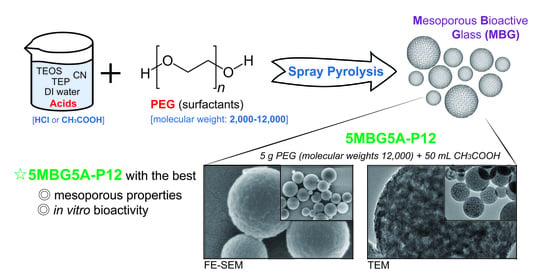
Mesoporous Properties of Bioactive Glass Synthesized by Spray Pyrolysis with Various Polyethylene Glycol and Acid Additions
Abstract
:1. Introduction
2. Materials and Methods
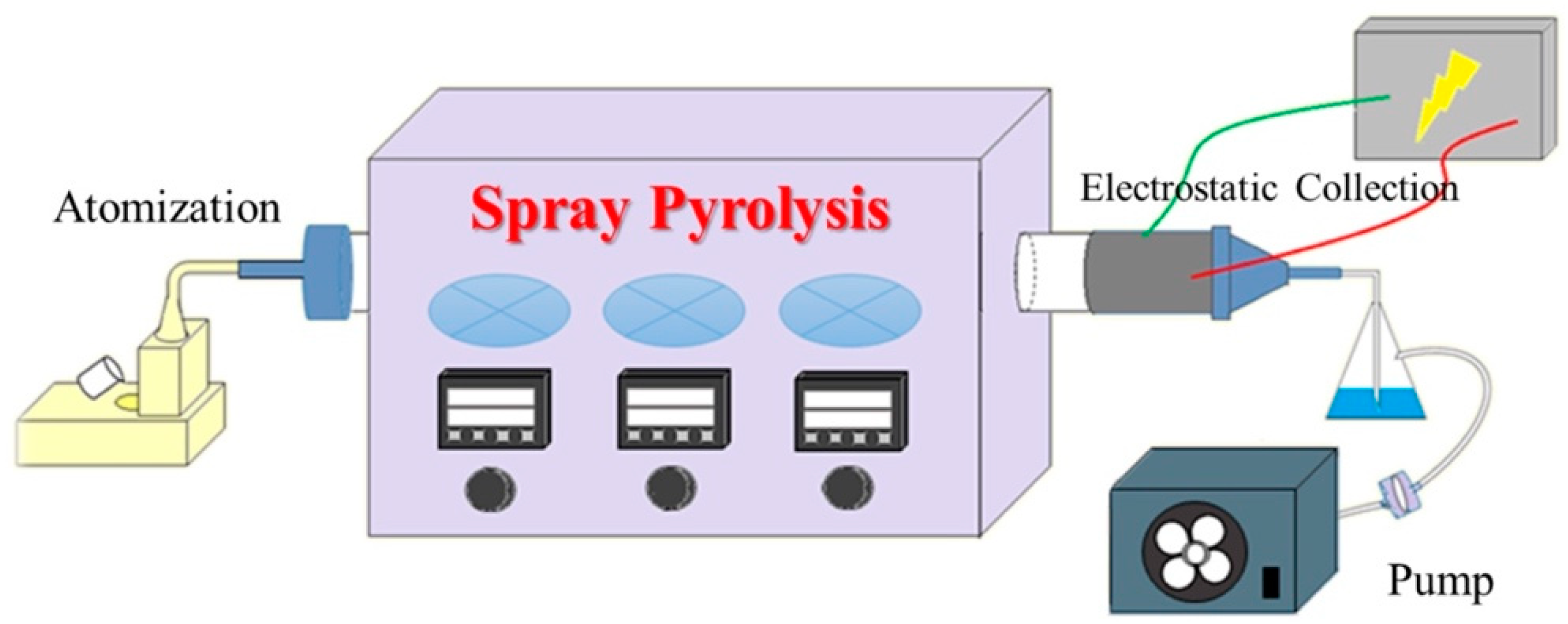
2.1. MBG Particles Synthesis
2.2. Precursor Solution Preparation Conditions
2.3. Characterizations of MBG Particles
2.4. In Vitro Bioactivity Analysis
3. Results
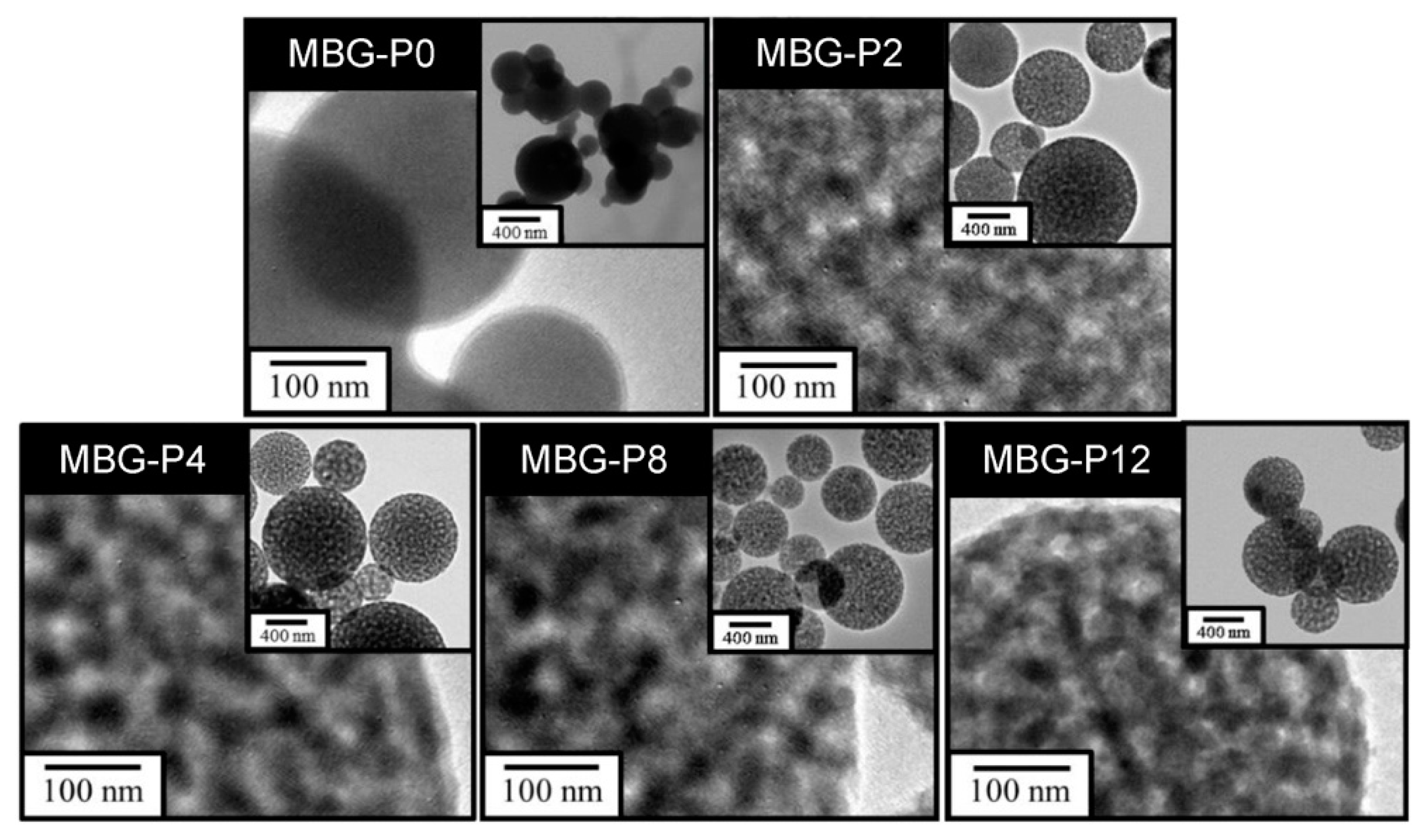
3.1. Different Molecular Weights of PEG
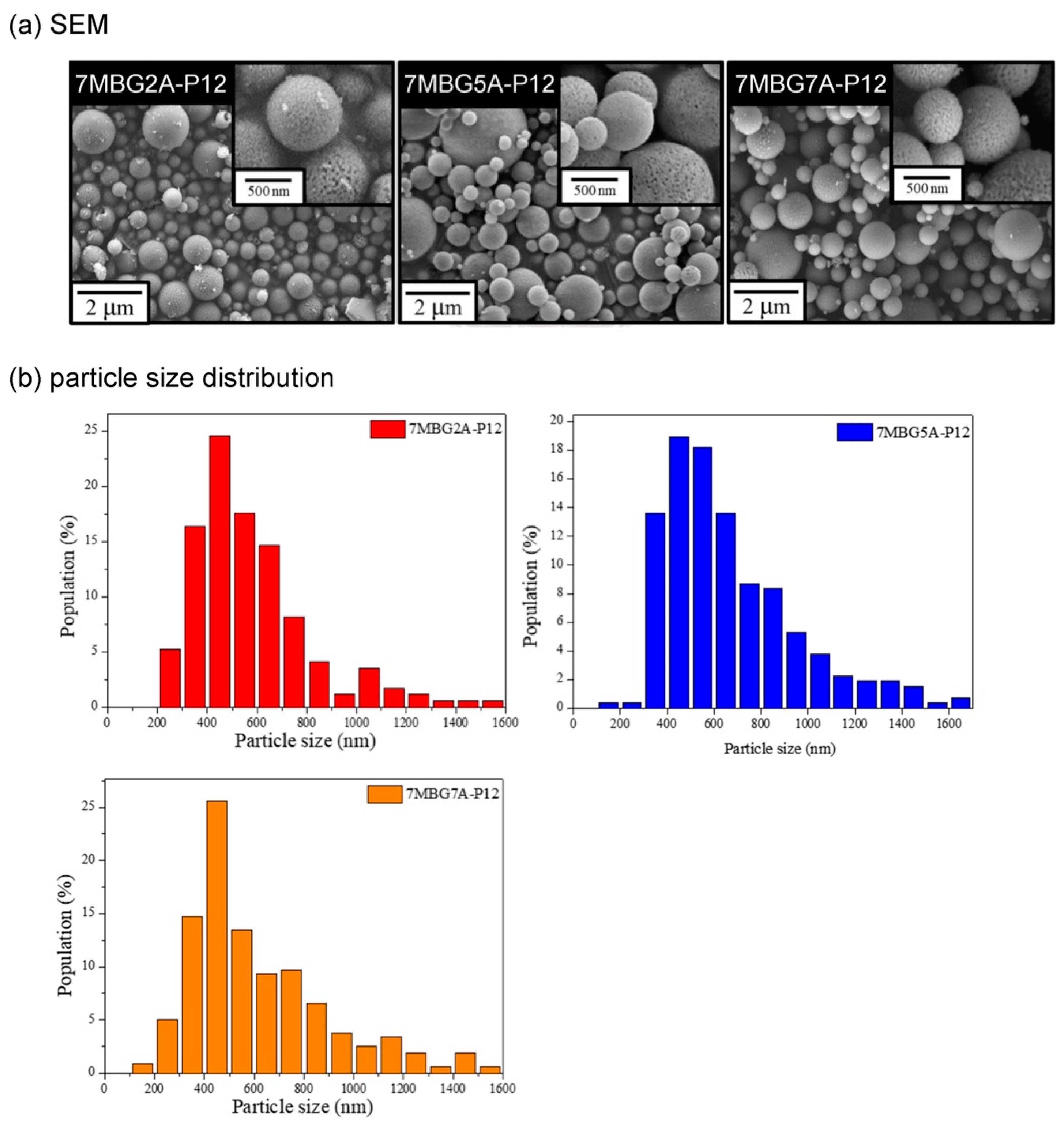
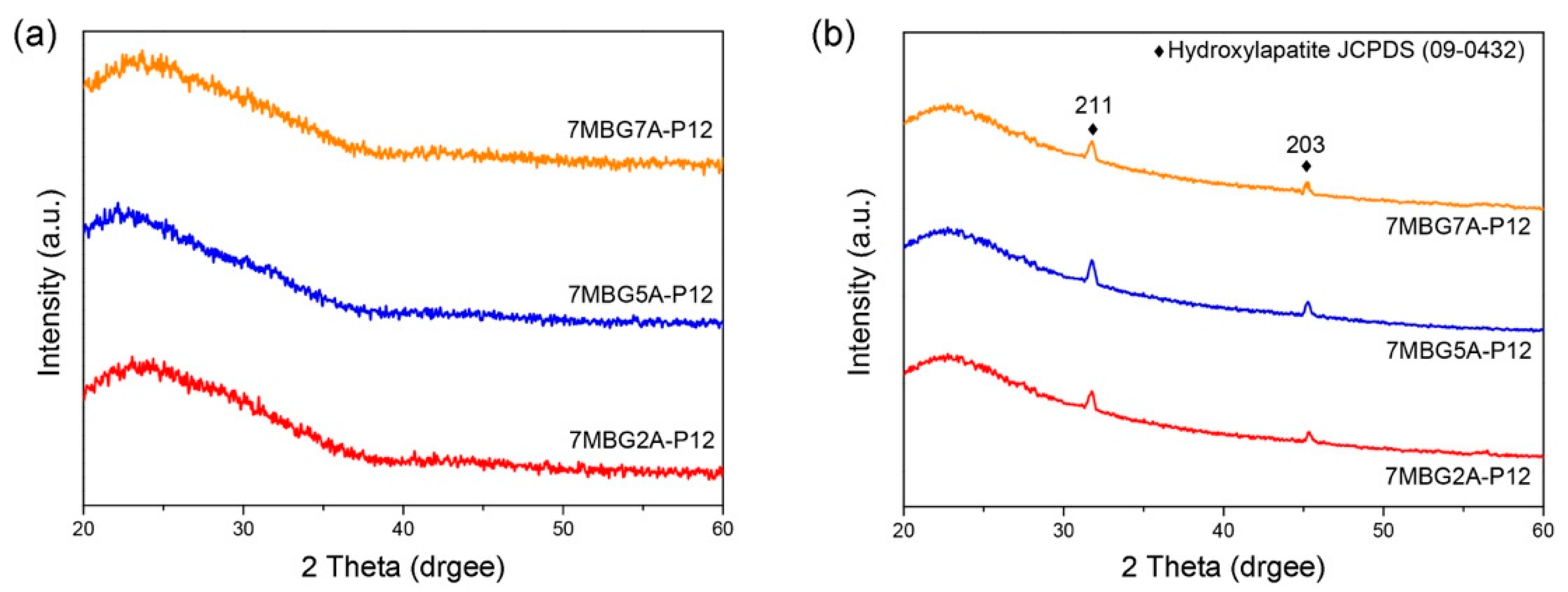
3.2. Different Concentrations of Acetic Acid (CH3COOH)
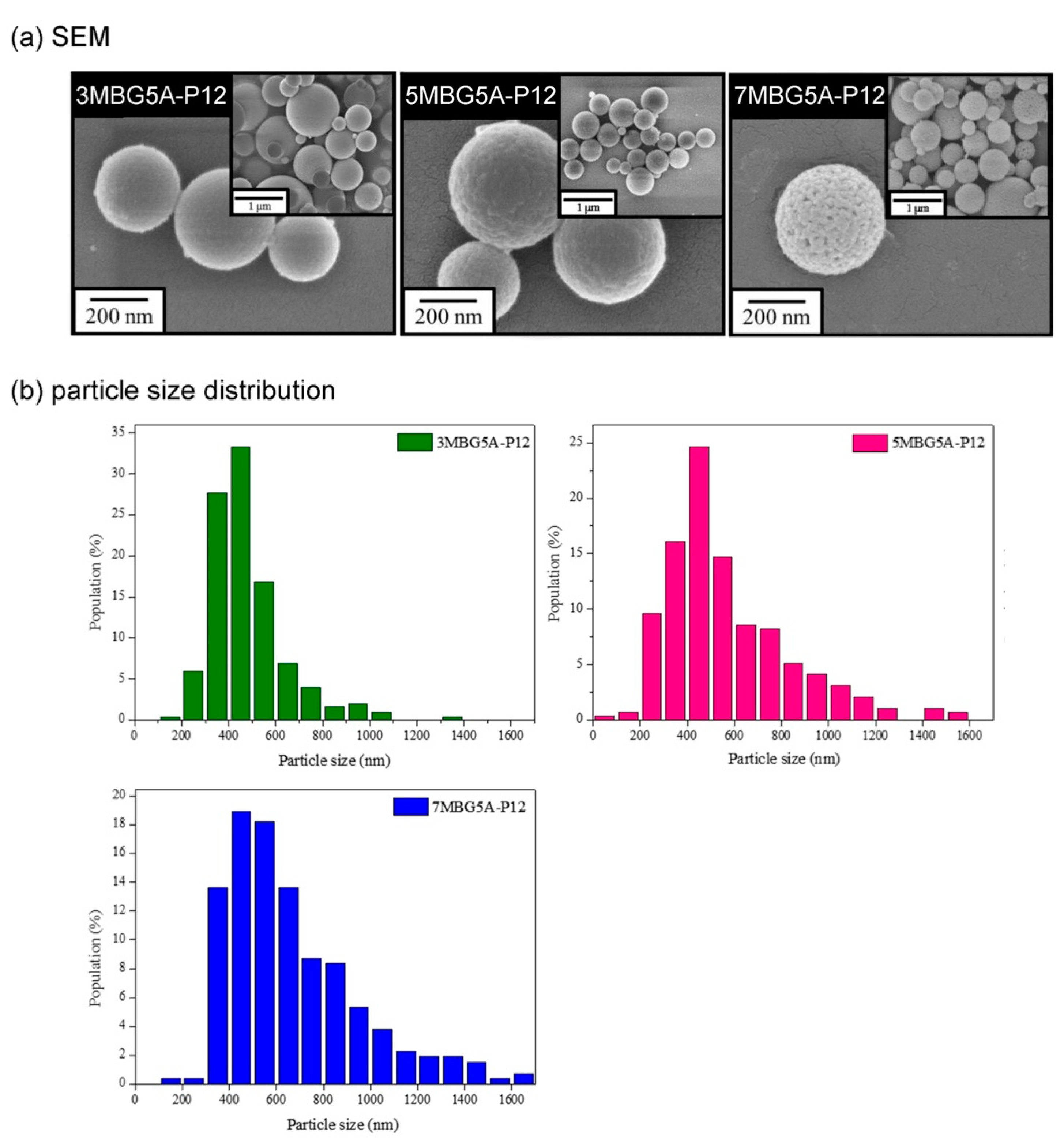
3.3. Different Concentrations of PEG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Lombardi, M.; Gremillard, L.; Chevalier, J.; Lefebvre, L.; Cacciotti, I.; Bianco, A.; Montanaro, L. A comparative study be-tween melt-derived and sol-gel synthesized 45s5 bioactive glasses. Key Eng. Mater. 2013, 541, 15–30. [Google Scholar] [CrossRef]
- Daguano, J.K.M.F.; Rogero, S.O.; Crovace, M.C.; Peitl, O.; Strecker, K.; dos Santos, C. Bioactivity and cytotoxicity of glass and glass–ceramics based on the 3CaO·P2O5–SiO2–MgO system. J. Mater. Sci. Mater. Med. 2013, 24, 2171–2180. [Google Scholar] [CrossRef]
- Magri, A.M.P.; Fernandes, K.R.; Ueno, F.R.; Kido, H.W.; Da Silva, A.C.; Braga, F.J.C.; Granito, R.N.; Gabbai-Armelin, P.R.; Rennó, A.C.M. Osteoconductive properties of two different bioactive glass forms (powder and fiber) combined with collagen. Appl. Surf. Sci. 2017, 423, 557–565. [Google Scholar] [CrossRef]
