Polymers under Load and Heating Deformability: Modelling and Predicting
Abstract
:1. Introduction
- -
- Thermo-gravimetrical research of glassed polymers aimed at determining entropy change;
- -
- Experimental study of the polymer “deformation modulus-temperature” dependence;
- -
- Formulation and testing of the calculation model for layer-bonded polymer deformability depending on temperature factor.
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
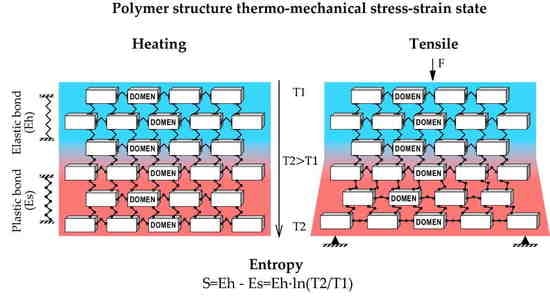
3.1. Layer-Bonded Polymer Deformability Calculation Model Formed on the Basis of the Research in Polymer Elasticity and Entropy under Heating
3.2. Formulation and Testing of Layer-Bonded Model in Polymer Elasticity Modulus in Relation with Temperature and Entropy Factor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slutsker, A.I.; Sanphirova, T.P.; Yastrebinskii, A.A.; Kuksenko, V.S. Structure, and reversible deformability of oriented crystallizing polymers. J. Polym. Sci. Part C Polym. Symp. 1967, 16, 4093–4101. [Google Scholar] [CrossRef]
- Flory, P.J.; Erman, B.; Flory, P.J.; Erman, B.; Erman, B. Theory of Elasticity of Polymer Networks. 3. Macromolecules 1982, 15, 800–806. [Google Scholar] [CrossRef]
- Dill, K.A.; Bromberg, S.; Stigter, D. Polymer Elasticity & Collapse. In Molecular Driving Forces; Taylor &. Francis Group: Boca Raton, FL, USA, 2010; ISBN 9780203809075. [Google Scholar] [CrossRef]
- Lomakin, V.A.; Ogibalov, P.M.; Teters, G.A. Problems of the theory of deformation of polymeric materials. Polym. Mech. 1972, 8, 377–385. [Google Scholar] [CrossRef]
- Vashisth, A.; Ashraf, C.; Bakis, C.E.; van Duin, A.C.T. Effect of chemical structure on thermo-mechanical properties of epoxy polymers: Comparison of accelerated ReaxFF simulations and experiments. Polymer 2018, 158, 354–363. [Google Scholar] [CrossRef]
- Chan, C.H.; Chia, C.H.; Thomas, S. Physical Chemistry of Macromolecules: Macro to Nanoscales; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781482234190. [Google Scholar]
- Guo, Q. Polymer Morphology: Principles, Characterization, and Processing; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9781118892756. [Google Scholar]
- Knauert, S.T.; Douglas, J.F.; Starr, F.W. The effect of nanoparticle shape on polymer-nanocomposite rheology and tensile strength. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1882–1897. [Google Scholar] [CrossRef] [Green Version]
- Kuperman, A.M.; Turusov, R.A.; Gorenberg, A.Y. Study of elastic and strength properties of hybrid and gradient polymer composites. Compos. Mech. Comput. Appl. 2010, 1. [Google Scholar] [CrossRef]
- Rousseau, I.A.; Xie, T. Shape memory epoxy: Composition, structure, properties and shape memory performances. J. Mater. Chem. 2010, 20, 3431–3441. [Google Scholar] [CrossRef]
- Zhang, C.; Hankett, J.; Chen, Z. Molecular-level understanding of adhesion mechanisms at the epoxy/polymer interphases. ACS Appl. Mater. Interphases 2012, 4, 3730–3737. [Google Scholar] [CrossRef]
- Miller, R.; Ferri, J.K.; Javadi, A.; Krägel, J.; Mucic, N.; Wüstneck, R. Rheology of interfacial layers. Colloid Polym. Sci. 2010, 288, 937–950. [Google Scholar] [CrossRef]
- Solar, M.; Qin, Z.; Buehler, M.J. Molecular mechanics and performance of crosslinked amorphous polymer adhesives. J. Mater. Res. 2014, 29, 1077. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Doren, A.; Dewez, J.L.; Heuschling, O. Chemical composition and physico-chemical properties of polymer surfaces. Prog. Org. Coat. 1993, 22, 327–344. [Google Scholar] [CrossRef]
