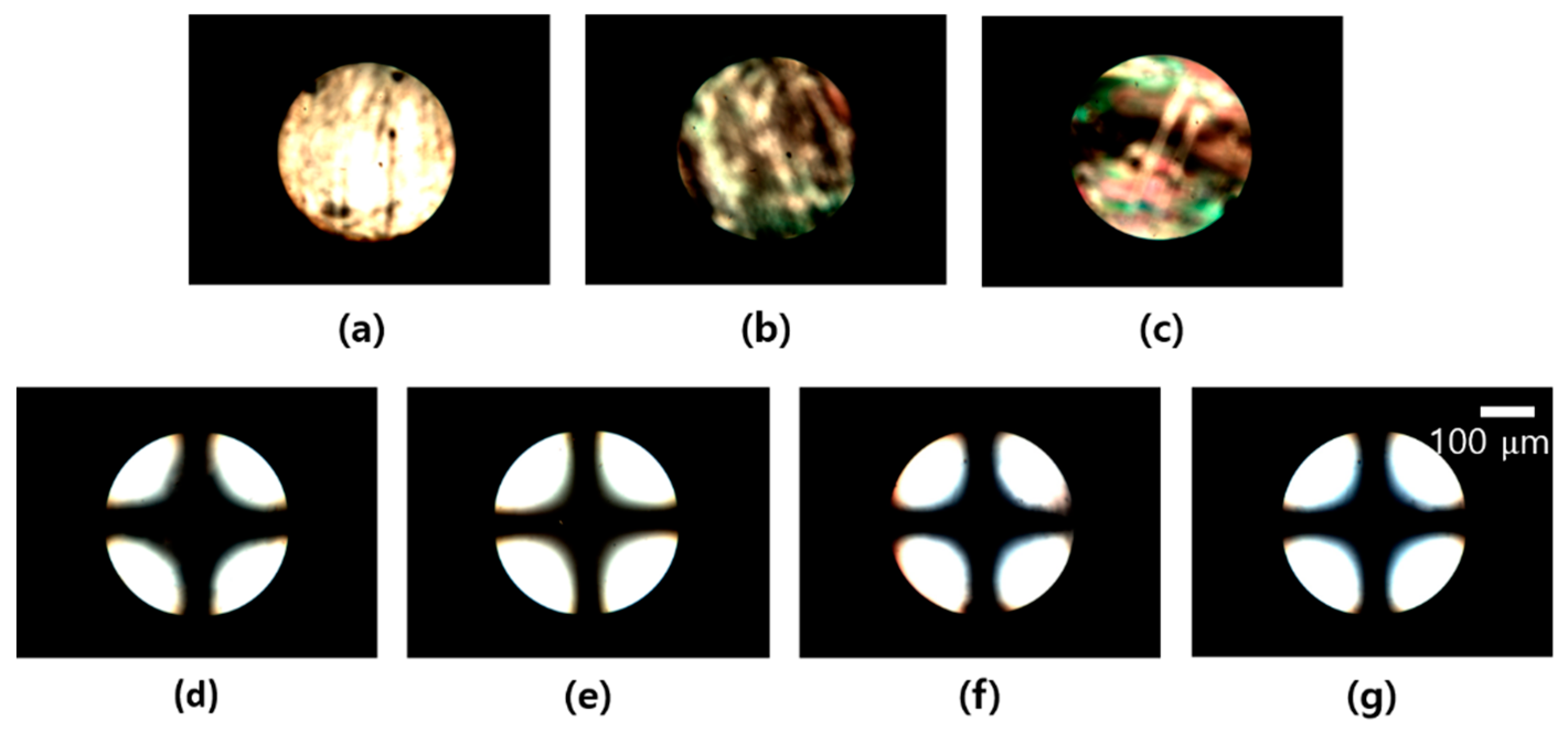
3.2. LC Alignment Behavior of the LC Cell Fabricated with Chavicol-Modified Polystyrene Film
3.3. Surface Properties of Chavicol-Modified Polystyrene Films
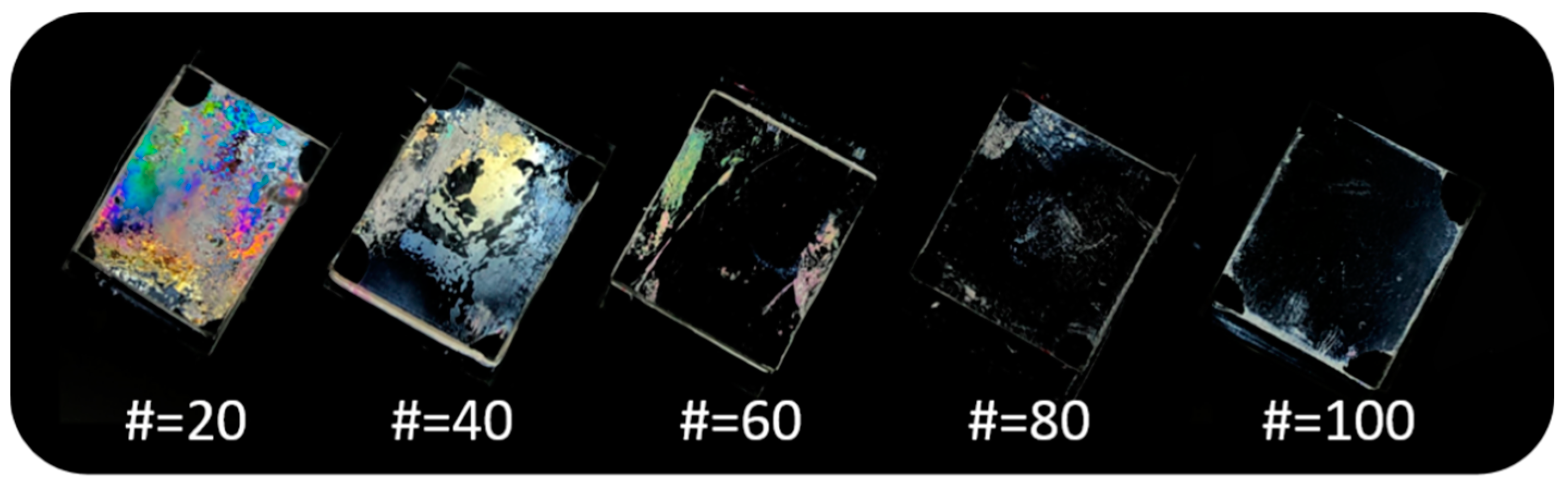
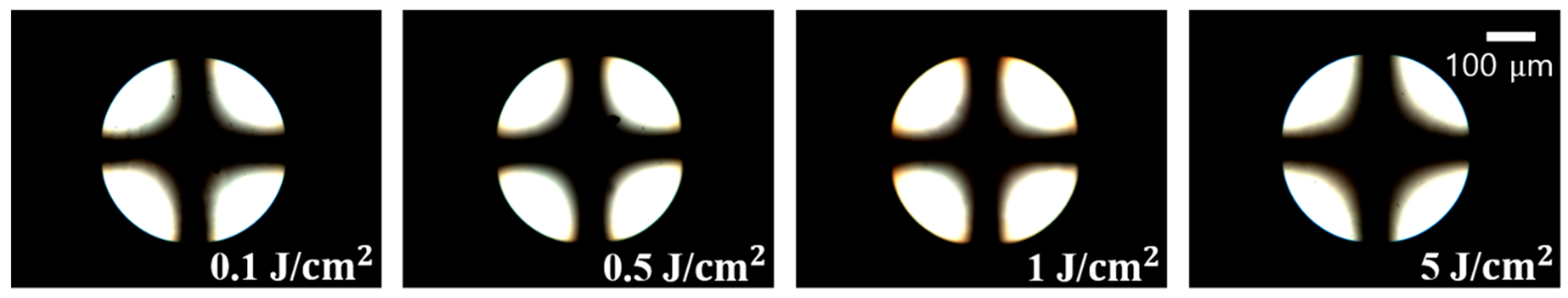
3.4. Reliability and Electro-Optical Performance of the LC Cells Fabricated with Chavicol-Modified Polystyrene Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Gennes, P.-G.; Prost, J. The Physics of Liquid Crystals, 2nd ed.; Oxford University Press: England, UK, 1995; pp. 1–2. [Google Scholar]
- Meyer, R.B. Effects of electric and magnetic fields on the structure of cholesteric liquid crystals. Appl. Phys. Lett. 1968, 12, 281–282. [Google Scholar] [CrossRef]
- Geary, J.M.; Goodby, J.W.; Kmetz, A.R.; Patel, J.S. The mechanism of polymer alignment of liquid-crystal materials. J. Appl. Phys. 1987, 62, 4100–4108. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S. Wavelength and bandwidth based on cholesteric liquid crystals. Opt. Lett. 2011, 36, 4563–4565. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Takasu, T. Hybrid aligned nematic liquid crystal smart glass with asymmetrical daylight controls. J. Soc. Inf. Disp. 2015, 23, 365–370. [Google Scholar] [CrossRef]
- Samsung Display Co., Ltd. Liquid Crystal Display. U.S. Patent 10,712,596, 14 July 2020. [Google Scholar]
- Yin, K.; He, Z.; Wu, S. Reflective polarization volume lens with small f-number and large diffraction angle. Adv. Opt. Mater. 2020, 8, 2000170. [Google Scholar] [CrossRef]
- Smith, C.; Sabatino, D.; Praisner, T. Temperature sensing with thermochromic liquid crystals. Exp. Fluids 2001, 30, 190–200. [Google Scholar] [CrossRef]
- Han, Y.; Pacheco, K.; Bastiaansen, C.W.; Broer, D.J.; Sijbesma, R.P. Optical monitoring of gases with cholesteric liquid crystals. J. Am. Chem. Soc. 2010, 132, 2961–2967. [Google Scholar] [CrossRef]
- Saha, A.; Tanaka, Y.; Han, Y.; Bastiaansen, C.M.; Broer, D.J.; Sijbesma, R.P. Irreversible visual sensing of humidity using a cholesteric liquid crystal. Chem. Commun. 2012, 48, 4579–4581. [Google Scholar] [CrossRef]