- Zheng, K.; Torre, E.; Bari, A.; Taccardi, N.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Iviglia, G.; Boccaccini, A.R. Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater. Today Biol. 2020, 5, 100041. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [Green Version]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and Promising Applications of Strontium (Sr)-Containing Bioactive Glasses in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.; Chen, H.; Huang, L.; Lu, P.; Chang, H.; Chang, I. Synthesis and in vitro bioactivity of mesoporous bioactive glass scaffolds. Mater. Sci. Eng. C 2010, 30, 657–663. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E. 3D Printing of Hierarchical Scaffolds Based on Mesoporous Bioactive Glasses (MBGs)—Fundamentals and Applications. Materials 2020, 13, 1688. [Google Scholar] [CrossRef] [Green Version]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Deliormanlı, A.M.; Türk, M. Flow Behavior and Drug Release Study of Injectable Pluronic F-127 Hydrogels containing Bioactive Glass and Carbon-Based Nanopowders. J. Inorg. Organomet. Polym. Mater. 2019, 30, 1184–1196. [Google Scholar] [CrossRef]
- Tseng, C.-F.; Fei, Y.-C.; Chou, Y.-J. Investigation of in vitro bioactivity and antibacterial activity of manganese-doped spray pyrolyzed bioactive glasses. J. Non-Cryst. Solids 2020, 549, 120336. [Google Scholar] [CrossRef]
