Bonding and Thermal Cycling Performances of Two (Poly)Aryl–Ether–Ketone (PAEKs) Materials to an Acrylic Denture Base Resin
Abstract
:1. Introduction
2. Materials and Methods
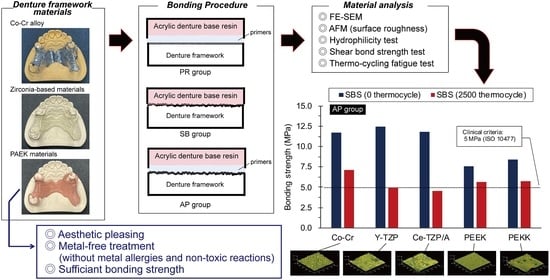
2.1. Specimen Preparation and Surface Pretreatment
2.2. Surface Roughness and Morphologies
2.3. Hydrophilicity (Contact Angle)
2.4. Bonding Procedure and Thermal Cycling Fatigue Test
2.5. Shear Bonding Strength (SBS) Testing
2.6. Analysis of Residual Adhesives
2.7. Statistic Analysis
3. Results
3.1. Characterizations of the Material Surfaces
3.1.1. Surface Roughness and Topography
3.1.2. Surface Morphology
3.1.3. Hydrophilicity
3.2. Bonding Properties
3.2.1. Shear Bond Strength (SBS)
3.2.2. SBS after Thermal Cycling Fatigue Test
3.2.3. Mode of Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.M.U.; Dwivedi, P.; Tiwari, R.V.C.; Virk, I.; Lahoti, A.; Kumar, S.; Pandey, P.R. Aesthetic in Complete Denture—A Review. J. Adv. Med. Dent. Sci. Res. 2020, 8, 187–189. [Google Scholar] [CrossRef]
- Zotti, F.; Pappalardo, D.; Capocasale, G.; Sboarina, A.; Bertossi, D.; Albanese, M. Aesthetic Dentistry, How You Say and How You See: A 500-People Survey on Digital Preview and Color Perception. Clin. Cosmet. Investig. Dent. 2020, 12, 377–389. [Google Scholar] [CrossRef]
- Horita, S.; Sugiura, T.; Yamamoto, K.; Murakami, K.; Imai, Y.; Kirita, T. Biomechanical analysis of immediately loaded implants according to the “All-on-Four” concept. J. Prosthodont. Res. 2017, 61, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kitagawa, N.; Isobe, A. Current Consensus of Dental Implants in the Elderly—What Are the Limitations? Curr. Oral. Health Rep. 2020, 7, 321–326. [Google Scholar] [CrossRef]
- Muller, F.; Shimazaki, Y.; Kahabuka, F.; Schimmel, M. Oral health for an ageing population: The importance of a natural dentition in older adults. Int. Dent. J. 2017, 67 (Suppl. 2), 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.H.; Chow, T.W. Esthetic designs of removable partial dentures. Gen. Dent. 2003, 51, 322–324. [Google Scholar] [PubMed]
- Uriciuc, W.A.; Vermesan, H.; Tiuc, A.E.; Ilea, A.; Bosca, A.B.; Popa, C.O. Casting over Metal Method Used in Manufacturing Hybrid Cobalt-Chromium Dental Prosthetic Frameworks Assembles. Materials 2021, 14, 539. [Google Scholar] [CrossRef]
- Polychronakis, N.; Lagouvardos, P.; Polyzois, G.; Sykaras, N.; Zoidis, P. Color changes of polyetheretherketone (PEEK) and polyoxymethelene (POM) denture resins on single and combined staining/cleansing action by CIELab and CIEDE2000 formulas. J. Prosthodont. Res. 2020, 64, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.E.; Ercoli, C.; Rispoli, L.; Maiorana, C.; Esposito, M. Metal-free materials for fixed prosthodontic restorations. Cochrane Database Syst. Rev. 2017, 12, CD009606. [Google Scholar] [CrossRef] [PubMed]
- Baba, K. Paradigm shifts in prosthodontics. J. Prosthodont. Res. 2014, 58, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontolliet, A.; Husain, N.A.-H.; Özcan, M. Wear analysis and topographical properties of monolithic zirconia and CoCr against human enamel after polishing and glazing procedures. J. Mech. Behav. Biomed. Mater. 2020, 105, 103712. [Google Scholar] [CrossRef]
