Enhancing Lignin Dissolution and Extraction: The Effect of Surfactants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Conductivity Measurements
2.3. Dissolution Efficiency
2.4. Lignin Extraction and Purity Determination
3. Results and Discussion
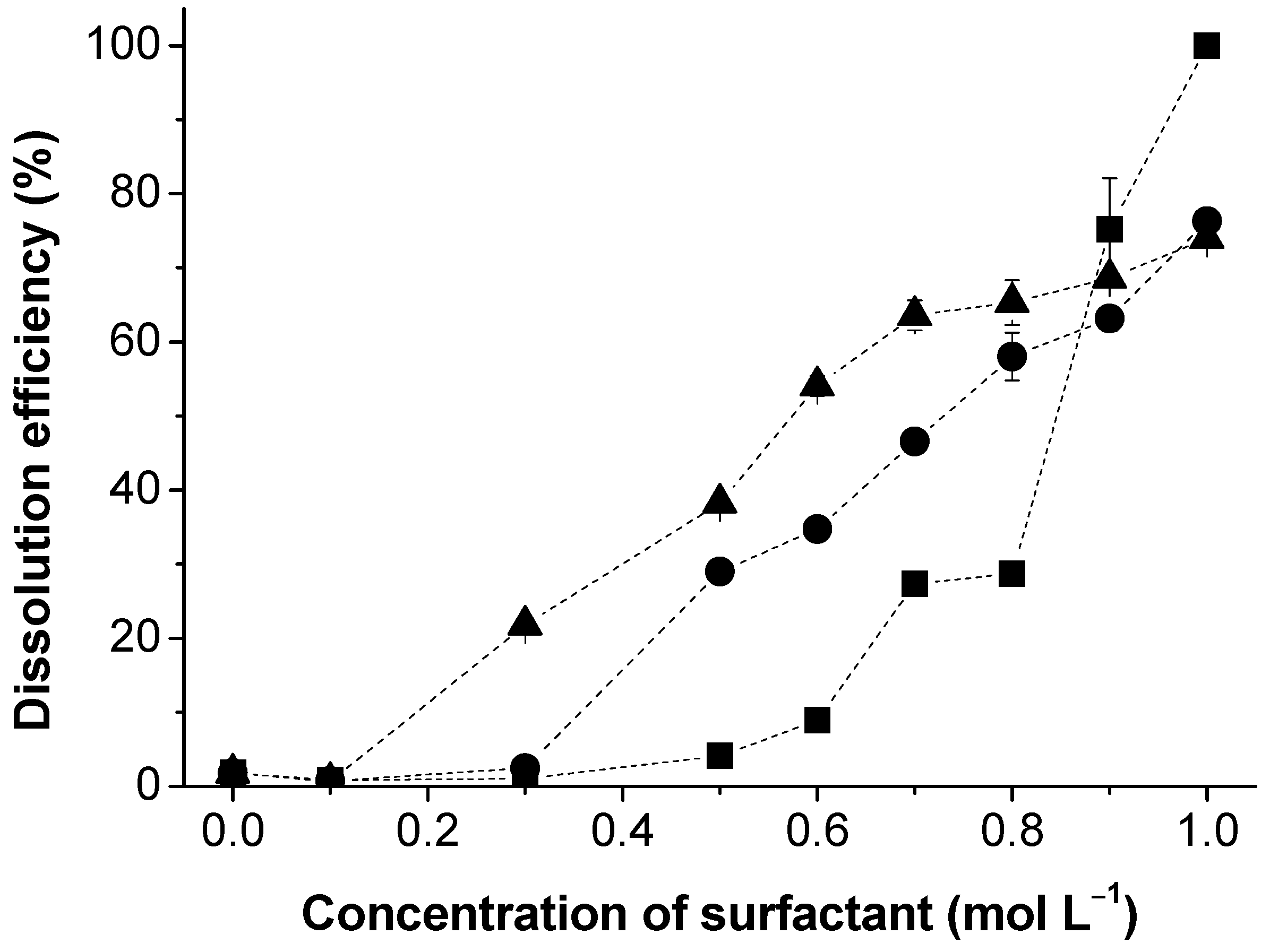
3.1. Lignin Dissolution
3.2. Lignin Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goddard, E.D.; Ananthapadmanabhan, K.P. Interactions of Surfactants with Polymers and Proteins; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351082235. [Google Scholar]
- Kwak, J.C.T. Polymer-Surfactant Systems; CRC Press: Boca Raton, FL, USA, 2020; ISBN 1000110206. [Google Scholar]
- Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surface Chemistry of Surfactants and Polymers; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; ISBN 9781118695968. [Google Scholar]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Zhang, L.; Gellerstedt, G. NMR observation of a new lignin structure, a spiro-dienone. Chem. Commun. 2001, 24, 2744–2745. [Google Scholar] [CrossRef]
- Melro, E.; Alves, L.; Antunes, F.E.; Medronho, B. A brief overview on lignin dissolution. J. Mol. Liq. 2018, 265, 578–584. [Google Scholar] [CrossRef]
- Jiang, F.; Qian, C.; Esker, A.R.; Roman, M. Effect of Nonionic Surfactants on Dispersion and Polar Interactions in the Adsorption of Cellulases onto Lignin. J. Phys. Chem. B 2017, 121, 9607–9620. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Gao, Y.; Lu, L.; Liu, X.; Tong, Z. Aggregates of alginates binding with surfactants of single and twin alkyl chains in aqueous solutions: Fluorescence and dynamic light scattering studies. Carbohydr. Polym. 2006, 66, 266–273. [Google Scholar] [CrossRef]
- Xu, A.; Li, W.; Zhang, Y.; Xu, H. Eco-friendly polysorbate aqueous solvents for efficient dissolution of lignin. RSC Adv. 2016, 6, 8377–8379. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Stabilisation of kraft lignin solutions by surfactant additions. Colloids Surf. Physicochem. Eng. Asp. 2001, 194, 239–248. [Google Scholar] [CrossRef]
- Singh, S.K. Biological treatment of plant biomass and factors affecting bioactivity. J. Clean. Prod. 2020, 279, 123546. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, M.; Zhou, Q.; Liu, H. Effect of lignin degradation product sinapyl alcohol on laccase catalysis during lignin degradation. Ind. Crops Prod. 2019, 139, 111544. [Google Scholar] [CrossRef]
- Wang, B.; Yang, G.; Wang, Q.; Liu, S.; Chen, J.; Fang, G. A new surfactant assisted acid prehydrolysis process for enhancing biomass pretreatment. Cellulose 2020, 27, 2149–2160. [Google Scholar] [CrossRef]
- Qing, Q.; Yang, B.; Wyman, C.E. Impact of surfactants on pretreatment of corn stover. Bioresour. Technol. 2010, 101, 5941–5951. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, Y.; Abyaz, A.; Niaraki, M.O.S.M.; Abdulkhani, A. Application of surfactants as pulping additives in soda pulping of bagasse. BioResources 2009, 4, 1267–1275. [Google Scholar] [CrossRef]
- Norgren, M.; Mackin, S. Sulfate and surfactants as boosters of kraft lignin precipitation. Ind. Eng. Chem. Res. 2009, 48, 5098–5104. [Google Scholar] [CrossRef]