- Lodge, T.P.; Muthukumar, M. Physical chemistry of polymers: Entropy, interactions, and dynamics. J. Phys. Chem. 1996, 100, 13275–13292. [Google Scholar] [CrossRef]
- Holzapfel, G.A.; Simo, J.C. Entropy elasticity of isotropic rubber-like solids at finite strains. Comput. Methods Appl. Mech. Eng. 1996, 132, 17–44. [Google Scholar] [CrossRef]
- Green, A.E.; Naghdi, P.M. Thermoelasticity without energy dissipation. J. Elast. 1993, 31, 189–208. [Google Scholar] [CrossRef]
- Kuksenko, V.S.; Ovchinnikov, V.A.; Slutsker, A.I. Elasticity of the intercrystallite zones and the mechanical properties of oriented polymers. Polym. Mech. 1969, 5, 891–895. [Google Scholar] [CrossRef]
- Potemkin, I.I.; Khokhlov, A.R.; Prokhorova, S.; Sheiko, S.S.; Möller, M.; Beers, K.L.; Matyjaszewski, K. Spontaneous curvature of comblike polymers at a flat interphase. Macromolecules 2004, 37, 3918–3923. [Google Scholar] [CrossRef]
- Shaw, M.T.; MacKnight, W.J. Introduction to Polymer Viscoelasticity: Third Edition; John Wiley & Sons. Inc.: Hoboken. NJ, USA, 2005; ISBN 9780471741831. [Google Scholar] [CrossRef]
- Sogolova, T.I.; Demina, M.I. Temperature dependence of the mechanical properties of polymers of different chemical structure in the temperature range from 4.2 to 300°K. Polym. Mech. 1977, 13, 333–337. [Google Scholar] [CrossRef]
- Tanaka, F.; Edwards, S.F. Viscoelastic Properties of Physically Crosslinked Networks. Transient Network Theory. Macromolecules 1992, 25, 1516–1523. [Google Scholar] [CrossRef]
- Walley, S.M.; Field, J.E.; Pope, R.; Safford, N.A. The rapid deformation behaviour of various polymers. J. Phys. III 1991, 1, 1889–1925. [Google Scholar] [CrossRef]
- Moroz, J.D.; Nelson, P. Entropic elasticity of twist-storing polymers. Macromolecules 1998, 31, 6333–6347. [Google Scholar] [CrossRef] [Green Version]
- Shcherbak, V.V.; Gol’ dman, A.Y. Prediction of the nonlinear creep and long-term strength of isotropic and anisotropic polymer materials. Strength Mater. 1986, 18, 878–885. [Google Scholar] [CrossRef]
- Rezanova, V.G.; Pridatchenko, Y.V.; Tsebrenko, M.V. Mathematical description of deformation of a dispersed phase polymer in flow of melts of polymer blends. Fibre Chem. 2003, 35, 468–474. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Wu, Y. Computational thermomechanical properties of silica-epoxy nanocomposites by molecular dynamic simulation. Polymers 2017, 9, 430. [Google Scholar] [CrossRef]
- Gol’ dman, A.Y.; Murzakhanov, G.K.; Soshina, O.A. Temperature—Time analogy for thermorheologically complex polymeric materials—1. Mixtures of partially crystalline polymers with elastomers. Polym. Mech. 1977, 13, 516–522. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Fei, F.; Wang, Y.; Liu, Y.; Leng, J. Modeling mechanical behavior of epoxy-shape memory polymers. Behav. Mech. Multifunct. Mater. Compos. 2013, 8689, 86890L. [Google Scholar] [CrossRef]
- Khan, A.S.; Lopez-Pamies, O.; Kazmi, R. Thermo-mechanical large deformation response and constitutive modeling of viscoelastic polymers over a wide range of strain rates and temperatures. Int. J. Plast. 2006, 22, 581–601. [Google Scholar] [CrossRef]
- Cheng, H.L.; Wang, J.; Huang, Z.P. A thermo-viscoelastic constitutive model for compressible amorphous polymers. Mech. Time-Depend. Mater. 2010, 14, 261–275. [Google Scholar] [CrossRef]
- Russian State Standard GOST 25.604-82 Design Calculation and Strength Testings. Methods of Mechanical Testing of Polymeric Composite Materials. Test for Bending Properties at Normal, Elevated, and Low Temperatures. Available online: http://docs.cntd.ru/document/1200012862 (accessed on 28 January 2021).