- Sawada, T.; Wagner, V.; Schille, C.; Spintzyk, S.; Schweizer, E.; Geis-Gerstorfer, J. Effect of slow-cooling protocol on biaxial flexural strengths of bilayered porcelain-ceria-stabilized zirconia/alumina nanocomposite (Ce-TZP/A) disks. Dent. Mater. 2019, 35, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Kang, K.-H.; Att, W. Effect of aging process on some properties of conventional and multilayered translucent zirconia for monolithic restorations. Ceram. Int. 2020, 46, 1854–1868. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Skirbutis, G.; Dzingutė, A.; Masiliūnaitė, V.; Šulcaitė, G.; Žilinskas, J. A review of PEEK polymer’s properties and its use in prosthodontics. Stomatologija 2017, 19, 19–23. [Google Scholar] [PubMed]
- Gama, L.T.; Duque, T.M.; Özcan, M.; Philippi, A.G.; Mezzomo, L.A.M.; Gonçalves, T.M.S.V. Adhesion to high-performance polymers applied in dentistry: A systematic review. Dent. Mater. 2020, 36, e93–e108. [Google Scholar] [CrossRef]
- Qin, L.; Yao, S.; Zhao, J.; Zhou, C.; Oates, T.W.; Weir, M.D.; Wu, J.; Xu, H.H.K. Review on Development and Dental Applications of Polyetheretherketone-Based Biomaterials and Restorations. Materials 2021, 14, 408. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Karthik, N.; Xiong, D.; Liu, Y. Bio-inspired surface modification of PEEK through the dual cross-linked hydrogel layers. J. Mech. Behav. Biomed. Mater. 2020, 112, 104032. [Google Scholar] [CrossRef]
- Peng, T.-Y.; Shimoe, S.; Tanoue, N.; Akebono, H.; Murayama, T.; Satoda, T. Fatigue resistance of yttria-stabilized tetragonal zirconia polycrystal clasps for removable partial dentures. Eur. J. Oral. Sci. 2019, 127, 269–275. [Google Scholar] [CrossRef]
- Urano, S.; Hotta, Y.; Miyazaki, T.; Baba, K. Bending properties of Ce-TZP/A nanocomposite clasps for removable partial dentures. Int. J. Prosthodont. 2015, 28, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.-Y.; Ogawa, Y.; Akebono, H.; Iwaguro, S.; Sugeta, A.; Shimoe, S. Finite-element analysis and optimization of the mechanical properties of polyetheretherketone (PEEK) clasps for removable partial dentures. J. Prosthodont. Res. 2020, 64, 250–256. [Google Scholar] [CrossRef]
- Iwaguro, S.; Shimoe, S.; Hirata, I.; Murayama, T.; Satoda, T. Effect of microslit retention on the bond strength of zirconia to dental materials. Dent. Mater. J. 2019, 38, 1043–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoe, S.; Peng, T.-Y.; Wakabayashi, Y.; Takenaka, H.; Iwaguro, S.; Kaku, M. Laser-milled microslits improve the bonding strength of acrylic resin to zirconia ceramics. Polymers 2020, 12, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoe, S.; Peng, T.-Y.; Otaku, M.; Tsumura, N.; Iwaguro, S.; Satoda, T. Influence of various airborne-particle abrasion conditions on bonding between zirconia ceramics and an indirect composite resin material. J. Prosthet. Dent. 2019, 122, 491.e1–491.e9. [Google Scholar] [CrossRef]
- Lin, D.-J.; Fuh, L.-J.; Chen, W.-C. Nano-morphology, crystallinity and surface potential of anatase on micro-arc oxidized titanium affect its protein adsorption, cell proliferation and cell differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110204. [Google Scholar] [CrossRef]
- ISO 10477: 2018—Dentistry—Polymer-Based Crown and Veneering Materials. Available online: https://www.iso.org/standard/68235.html (accessed on 8 February 2021).