- Wang, W.; Zhuang, X.; Tan, X.; Wang, Q.; Chen, X.; Yu, Q.; Qi, W.; Wang, Z.; Yuan, Z. Dual Effect of Nonionic Surfactants on Improving the Enzymatic Hydrolysis of Lignocellulose. Energy Fuels 2018, 32, 5951–5959. [Google Scholar] [CrossRef]
- Mesquita, J.F.; Ferraz, A.; Aguiar, A. Alkaline-sulfite pretreatment and use of surfactants during enzymatic hydrolysis to enhance ethanol production from sugarcane bagasse. Bioprocess. Biosyst. Eng. 2016, 39, 441–448. [Google Scholar] [CrossRef]
- Zhang, H.; Lyu, G.; Zhang, A.; Li, X.; Xie, J. Effects of ferric chloride pretreatment and surfactants on the sugar production from sugarcane bagasse. Bioresour. Technol. 2018, 265, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Chen, X.; Wan, Y. Pretreatment of wheat straw by nonionic surfactant-assisted dilute acid for enhancing enzymatic hydrolysis and ethanol production. Bioresour. Technol. 2010, 101, 4875–4883. [Google Scholar] [CrossRef]
- Parnthong, J.; Kungsanant, S.; Chavadej, S. The Influence of Nonionic Surfactant Adsorption on Enzymatic Hydrolysis of Oil Palm Fruit Bunch. Appl. Biochem. Biotechnol. 2018, 186, 895–908. [Google Scholar] [CrossRef]
- Alkasrawi, M.; Eriksson, T.; Börjesson, J.; Wingren, A.; Galbe, M.; Tjerneld, F.; Zacchi, G. The effect of Tween-20 on simultaneous saccharification and fermentation of softwood to ethanol. Enzyme Microb. Technol. 2003, 33, 71–78. [Google Scholar] [CrossRef]
- Li, K.; Wan, J.; Wang, X.; Wang, J.; Zhang, J. Comparison of dilute acid and alkali pretreatments in production of fermentable sugars from bamboo: Effect of Tween 80. Ind. Crops Prod. 2016, 83, 414–422. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, L.; Ma, G.; Jin, D.; Zhao, L.; Ouyang, X. Lignin Removal from Tobacco Stem with Laccase Improved by Synergistic Action of Weak Alkali and Tween 80. Waste Biomass Valorization 2019, 10, 3343–3350. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Z.; Zhang, R.; Wang, D.; Jenkins, B. Non-Ionic Surfactants and Non-Catalytic Protein Treatment on Enzymatic Hydrolysis of Pretreated Creeping Wild Ryegrass; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Méndez Arias, J.; de Oliveira Moraes, A.; Modesto, L.F.A.; de Castro, A.M.; Pereira, N. Addition of Surfactants and Non-Hydrolytic Proteins and Their Influence on Enzymatic Hydrolysis of Pretreated Sugarcane Bagasse. Appl. Biochem. Biotechnol. 2017, 181, 593–603. [Google Scholar] [CrossRef]
- Chen, Y.A.; Zhou, Y.; Liu, D.; Zhao, X.; Qin, Y. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and lignin structure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.B.; Kim, C.J. The effects of nonionic surfactants on the pretreatment and enzymatic hydrolysis of recycled newspaper. Biotechnol. Bioprocess. Eng. 2007, 12, 147–151. [Google Scholar] [CrossRef]
- Barthel, J.; Feuerlein, F.; Neueder, R.; Wachter, R. Calibration of conductance cells at various temperatures. J. Solut. Chem. 1980, 9, 209–219. [Google Scholar] [CrossRef]
- Templeton, D.; Ehrman, T. Chemical Analysis and Testing Task: LAP-003 (Determination of Acid-Insoluble Lignin in Biomass); National Renewable Energy Laboratory: Golden, CO, USA, 1995.