- Su, H.; Shi, S.; Zhu, M.; Crump, D.; Letcher, R.J.; Giesy, J.P.; Su, G. Persistent, bioaccumulative, and toxic properties of liquid crystal monomers and their detection in indoor residential dust. Proc. Natl. Acad. Sci. USA 2019, 116, 26450–26458. [Google Scholar] [CrossRef]
- Lin, C.-M.; Wu, P.-C.; Lee, M.-J.; Lee, W. Label-free protein quantitation by dielectric spectroscopy of dual-frequency liquid crystal. Sens. Actuators B Chem. 2019, 282, 158–163. [Google Scholar] [CrossRef]
- Lee, M.-J.; Chang, C.-H.; Lee, W. Label-free protein sensing by employing blue phase liquid crystal. Biomed. Opt. Express 2017, 8, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Brake, J.M.; Mezera, A.D.; Abbott, N.L. Effect of surfactant structure on the orientation of liquid crystals at aqueous-liquid crystal interfaces. Langmuir 2003, 19, 6436–6442. [Google Scholar] [CrossRef]
- Lockwood, N.A.; De pablo, J.J.; Abbott, N.L. Influence of surfactant tail branching and organization on the orientation of liquid crystals at aqueous-liquid crystal interfaces. Langmuir 2005, 21, 6805–6814. [Google Scholar] [CrossRef] [PubMed]
- Meli, M.V.; Lin, I.H.; Abbott, N.L. Preparation of microscopic and planar oil-water interfaces that are decorated with prescribed densities of insoluble amphiphiles. J. Am. Chem. Soc. 2008, 130, 4326–4333. [Google Scholar] [CrossRef] [PubMed]
- Price, A.D.; Schwartz, D.K. Fatty-acid monolayers at the nematic/water interface: Phases and liquid-crystal alignment. J. Phys. Chem. B 2007, 111, 1007–1015. [Google Scholar] [CrossRef]
- Sivakumar, S.; Wark, K.L.; Gupta, J.K.; Abbott, N.L.; Caruso, F. Liquid crystal emulsions as the basis of biological sensors for the optical detection of bacteria and viruses. Adv. Funct. Mater. 2009, 19, 2260–2265. [Google Scholar] [CrossRef]