- Koko, M.; Takagaki, T.; Abdou, A.; Inokoshi, M.; Ikeda, M.; Wada, T.; Uo, M.; Nikaido, T.; Tagami, J. Effects of the ratio of silane to 10-methacryloyloxydecyl dihydrogenphosphate (MDP) in primer on bonding performance of silica-based and zirconia ceramics. J. Mech. Behav. Biomed. Mater. 2020, 112, 104026. [Google Scholar] [CrossRef]
- Steling Rego, M.E.; Nunes Guimarães Paes, P.; Ribeiro da Silva Schanuel, F.; Mendes Jardim, P. Acid etching and silica coating effects on Y-TZP topography and ceramic/resin cement bond strength. Ceram. Int. 2021, 47, 5235–5243. [Google Scholar] [CrossRef]
- Byeon, S.M.; Jang, Y.S.; Lee, M.H.; Bae, T.S. Improvement in the tensile bond strength between 3Y-TZP ceramic and enamel by surface treatments. Materials 2016, 9, 702. [Google Scholar] [CrossRef] [Green Version]
- Akazawa, N.; Koizumi, H.; Nogawa, H.; Nakayama, D.; Kodaira, A.; Matsumura, H. Effect of mechanochemical surface preparation on bonding to zirconia of a tri-n-butylborane initiated resin. Dent. Mater. J. 2017, 36, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Lümkemann, N.; Eichberger, M.; Murphy, R.J.; Stawarczyk, B. Suitability of the new Aryl-Ketone-Polymer indicated for removable partial dentures: Analysis of elastic properties and bond strength to denture resin. Dent. Mater. J. 2020, 39, 539–546. [Google Scholar] [CrossRef]
- Kwon, T.-Y.; Ha, J.-Y.; Chun, J.-N.; Son, J.S.; Kim, K.-H. Effects of Prepolymerized Particle Size and Polymerization Kinetics on Volumetric Shrinkage of Dental Modeling Resins. Biomed. Res. Int. 2014, 2014, 914739. [Google Scholar] [CrossRef] [PubMed]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. (Eds.) Phillips’ Science of Dental Materials, 12th ed.; Elsevier: Amsterdam, The Netherlands, 2012; p. 592. [Google Scholar]
- Soon, G.; Pingguan-Murphy, B.; Lai, K.W.; Akbar, S.A. Review of zirconia-based bioceramic: Surface modification and cellular response. Ceram. Int. 2016, 42, 12543–12555. [Google Scholar] [CrossRef]
- Lee, K.S.; Shin, M.S.; Lee, J.Y.; Ryu, J.J.; Shin, S.W. Shear bond strength of composite resin to high performance polymer PEKK according to surface treatments and bonding materials. J. Adv. Prosthodont. 2017, 9, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Matsui, N.; Takagaki, T.; Sadr, A.; Ikeda, M.; Ichinose, S.; Nikaido, T.; Tagami, J. The role of MDP in a bonding resin of a two-step self-etching adhesive system. Dent. Mater. J. 2015, 34, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP based dental adhesives: Adhesive interface characterization and adhesive stability-a systematic review. Materials 2019, 12, 790. [Google Scholar] [CrossRef] [Green Version]
- Kurahashi, K.; Matsuda, T.; Ishida, Y.; Ichikawa, T. Effect of surface treatments on shear bond strength of polyetheretherketone to autopolymerizing resin. Dent. J. 2019, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Barkarmo, S.; Longhorn, D.; Leer, K.; Johansson, C.B.; Stenport, V.; Franco-Tabares, S.; Kuehne, S.A.; Sammons, R. Biofilm formation on polyetheretherketone and titanium surfaces. Clin. Exp. Dent. Res. 2019, 5, 427–437. [Google Scholar] [CrossRef] [PubMed]





| Groups | Materials (Dentification) | Main Composition * | Manufactures (Batch No.) |
|---|---|---|---|