- Ehrman, T. Determination of Acid Soluble Lignin in Biomass, Chemical Analysis and Testing Task, Laboratory Analytical Procedure (LAP-004); Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 1996.
- Ansari, A.A.; Kamil, M.; Kabir-ud-Din. Polymer-Surfactant Interactions and the Effect of Tail Size Variation on Micellization Process of Cationic ATAB Surfactants in Aqueous Medium. J. Dispers. Sci. Technol. 2013, 34, 722–730. [Google Scholar] [CrossRef]
- Banipal, T.S.; Kaur, H.; Banipal, P.K.; Sood, A.K. Effect of head groups, temperature, and polymer concentration on surfactant—Polymer interactions. J. Surfactants Deterg. 2014, 17, 1181–1191. [Google Scholar] [CrossRef]
- Ruiz, C.C. Thermodynamics of micellization of tetradecyltrimethylammonium bromide in ethylene glycol-water binary mixtures. Colloid Polym. Sci. 1999, 277, 701–707. [Google Scholar] [CrossRef]
- Jang, J.; Bae, J.; Park, E. Selective fabrication of poly(3,4-ethylenedioxythiophene) nanocapsules and mesocellular foams using surfactant-mediated interfacial polymerization. Adv. Mater. 2006, 18, 354–358. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Notley, S.M.; Norgren, M. Adsorption of a strong polyelectrolyte to model lignin surfaces. Biomacromolecules 2008, 9, 2081–2086. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, S.; Qiu, X.; Luo, Y.; Lou, H.; Huang, J. Preparation of Lignin/Sodium Dodecyl Sulfate Composite Nanoparticles and Their Application in Pickering Emulsion Template-Based Microencapsulation. J. Agric. Food Chem. 2017, 65, 11011–11019. [Google Scholar] [CrossRef]