- Chou, Y.-J.; Hsiao, C.-W.; Tsou, N.-T.; Wu, M.-H.; Shih, S.-J. Preparation and in Vitro Bioactivity of Micron-sized Bioactive Glass Particles Using Spray Drying Method. Appl. Sci. 2018, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Dang, H.; Bui, H.; Guseva, E.; Ta, A.; Nguyen, A.; Trong, T.; Hoang, H.; Bui, X. Characterization of bioactive glass synthesized by sol-gel process in hot water. Crystals 2020, 10, 529. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of melt-derived 45S5 and sol-gel-derived 58S bioactive glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Sharifianjazi, F.; Moradi, M.; Abouchenari, A.; Pakseresht, A.H.; Esmaeilkhanian, A.; Shokouhimehr, M.; Asl, M.S. Effects of Sr and Mg dopants on biological and mechanical properties of SiO2–CaO–P2O5 bioactive glass. Ceram. Int. 2020, 46, 22674–22682. [Google Scholar] [CrossRef]
- Hong, B.-J.; Hsiao, C.-W.; Bakare, F.F.; Sun, J.-T.; Shih, S.-J. Effect of Acetic Acid Concentration on Pore Structure for Mesoporous Bioactive Glass during Spray Pyrolysis. Materials 2018, 11, 963. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chang, J.; Xiao, Y. Mesoporous bioactive glasses as drug delivery and bone tissue regeneration platforms. Ther. Deliv. 2011, 2, 1189–1198. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.-J.; Hong, B.-J.; Lin, Y.-C.; Wang, C.-Y.; Shih, S.-J. The correlation of pore size and bioactivity of spray-pyrolyzed mes-oporous bioactive glasses. Materials 2017, 10, 488. [Google Scholar] [CrossRef]