| Co-Cr | Framework: Wironit Extra-hard | Co, Cr, Mo | BEGO, Bremen, Germany (132300912) |
| Primer: Clearfil Ceramic Primer Plus | MDP, silane, ethanol | Kuraray Medical Inc., Tokyo, Japan (AV0049) | |
| Y-TZP | Framework: TZ-3YB-E | ZrO2, Y2O3 | Tosoh Corporation, Tokyo, Japan (S305374B) |
| Primer: Clearfil Ceramic Primer Plus | MDP, silane, ethanol | Kuraray Medical Inc., Tokyo, Japan (AV0049) | |
| Ce-TZP/A | Framework: Cpro NANO Zirconia | ZrO2, Al2O3, CeO2 | YAMAKIN Co., Ltd., Osaka, Japan (CD90011076J) |
| Primer: Clearfil Ceramic Primer Plus | MDP, silane, ethanol | Kuraray Medical Inc., Tokyo, Japan (AV0049) | |
| PEEK | Framework: VESTAKEEP | poly(ether–ether–ketone) | Evonik Japan Co., Tokyo, Japan (57781699) |
| Primer: visio. link | PETIA, MMA, photo initiators | Bredent, Senden, Germany (700388) | |
| PEKK | Framework: Pekkton ivory | poly(ether–ketone–ketone) | Cendres+Métaux SA, Biel/Bienne, Switzerland (335773) |
| Primer: visio. link | PETIA, MMA, photo initiators | Bredent, Senden, Germany (700388) |
| Condition | Group | 0 Thermal Cycle | 2500 Thermal Cycles | Reduction | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Failure Mode A/AC/C | Mean ± SD | Failure Mode A/AC/C | |||
| SB | Co-Cr | 4.62 ± 1.24 a, b | 10/0/0 | 2.49 ± 0.59 α | 10/0/0 | 46.08% * |
| Y-TZP | 2.00 ± 0.44 a | 10/0/0 | 0.00 ± 0.00 β | 10/0/0 | 100.00% * | |
| Ce-TZP/A | 1.83 ± 0.48 a | 10/0/0 | 0.00 ± 0.00 β | 10/0/0 | 100.00% * | |
| PEEK | 3.07 ± 0.45 a | 10/0/0 | 2.76 ± 0.45 α | 10/0/0 | 10.09% | |
| PEKK | 4.75 ± 1.15 a, b | 10/0/0 | 3.35 ± 0.72 α, γ | 10/0/0 | 29.43% * | |
| PR | Co-Cr | 9.76 ± 1.17 c, d, e, h | 8/2/0 | 7.01 ± 0.84 δ | 10/0/0 | 28.24% * |
| Y-TZP | 9.50 ± 3.52 c, d, e, h | 9/1/0 | 3.78 ± 1.77 α, ε, ϝ | 10/0/0 | 60.27% * | |
| Ce-TZP/A | 8.36 ± 2.61 f, g, h | 9/1/0 | 3.02 ± 1.58 α, ϝ | 9/1/0 | 63.92% * | |
| PEEK | 3.82 ± 0.32 a | 10/0/0 | 3.50 ± 1.19 α, ϝ, η | 10/0/0 | 8.39% | |
| PEKK | 3.78 ± 0.63 a | 10/0/0 | 2.96 ± 1.20 α,ϝ | 10/0/0 | 12.60% | |
| AP | Co-Cr | 11.73 ± 1.82 c, d, f | 8/2/0 | 7.14 ± 2.05 δ | 10/0/0 | 39.14% * |
| Y-TZP | 12.47 ± 4.84 c | 8/2/0 | 4.93 ± 1.28 γ, ε, η, ω | 10/0/0 | 60.43% * | |
| Ce-TZP/A | 11.80 ± 4.63 c, d, f | 8/2/0 | 4.54 ± 1.47 γ, ϝ, ω | 10/0/0 | 61.49% * | |
| PEEK | 7.60 ± 1.99 b, e, g, i | 10/0/0 | 5.64 ± 0.64 δ, ω | 10/0/0 | 25.86% | |
| PEKK | 8.38 ± 1.92 d, f, i | 10/0/0 | 5.77 ± 0.61 δ, ω | 10/0/0 | 31.11% * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.-Y.; Shimoe, S.; Fuh, L.-J.; Lin, C.-K.; Lin, D.-J.; Kaku, M. Bonding and Thermal Cycling Performances of Two (Poly)Aryl–Ether–Ketone (PAEKs) Materials to an Acrylic Denture Base Resin. Polymers 2021, 13, 543. https://doi.org/10.3390/polym13040543
Peng T-Y, Shimoe S, Fuh L-J, Lin C-K, Lin D-J, Kaku M. Bonding and Thermal Cycling Performances of Two (Poly)Aryl–Ether–Ketone (PAEKs) Materials to an Acrylic Denture Base Resin. Polymers. 2021; 13(4):543. https://doi.org/10.3390/polym13040543
Chicago/Turabian StylePeng, Tzu-Yu, Saiji Shimoe, Lih-Jyh Fuh, Chung-Kwei Lin, Dan-Jae Lin, and Masato Kaku. 2021. "Bonding and Thermal Cycling Performances of Two (Poly)Aryl–Ether–Ketone (PAEKs) Materials to an Acrylic Denture Base Resin" Polymers 13, no. 4: 543. https://doi.org/10.3390/polym13040543