- Jang, C.-H.; Cheng, L.-L.; Olsen, C.W.; Abbott, N.L. Anchoring of nematic liquid crystals on viruses with different envelope structures. Nano Lett. 2006, 6, 1053–1058. [Google Scholar] [CrossRef]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938. [Google Scholar] [CrossRef]
- Chigrinov, V.G.; Kozenkov, V.M.; Kwok, H. Photoalignment of Liquid Crystalline Materials: Physics and Applications; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Dyadyusha, A.; Khizhnyak, A.; Marusii, T.; Reznikov, Y.; Yaroshchuk, O.; Reshetnyak, V.; Park, W.; Kwon, S.; Shin, H.; Kang, D. An oblique orientation of nematic liquid crystals on a photosensitive aligning polymer. Mol. Cryst. Liq. Cryst. 1995, 263, 399–413. [Google Scholar] [CrossRef]
- Yin, K.; Xiong, J.; He, Z.; Wu, S. Patterning liquid-crystal alignment for ultrathin flat optics. ACS Omega 2020, 5, 31485–31489. [Google Scholar] [CrossRef]
- Seo, D.S.; Matsuda, H.; Oh-ide, T.; Kobayashi, S. Alignment of nematic liquid crystal(5CB) on the treated substrates: Characterization of orientation films, generation of pretilt angles, and surface anchoring strength. Mol. Cryst. Liq. Cryst. 1993, 224, 13–31. [Google Scholar] [CrossRef]
- Weng, L.; Liao, P.-C.; Lin, C.-C.; Ting, T.-L.; Hsu, W.-H.; Su, J.-J.; Chien, L.-C. Anchoring energy enhancement and pretilt angle control of liquid crystal alignment on polymerized surfaces. AIP Adv. 2015, 5, 0987218. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.-S. Generation of high pretilt angle and surface anchoring strength in nematic liquid crystal on a rubbed polymer surface. J. Appl. Phys. 1999, 86, 3594–3597. [Google Scholar] [CrossRef]
- Park, K.-S.; Baek, J.-H.; Lee, Y.-J.; Kim, J.-H.; Yu, C.-J. Effects of pretilt angle and anchoring energy on alignment of uniformly lying helix mode. Liq. Cryst. 2016, 43, 1184–1189. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Mittal, K.L. Polyimide: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996; pp. 806–807. [Google Scholar]
- Feller, M.B.; Chen, W.; Shen, Y.R. Investigation of surface-induced alignment of liquid-crystal molecules by optical second-harmonic generation. Phys. Rev. A 1991, 43, 6778–6792. [Google Scholar] [CrossRef]