- Lei, B.; Chen, X.; Wang, Y.; Zhao, N.; Miao, G.; Li, Z.; Lin, C. Fabrication of porous bioactive glass particles by one step sintering. Mater. Lett. 2010, 64, 2293–2295. [Google Scholar] [CrossRef]
- Nommeots-Nomm, A.; Lee, P.D.; Jones, J.R. Direct ink writing of highly bioactive glasses. J. Eur. Ceram. Soc. 2018, 38, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Rainer, A.; Giannitelli, S.M.; Abbruzzese, F.; Traversa, E.; Licoccia, S.; Trombetta, M. Fabrication of bioactive glass–ceramic foams mimicking human bone portions for regenerative medicine. Acta Biomater. 2008, 4, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Chen, X.; Wang, Y.; Zhao, N. Synthesis and in vitro bioactivity of novel mesoporous hollow bioactive glass micro-spheres. Mater. Lett. 2009, 63, 1719–1721. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Gulik, A.; Bourdon, O.; Gref, R.; Labarre, D.; Barratt, G. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel PEG-modified nanocapsules. Biomaterials 2001, 22, 2967–2979. [Google Scholar] [CrossRef]
- Mahaleh, Y.B.M.; Sadrnezhaad, S.K.; Hosseini, D. NiO Nanoparticles Synthesis by Chemical Precipitation and Effect of Applied Surfactant on Distribution of Particle Size. J. Nanomater. 2008, 2008, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Carlisle, E.M. Silicon: A Possible Factor in Bone Calcification. Science 1970, 167, 279–280. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Julien, M.; Magne, D.; Masson, M.; Rolli-Derkinderen, M.; Chassande, O.; Cario-Toumaniantz, C.; Cherel, Y.; Weiss, P.; Guicheux, J. Phosphate Stimulates Matrix Gla Protein Expression in Chondrocytes through the Extracellular Signal Regulated Kinase Signaling Pathway. Endocrinology 2007, 148, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Shih, S.-J.; Chou, Y.-J.; Chien, I.-C. One-step synthesis of bioactive glass by spray pyrolysis. J. Nanopart. Res. 2012, 14, 1299. [Google Scholar] [CrossRef]
- Lei, B.; Chen, X.; Wang, Y.; Zhao, N.; Du, C.; Zhang, L. Acetic acid derived mesoporous bioactive glasses with an enhanced in vitro bioactivity. J. Non-Cryst. Solids 2009, 355, 2583–2587. [Google Scholar] [CrossRef]
- Su, J.; Zhang, T.; Li, Y.; Chen, Y.; Liu, M. Photocatalytic Activities of Copper Doped Cadmium Sulfide Microspheres Prepared by a Facile Ultrasonic Spray-Pyrolysis Method. Molecules 2016, 21, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, A.; Ahmad, T.; Ganguli, A.K. Silica mesostructures: Control of pore size and surface area using a surfac-tant-templated hydrothermal process. Langmuir 2010, 26, 14901–14908. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Messing, G.L.; Borden, M. Synthesis of Solid, Spherical Zirconia Particles by Spray Pyrolysis. J. Am. Ceram. Soc. 1990, 73, 61–67. [Google Scholar] [CrossRef]