- Notley, S.M.; Norgren, M. Measurement of interaction forces between lignin and cellulose as a function of aqueous electrolyte solution conditions. Langmuir 2006, 22, 11199–11204. [Google Scholar] [CrossRef]
- Helenius, A.; Simons, K. Solubilization of membranes by detergents. BBA Rev. Biomembr. 1975, 415, 29–79. [Google Scholar] [CrossRef]
- Fijan, R.; Šostar-Turk, S.; Lapasin, R. Rheological study of interactions between non-ionic surfactants and polysaccharide thickeners used in textile printing. Carbohydr. Polym. 2007, 68, 708–717. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Qiu, X.; Liu, D.; Yang, D.; Liu, W.; Qian, Y. Dissolution of lignin in green urea aqueous solution. Appl. Surf. Sci. 2017, 425, 736–741. [Google Scholar] [CrossRef]
- Appell, J.; Porte, G.; Rawiso, M. Interactions between Nonionic Surfactant Micelles Introduced by a Telechelic Polymer. A Small Angle Neutron Scattering Study. Langmuir 1998, 14, 4409–4414. [Google Scholar] [CrossRef]
- Winnik, F.M.; Regismond, S.T.A. Fluorescence methods in the study of the interactions of surfactants with polymers. Colloids Surf. Physicochem. Eng. Asp. 1996, 118, 1–39. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Sachar, S. Surfactant polymer interactions between strongly interacting cationic surfactants and anionic polyelectrolytes from conductivity and turbidity measurements. Colloid Polym. Sci. 2004, 282, 993–999. [Google Scholar] [CrossRef]