- Van Aerle, N.; Tol, A.J.W. Molecular orientation in rubbed polyimide alignment layers used for liquid-crystal displays. Macromolecules 1994, 27, 6520–6526. [Google Scholar] [CrossRef]
- Lee, K.-W.; Paek, S.-H.; Lien, A.; Durning, C.; Fukuro, H. Microscopic molecular reorientation of alignment layer polymer surfaces induced by rubbing and its effects on LC pretilt angles. Macromolecules 1996, 29, 8894–8899. [Google Scholar] [CrossRef]
- Weiss, K.; Wöll, C.; Höhm, E.; Fiebranz, B.; Forstmann, G.; Peng, B.; Scheumann, V.; Johannsmann, D. Molecular orientation at rubbed polyimide surfaces determined with X-ray absorption spectroscopy: Relevance for liquid crystal alignment. Macromolecules 1998, 31, 1930–1936. [Google Scholar] [CrossRef]
- Stöhr, J.; Samant, M.G.; Cossy-Favre, A.; Diaz, J.; Momoi, Y.; Odahara, S.; Nagata, T. Microscopic origin of liquid crystal alignment on rubbed polymer surfaces. Macromolecules 1998, 31, 1942–1946. [Google Scholar] [CrossRef]
- Meister, R.; Jérôme, B. The conformation of a rubbed polyimide. Macromolecules 1999, 32, 480–486. [Google Scholar] [CrossRef]
- Ge, J.J.; Li, C.Y.; Xue, G.; Mann, I.K.; Zhang, D.; Wang, S.-Y.; Harris, F.W.; Cheng, S.Z.; Hong, S.-C.; Zhuang, X. Rubbing-induced molecular reorientation on an alignment surface of an aromatic polyimide containing cyanobiphenyl side chains. J. Am. Chem. Soc. 2001, 123, 5768–5776. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Oh-e, M.; Shen, Y.R. Rubbed polyimide surface studied by sum-frequency vibrational spectroscopy. Macromolecules 2001, 34, 9125–9129. [Google Scholar] [CrossRef]
- Vaughn, K.E.; Sousa, M.; Kang, D.; Rosenblatt, C. Continuous control of liquid crystal pretilt angle from homotropic to planar. Appl. Phys. Lett. 2007, 90, 194102. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Kim, S.I.; Park, Y.H.; Reea, M.; Rim, Y.N.; Yoon, H.J.; Kim, H.C.; Kim, Y.B. Liquid-crystal alignment on the rubbed film surface of semi-flexible copolyimides containing n-alkyl side groups. Mol. Cryst. Liq. Cryst. 