- Guo, W.; Sun, Y.; Luo, G.; Wang, Y. Interaction of PEG with ionic surfactant SDS to form template for mesoporous material. Colloids Surf. A Physicochem. Eng. Asp. 2005, 252, 71–77. [Google Scholar] [CrossRef]
- Gillespie, T. The effect of aggregation and particle size distribution on the viscosity of newtonian suspensions. J. Colloid Interface Sci. 1983, 94, 166–173. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.; Al-Othman, A.; Taylor, K.; Al-Ghamdi, A. Surface tension of HCl-based stimulation fluids at high temperatures. J. Pet. Sci. Eng. 2004, 43, 57–73. [Google Scholar] [CrossRef]
- Qun, G.; Ajun, W. Effects of molecular weight, degree of acetylation and ionic strength on surface tension of chitosan in dilute solution. Carbohydr. Polym. 2006, 64, 29–36. [Google Scholar] [CrossRef]
- Bleazard, J.G.; Sun, T.F.; Teja, A.S. The thermal conductivity and viscosity of acetic acid-water mixtures. Int. J. Thermophys. 1996, 17, 111–125. [Google Scholar] [CrossRef]
- Guo, J.; Legum, B.; Anasori, B.; Wang, K.; Lelyukh, P.; Gogotsi, Y.; Randall, C.A. Cold Sintered Ceramic Nanocomposites of 2D MXene and Zinc Oxide. Adv. Mater. 2018, 30, e1801846. [Google Scholar] [CrossRef]
- Vlădescu, A.; Pârâu, A.; Pană, I.; Cotruț, C.M.; Constantin, L.R.; Braic, V.; Vrânceanu, D.M. In Vitro Activity Assays of Sputtered HAp Coatings with SiC Addition in Various Simulated Biological Fluids. Coatings 2019, 9, 389. [Google Scholar] [CrossRef] [Green Version]