- Ali, M.S.; Suhail, M.; Ghosh, G.; Kamil, M.; Kabir-ud-Din. Interactions between cationic gemini/conventional surfactants with polyvinylpyrrolidone: Specific conductivity and dynamic light scattering studies. Colloids Surf. Physicochem. Eng. Asp. 2009, 350, 51–56. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, S.; Aswal, V.K.; Mahajan, R.K. Interactional and aggregation behavior of twin tail cationic surfactants with pluronic L64 in aqueous solution. Colloid Polym. Sci. 2012, 290, 127–139. [Google Scholar] [CrossRef]
- Cabane, B. Structure of some polymer-detergent aggregates in water. J. Phys. Chem. 1977, 81, 1639–1645. [Google Scholar] [CrossRef]
- Pettersson, E.; Topgaard, D.; Stilbs, P.; Söderman, O. Surfactant/Nonionic Polymer Interaction. A NMR Diffusometry and NMR Electrophoretic Investigation. Langmuir 2004, 20, 1138–1143. [Google Scholar] [CrossRef]
- Rivero, D.; Gouveia, L.M.; Müller, A.J.; Sáez, A.E. Shear-thickening behavior of high molecular weight poly(ethylene oxide) solutions. Rheol. Acta 2012, 51, 13–20. [Google Scholar] [CrossRef]
- Khan, M.Y.; Samanta, A.; Ojha, K.; Mandal, A. Interaction between aqueous solutions of polymer and surfactant and its effect on physicochemical properties. Asia-Pac. J. Chem. Eng. 2008, 3, 579–585. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Lobo, V.M.M.; Valente, A.J.M.; Azevedo, E.F.; Miguel, M.D.G.; Burrows, H.D. Transport properties of alkyltrimethylammonium bromide surfactants in aqueous solutions. Colloid Polym. Sci. 2004, 283, 277–283. [Google Scholar] [CrossRef]
- Evans, D.F.; Allen, M.; Ninham, B.W.; Fouda, A. Critical micelle concentrations for alkyltrimethylammonium bromides in water from 25 to 160 °C. J. Solut. Chem. 1984, 13, 87–101. [Google Scholar] [CrossRef]