2000, 349, 279–282. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.W.; Ha, J.D.; Oh, J.M.; Yi, M.H. Synthesis and characterization of novel polyimides with 1-octadecyl side chains for liquid crystal alignment layers. Polym. Adv. Technol. 2007, 18, 226–234. [Google Scholar] [CrossRef]
- Lee, S.W.; Chae, B.; Lee, B.; Choi, W.; Kim, S.B.; Kim, S.I. Rubbing-induced surface morphology and polymer segmental reorientations of a model brush polyimide and interactions with liquid crystals at the surface. Chem. Mater. 2003, 15, 3105–3112. [Google Scholar] [CrossRef]
- Lee, S.B.; Shin, G.J.; Chi, J.H.; Zin, W.-C.; Jung, J.C.; Hahm, S.G.; Ree, M.; Chang, T. Synthesis, characterization and liquid-crystal-aligning properties of novel aromatic polypyromellitimides bearing (n-alkyloxy)biphenyloxy side chains. Polymer 2006, 47, 6606–6621. [Google Scholar] [CrossRef]
- Kang, H.; Park, J.S.; Kang, D.; Lee, J.-C. Liquid crystal alignment property of n-alkylthiomethyl- or n-alkylsulfonylmethyl-substituted polystyrenes. Polym. Adv. Technol. 2009, 20, 878–886. [Google Scholar] [CrossRef]
- Evans, P.H.; Bowers, W.S.; Funk, E.J. Identification of fungicidal and nematocidal components in the leaves of piper betle (piperaceae). J. Agric. Food Chem. 1984, 32, 1254–1256. [Google Scholar] [CrossRef]
- Garg, S.C.; Jain, R. Biological activity of the essential oil of Piper betle L. J. Essent. Oil Res. 1992, 4, 601–606. [Google Scholar] [CrossRef]
- Lubis, R.R.; Marlisa; Wahyuni, D.D. Antibacterial activity of betle leaf (piper betle l.) extract on inhibiting staphylococcus aureus in conjunctivitis patient. Am. J. Clin. Exp. Immunol. 2020, 9, 1–5. [Google Scholar]
- Sarma, C.; Rasane, P.; Kaur, S.; Singh, J.; Singh, J.; Gat, Y.; Garba, U.; Kaur, D.; Dhawan, K. Antioxidant and antimicrobial potential of selected varieties of piper betle l. (betel leaf). An. Acad. Bras. Ciências 2018, 90, 3871–3878. [Google Scholar] [CrossRef]