- Ninni, L.; Burd, H.; Fung, W.H.; Meirelles, A.J.A. Kinematic Viscosities of Poly(ethylene glycol) Aqueous Solutions. J. Chem. Eng. Data 2003, 48, 324–329. [Google Scholar] [CrossRef]






| Medicaments (Abbr.) | Manufacturer | Chemical Formula | Concentrations |
|---|---|---|---|
| Tetraethyl orthosilicate (TEOS) | Alfa Aesar Co., Massachusetts, MA, USA | Si(OC2H5)4 | 98.0 wt% |
| Calcium nitrate tetrahydrate (CN) | Showa Corporation, Tokyo, Japan | Ca(NO3)2·4H2O | 98.5 wt% |
| Triethyl phosphate (TEP) | Alfa Aesar Co., Massachusetts, MA, USA | (C2H5)3PO4 | >98.0 wt% |
| Hydrochloric acid | Acros Organics, New Jersey, NJ, USA | HCl | 37.0 wt% |
| Polyethylene glycol (PEG) | Alfa Aesar Co., Massachusetts MA, USA | HO(CH2CH2O)nH | - |
| Acetic acid | Showa Corporation, Tokyo Japan | CH3COOH | 99.7 wt% |
| Acid | PEG | Sample Code | ||
|---|---|---|---|---|
| Medicaments | Concentrations | Molecular Weights | Concentrations | |
| I. Different Molecular Weights of PEG | ||||
| HCl | 0.5 M | - | 7 g/1000 mL | MBG-P0 |
| HCl | 0.5 M | 2000 | 7 g/1000 mL | MBG-P2 |
| HCl | 0.5 M | 4000 | 7 g/1000 mL | MBG-P4 |
| HCl | 0.5 M | 8000 | 7 g/1000 mL | MBG-P8 |
| HCl | 0.5 M | 12,000 | 7 g/1000 mL | MBG-P12 |
| CH3COOH | 25 mL/1000 mL | 12,000 | 7 g/1000 mL | 7MBG2A-P12 |
| CH3COOH | 50 mL/1000 mL | 12,000 | 7 g/1000 mL | 7MBG5A-P12 |
| CH3COOH | 75 mL/1000 mL | 12,000 | 7 g/1000 mL | 7MBG7A-P12 |
| CH3COOH | 50 mL/1000 mL | 12,000 | 3 g/1000 mL | 3MBG5A-P12 |