- Burrows, H.D.; Valente, A.J.M.; Costa, T.; Stewart, B.; Tapia, M.J.; Scherf, U. What conjugated polyelectrolytes tell us about aggregation in polyelectrolyte/surfactant systems. J. Mol. Liq. 2015, 210, 82–99. [Google Scholar] [CrossRef]
- Diamant, H.; Andelman, D. Onset of self-assembly in polymer-surfactant systems. Europhys. Lett. 1999, 48, 170. [Google Scholar] [CrossRef]
- Dal-Bó, A.G.; Laus, R.; Felippe, A.C.; Zanette, D.; Minatti, E. Association of anionic surfactant mixed micelles with hydrophobically modified ethyl(hydroxyethyl)cellulose. Colloids Surf. Physicochem. Eng. Asp. 2011, 380, 100–106. [Google Scholar] [CrossRef]
- Sood, A.K.; Singh, K.; Banipal, T.S. Study of micellization behavior of some alkyldimethylbenzyl ammonium chloride surfactants in the presence of polymers. J. Dispers. Sci. Technol. 2009, 31, 62–71. [Google Scholar] [CrossRef]
- Silva, S.M.C.; Antunes, F.E.; Sousa, J.J.S.; Valente, A.J.M.; Pais, A.A.C.C. New insights on the interaction between hydroxypropylmethyl cellulose and sodium dodecyl sulfate. Carbohydr. Polym. 2011, 86, 35–44. [Google Scholar] [CrossRef]
- Zanette, D.; Ruzza, Â.A.; Froehner, S.J.; Minatti, E. Polymer-surfactant interactions demonstrated by a kinetic probe: Degree of ionization. Colloids Surf. Physicochem. Eng. Asp. 1996, 108, 91–100. [Google Scholar] [CrossRef]
- Zhang, M.; Qi, W.; Liu, R.; Su, R.; Wu, S.; He, Z. Fractionating lignocellulose by formic acid: Characterization of major components. Biomass Bioenergy 2010, 34, 525–532. [Google Scholar] [CrossRef]
- Inkrod, C.; Raita, M.; Champreda, V.; Laosiripojana, N. Characteristics of Lignin Extracted from Different Lignocellulosic Materials via Organosolv Fractionation. Bioenergy Res. 2018, 11, 277–290. [Google Scholar] [CrossRef]