- Row, L.-C.M.; Ho, J.-C. The antimicrobial activity, mosquito larvicidal activity, antioxidant property and tyrosinase inhibition of piper betle. J. Chin. Chem. Soc. 2009, 56, 653–658. [Google Scholar] [CrossRef]
- Alam, B.; Akter, F.; Parvin, N.; Pia, R.S.; Akter, S.; Chowdhury, J.; Sifath-E-Jahan, K.; Haque, E. Antioxidant, analgesic and anti-inflammatory activities of the methanolic extract of piper betle leaves. Avicenna J. Phytomed. 2013, 3, 112–125. [Google Scholar]
- Rintu, D.; Shinjini, M.; Kaustab, M.; Pramathadhip, P.; Umesh, P.S.; Banerjee, E.R. Anti-oxidant and anti-inflammatory activities of different varieties of piper leaf extracts (piper beltle l.). J. Nutr. Food Sci. 2015, 5, 415. [Google Scholar]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food. Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Koeduka, T. The phenylpropene synthase pathway and its applications in the engineering of volatile phenylpropanoids in plants. Plant Biotechnol. 2014, 31, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.G. Phenylpropenes: Occurrence, distribution, and biosynthesis in fruit. J. Agric. Food Chem. 2018, 66, 2259–2272. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Gedde, U. Polymer Physics; Chapman and Hall: London, UK, 1995; pp. 78–79. [Google Scholar]
- Hayes, R.A. The relationship between glass temperature, molar cohesion, and polymer structure. J. Appl. Polym. Sci. 1961, 5, 318–321. [Google Scholar] [CrossRef]
- Wesslen, B.; Lenz, R.; Macknight, W.; Karasz, F. Glass transition temperatures of poly(ethyl α-chloroacrylates). Macromolecules 1971, 4, 24–26. [Google Scholar] [CrossRef]
- Lee, J.-C.; Litt, M.H.; Rogers, C.E. Oxyalkylene polymers with alkylsulfonylmethyl side chains: Gas barrier properties. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 75–83. [Google Scholar] [CrossRef]