| CH3COOH | 50 mL/1000 mL | 12,000 | 5 g/1000 mL | 5MBG5A-P12 |
| CH3COOH | 50 mL/1000 mL | 12,000 | 7 g/1000 mL | 7MBG5A-P12 |
| Sample Code | Specific Surface Area (m2/g) | Pore Size (nm) |
|---|---|---|
| MBG-P0 | 56.48 | - |
| MBG-P2 | 102.08 | 3.136 |
| MBG-P4 | 104.87 | 3.144 |
| MBG-P8 | 108.24 | 3.146 |
| MBG-P12 | 121.53 | 3.149 |
| 7MBG2A-P12 | 104.39 | 3.141 |
| 7MBG5A-P12 | 111.51 | 3.152 |
| 7MBG7A-P12 | 110.35 | 3.149 |
| 3MBG5A-P12 | 176.21 | 1.924 |
| 5MBG5A-P12 | 173.93 | 3.481 |
| 7MBG5A-P12 | 111.51 | 3.152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.-Y.; Tsai, P.-Y.; Chen, M.-S.; Mine, Y.; Wu, S.-H.; Chen, C.-Y.; Lin, D.-J.; Lin, C.-K. Mesoporous Properties of Bioactive Glass Synthesized by Spray Pyrolysis with Various Polyethylene Glycol and Acid Additions. Polymers 2021, 13, 618. https://doi.org/10.3390/polym13040618
Peng T-Y, Tsai P-Y, Chen M-S, Mine Y, Wu S-H, Chen C-Y, Lin D-J, Lin C-K. Mesoporous Properties of Bioactive Glass Synthesized by Spray Pyrolysis with Various Polyethylene Glycol and Acid Additions. Polymers. 2021; 13(4):618. https://doi.org/10.3390/polym13040618
Chicago/Turabian StylePeng, Tzu-Yu, Pei-Yun Tsai, May-Show Chen, Yuichi Mine, Shan-Hua Wu, Chin-Yi Chen, Dan-Jae Lin, and Chung-Kwei Lin. 2021. "Mesoporous Properties of Bioactive Glass Synthesized by Spray Pyrolysis with Various Polyethylene Glycol and Acid Additions" Polymers 13, no. 4: 618. https://doi.org/10.3390/polym13040618