- Erdocia, X.; Prado, R.; Corcuera, M.Á.; Labidi, J. Effect of different organosolv treatments on the structure and properties of olive tree pruning lignin. J. Ind. Eng. Chem. 2014, 20, 1103–1108. [Google Scholar] [CrossRef]
- Snelders, J.; Dornez, E.; Benjelloun-Mlayah, B.; Huijgen, W.J.J.; de Wild, P.J.; Gosselink, R.J.A.; Gerritsma, J.; Courtin, C.M. Biorefining of wheat straw using an acetic and formic acid based organosolv fractionation process. Bioresour. Technol. 2014, 156, 275–282. [Google Scholar] [CrossRef]
- Malkov, S.; Tikka, P.; Gullichsen, J. Towards complete impregnation of wood chips with aqueous solutions. Part I. A retrospective and critical evaluation of the penetration process. Pap. Puu/Paper Timber 2001, 83, 468–473. [Google Scholar]
- Parthasarathy, V.R.; Grygotis, R.C.; Wahoske, K.W.; Bryer, D.M. A sulfur-free, chlorine-free alternative to kraft pulping. Tappi J. 1996, 79, 189–198. [Google Scholar]
- Rashid, T.; Kait, C.F.; Regupathi, I.; Murugesan, T. Dissolution of kraft lignin using Protic Ionic Liquids and characterization. Ind. Crops Prod. 2016, 84, 284–293. [Google Scholar] [CrossRef]
- Sathawong, S.; Sridach, W.; Techato, K.A. Lignin: Isolation and preparing the lignin based hydrogel. J. Environ. Chem. Eng. 2018, 6, 5879–5888. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Sun, R.C.; Tomkinson, J.; Lloyd Jones, G. Fractional characterization of ash-AQ lignin by successive extraction with organic solvents from oil palm EFB fibre. Polym. Degrad. Stab. 2000, 68, 111–119. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; dos Santos Rosa, D.; Leao, A.; Sain, M. Thermal characteristics of lignin residue from industrial processes. BioResources 2014, 9, 725–737. [Google Scholar] [CrossRef] [Green Version]
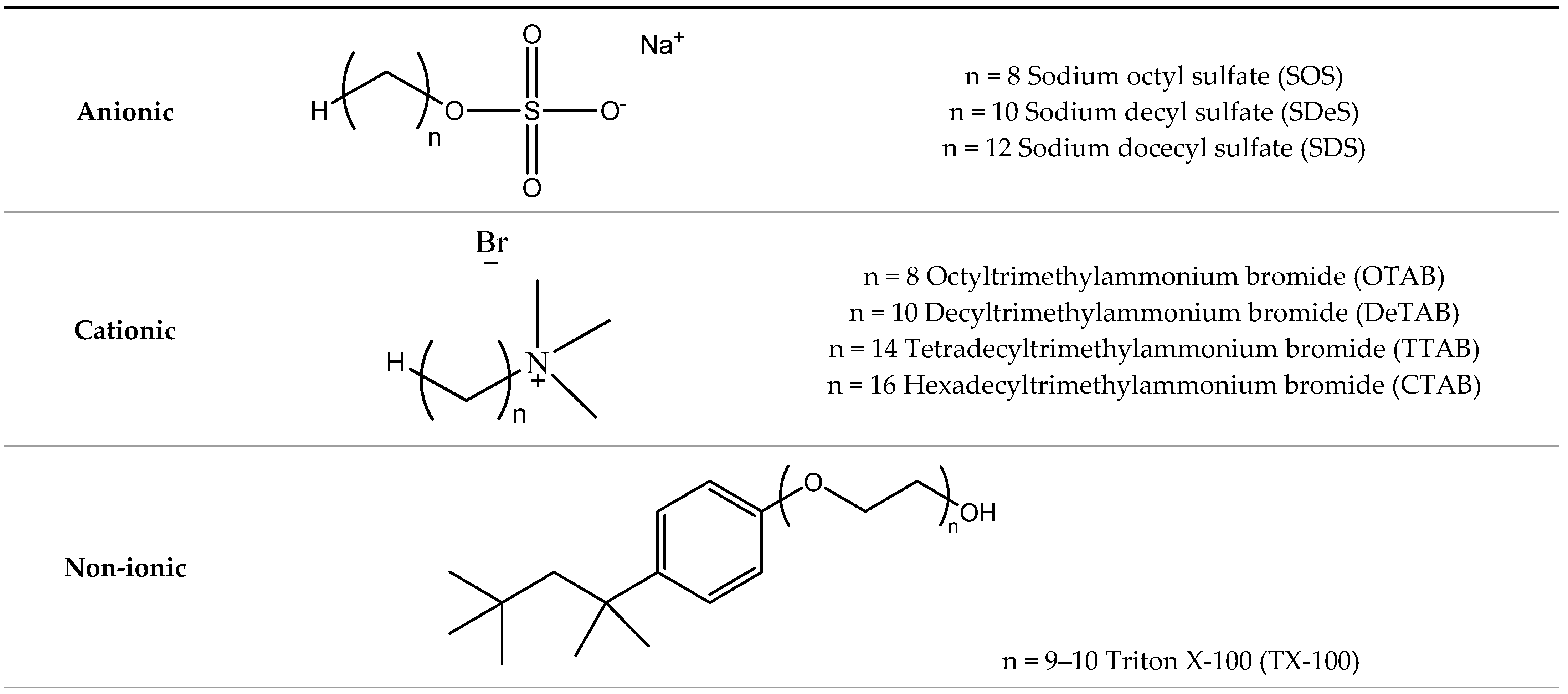
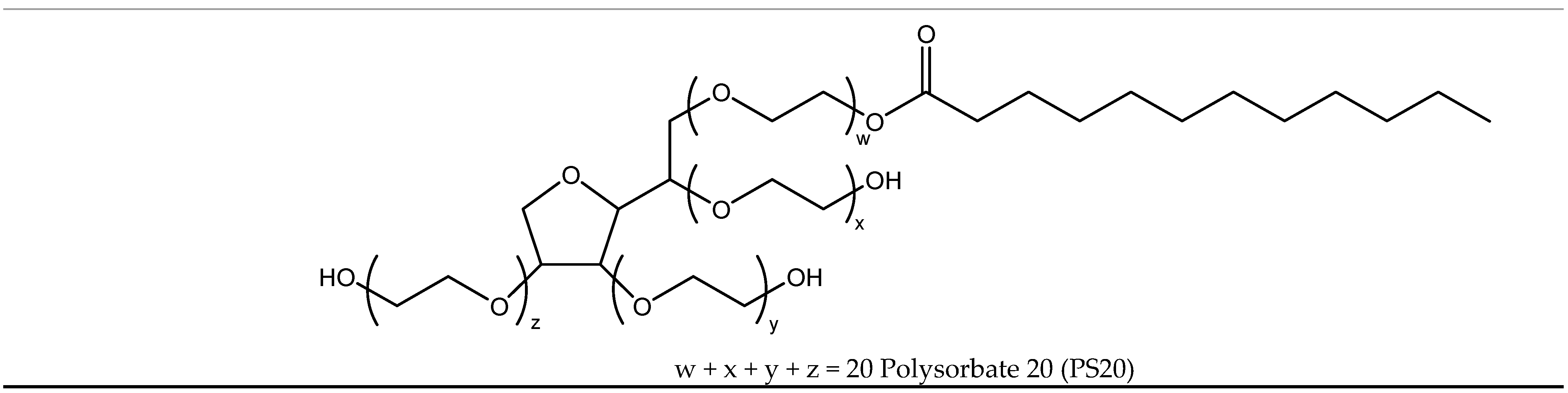
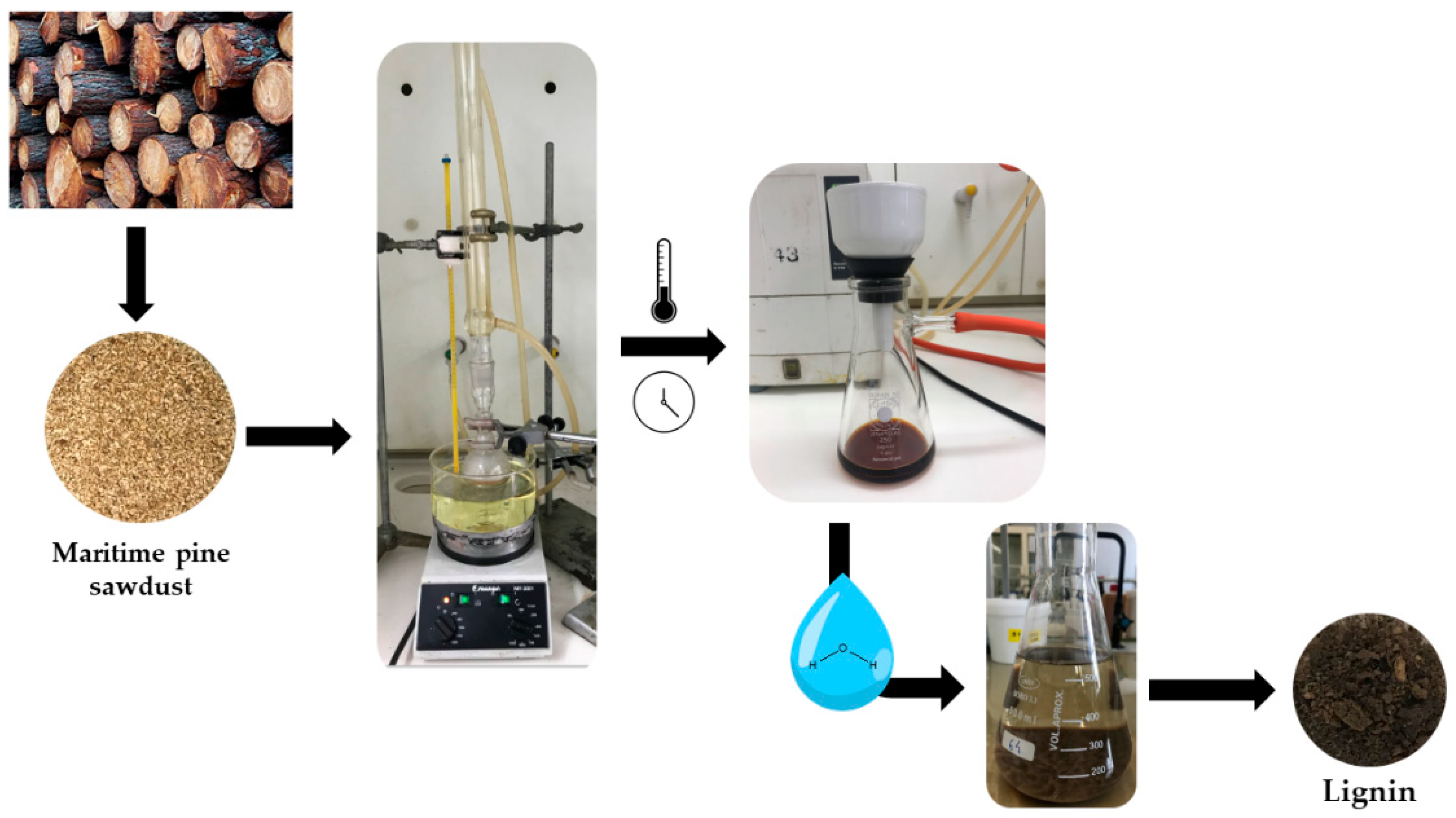



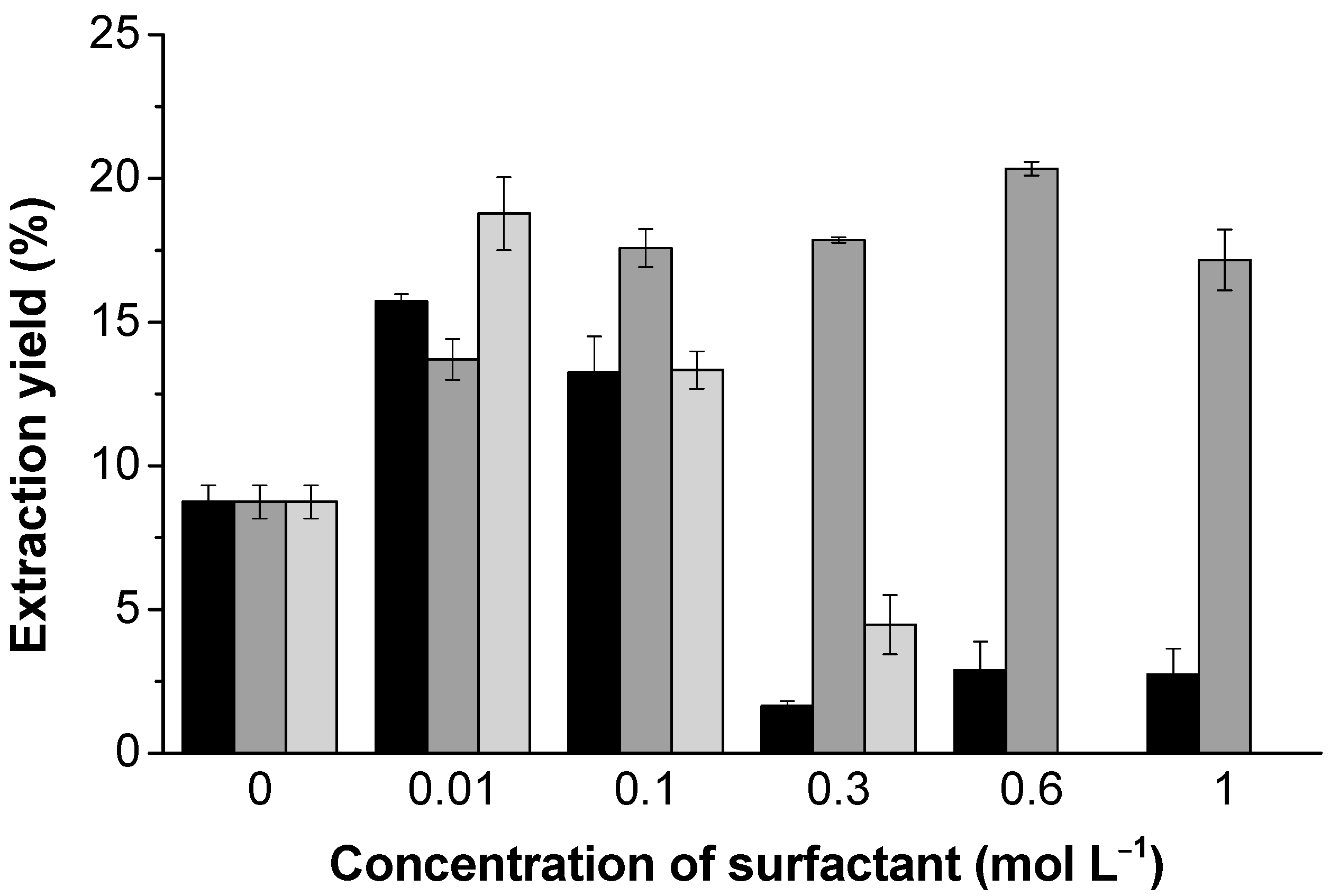
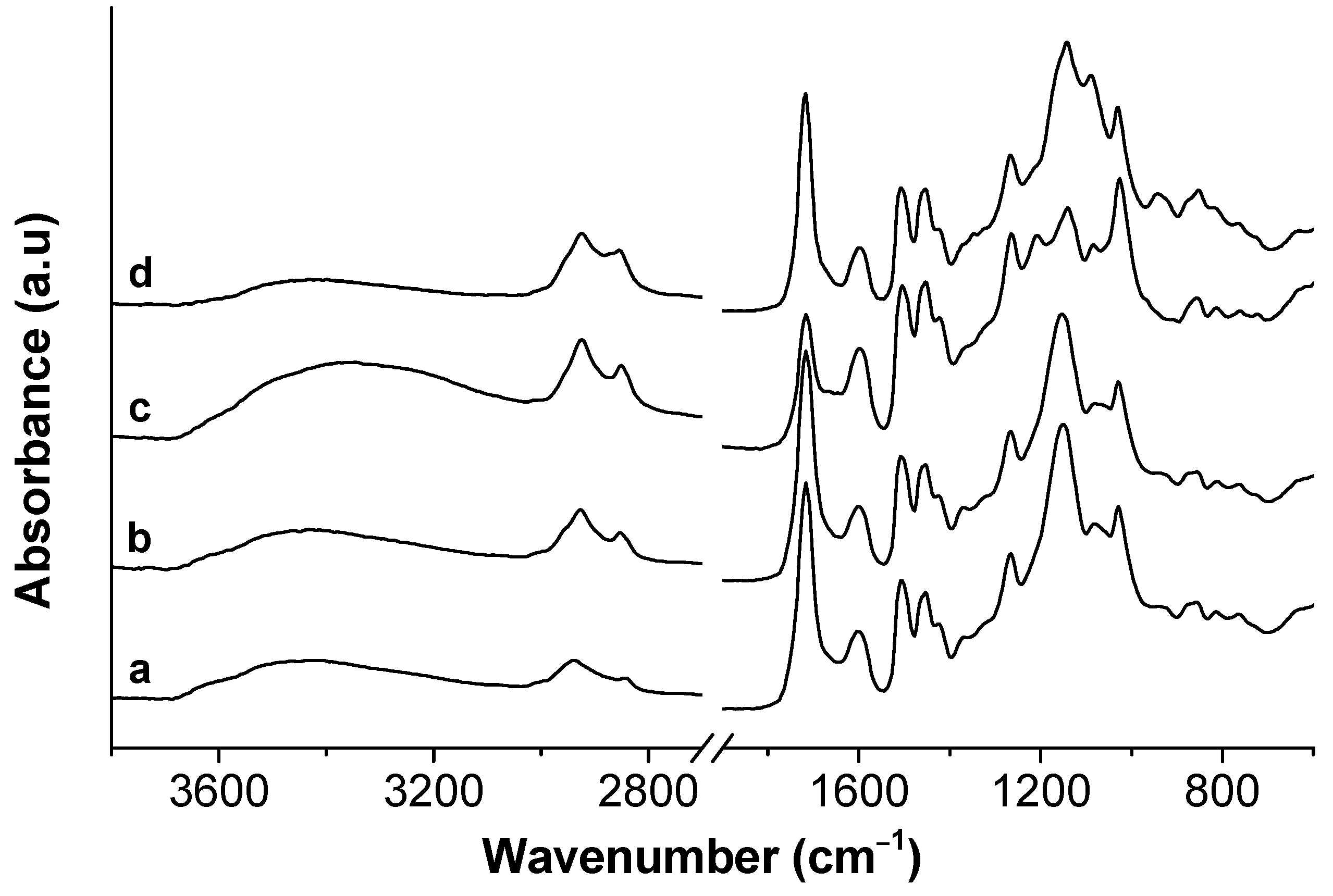

| Kraft Lignin (wt.%) | CAC (mol L−1) | PSP (mol L−1) | CMC’ (mmol L−1) | ΔG0m (kJ mol−1) |
|---|---|---|---|---|
| 0.0 | 0.065 ± 0.005 * | - | 64.6 | −29.8 |
| 0.1 | 0.020 ± 0.002 | 0.036 ± 0.004 | 48.0 | −33.9 |
| 1.0 | 0.020 ± 0.002 | 0.040 ± 0.006 | 70.0 | −31.2 |
| 2.5 | 0.018 ± 0.002 | 0.039 ± 0.006 | 52.0 | −32.5 |
| 5.0 | 0.018 ± 0.002 | 0.041 ± 0.007 | 72.0 | −30.9 |
| 10.0 | 0.015 ± 0.002 | 0.049 ± 0.002 | 53.0 | −28.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melro, E.; Valente, A.J.M.; Antunes, F.E.; Romano, A.; Medronho, B. Enhancing Lignin Dissolution and Extraction: The Effect of Surfactants. Polymers 2021, 13, 714. https://doi.org/10.3390/polym13050714
Melro E, Valente AJM, Antunes FE, Romano A, Medronho B. Enhancing Lignin Dissolution and Extraction: The Effect of Surfactants. Polymers. 2021; 13(5):714. https://doi.org/10.3390/polym13050714
Chicago/Turabian StyleMelro, Elodie, Artur J. M. Valente, Filipe E. Antunes, Anabela Romano, and Bruno Medronho. 2021. "Enhancing Lignin Dissolution and Extraction: The Effect of Surfactants" Polymers 13, no. 5: 714. https://doi.org/10.3390/polym13050714