- Lee, J.-B.; Lee, H.-K.; Park, J.-C.; Kim, Y.-B. The structural effects on the pretilt angle of alignment materials with alkylcyclohexylbenzene as a side chain in polyimides. Mol. Cryst. Liq. Cryst. 2005, 439, 161–172. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.J.; Hahm, S.G.; Lee, T.J.; Lee, B.; Cha, B.; Kim, S.B.; Jung, J.C.; Zin, W.C.; Sohn, B.H.; et al. Role of the n-alkyl end of bristles in governing liquid crystal alignment at rubbed films of brush polymer rods. Macromolecules 2005, 38, 4331–4338. [Google Scholar] [CrossRef]
- Paek, S.H.; Durning, C.J.; Lee, K.W.; Lien, A. A mechanistic picture of the effects of rubbing on polyimide surfaces and liquid crystal pretilt angles. J. Appl. Phys. 1998, 83, 1270–1280. [Google Scholar] [CrossRef]
- Ban, B.S.; Kim, Y.B. Surface energy and pretilt angle on rubbed polyimide surfaces. J. Appl. Polym. Sci. 1999, 74, 267–271. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, C.Y.; Lin, C.J.; Pan, R.P.; Lin, S.S.; Lee, C.D.; Kou, C.S. Mechanism in determining pretilt angle of liquid crystals aligned on fluorinated copolymer films. J. Phys. D Appl. Phys. 2009, 42, 155303. [Google Scholar] [CrossRef]

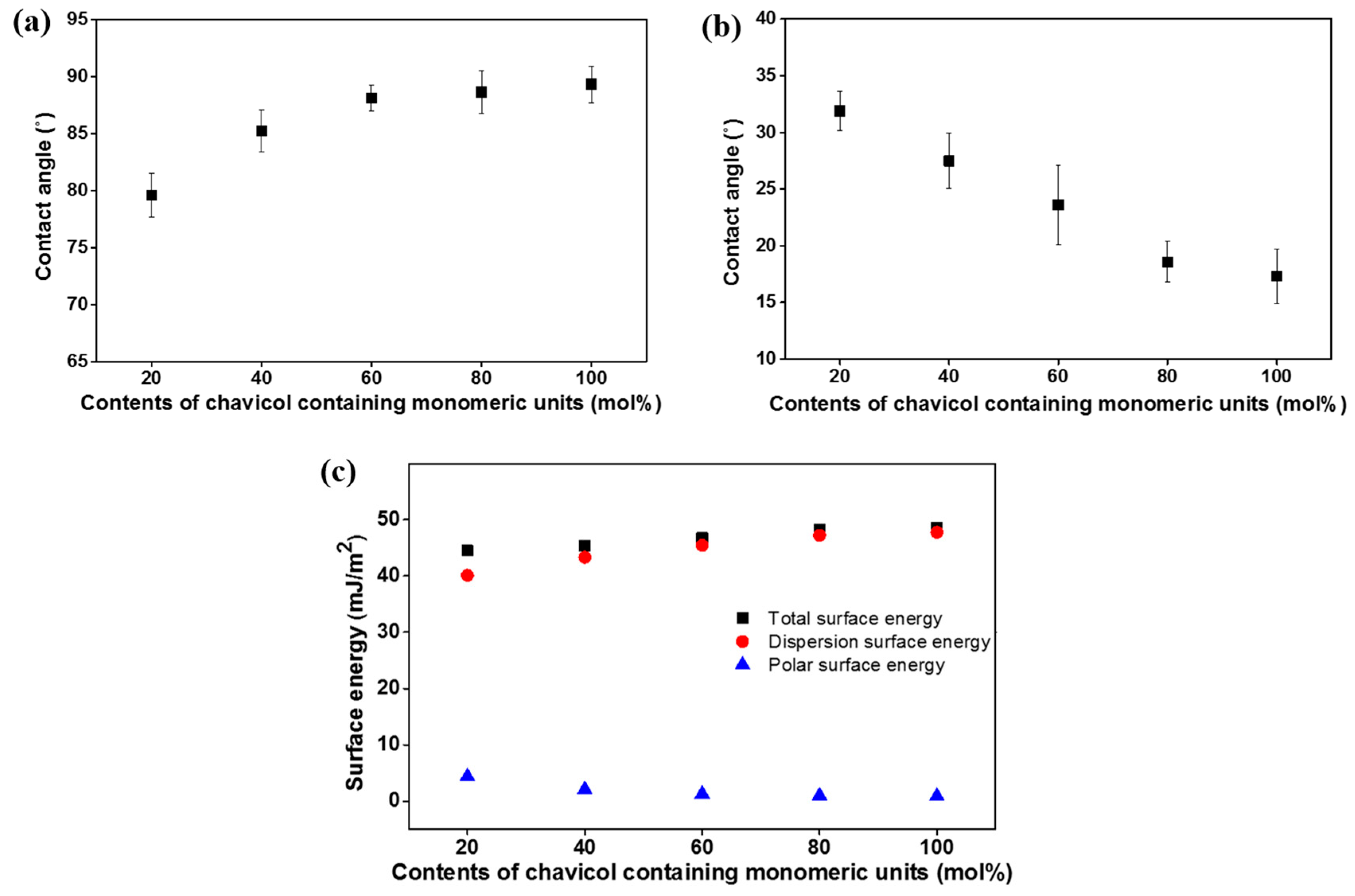
| Polymer Designation | Contact Angle (°) a | Surface Eergy (mJ m−2) b | LC Aligning Ability c | |||
|---|---|---|---|---|---|---|
| Water | Diiodomethane | Polar | Dispersion | Total | ||
| PCHA20 | 79.7 | 31.6 | 4.4 | 40.1 | 44.5 | X |
| PCHA40 | 83.9 | 29.1 | 2.1 | 43.3 | 45.4 | X |
| PCHA60 | 87.9 | 20.8 | 1.3 | 45.4 | 46.7 | O |
| PCHA80 | 88.5 | 20.7 | 1.0 | 47.2 | 48.2 | O |
| PCHA100 | 88.7 | 18.8 | 0.9 | 47.7 | 48.6 | O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.; Seo, K.; Kang, H. Vertical Alignment of Liquid Crystals on Comb-Like Renewable Chavicol-Modified Polystyrene. Polymers 2021, 13, 819. https://doi.org/10.3390/polym13050819
Moon J, Seo K, Kang H. Vertical Alignment of Liquid Crystals on Comb-Like Renewable Chavicol-Modified Polystyrene. Polymers. 2021; 13(5):819. https://doi.org/10.3390/polym13050819
Chicago/Turabian StyleMoon, Jihyeon, Kyutae Seo, and Hyo Kang. 2021. "Vertical Alignment of Liquid Crystals on Comb-Like Renewable Chavicol-Modified Polystyrene" Polymers 13, no. 5: 819. https://doi.org/10.3390/polym13050819