| No. | Compound | Thermo-Gravimetric Analysis | Three-Point Bending Test at Elevated Temperature |
|---|---|---|---|
| 1 | Epoxy resin (Ker 828 52.5% + MTHPA 44.5% + alkofen 3%) | + | + |
| 2 | Epoxy resin (No. 1) 81% + acetone 9% | + | - |
| 3 | Epoxy resin (No.1) 72% + micro-grained marble 28% | + | - |
| 4 | Epoxy resin (No. 1) 72% + micro-grained marble 24% + acetone 4% | + | - |
| 5 | Epoxy resin (No.1) 65% + micro-grained marble 35% | + | - |
| 6 | Epoxy resin (No. 1) 62% + micro-grained marble 33% + acetone 5% | + | - |
| 7 | Epoxy resin (No.1) 55% + micro-grained marble 45% | + | + |
| 8 | Epoxy resin (No. 1) 48% + micro-grained marble 48% + acetone 4% | + | - |
| 9 | Phenolic resin (SFZ-309) 100% | + | + |
| 10 | Epoxy-phenolic resin (ker 828 45% + SFZ-309 55%) | + | + |
| Compound | Temperature, °C | Efact, MPa | S, J/J | Kpl | Ecalc, MPa | % Derivation |
|---|---|---|---|---|---|---|
| Epoxy No. 1 | 25 | 2953 | 3.1 | 1.000 | - | - |
| 50 | 2700 | 0.750 | 2743.0 | 1.6 | ||
| 75 | 2350 | 0.519 | 2434.4 | 3.6 | ||
| 100 | 1700 | 0.304 | 1934.7 | 13.8 | ||
| 130 | 693 | 0.064 | 679.2 | −2.0 | ||
| Epoxy + micro-grained marble No. 7 | 25 | 5500 | 3.1 | 1.000 | 5666.9 | 3.0 |
| 50 | 5000 | 0.750 | 5108.9 | 2.2 | ||
| 75 | 4610 | 0.519 | 4534.1 | −1.6 | ||
| 100 | 3500 | 0.304 | 3603.4 | 3.0 | ||
| 120 | 2500 | 0.142 | 2303.5 | −7.9 | ||
| Phenolic No. 9 | 25 | 4242 | 1.45 | 1 | - | - |
| 50 | 3150 | 0.883 | 3746.4 | 18.9 | ||
| 100 | 2475 | 0.685 | 2909.7 | 17.5 | ||
| 150 | 1732 | 0.509 | 2162.8 | 24.8 | ||
| 200 | 1155 | 0.353 | 1499.2 | 29.8 | ||
| 250 | 1050 | 0.212 | 902.2 | −14.0 | ||
| 300 | 909 | 0.084 | 359.5 | −60.4 | ||
| Epoxy-phenolic No. 10 | 25 | 2940 | 2.1 | 1 | - | - |
| 50 | 2814 | 0.831 | 2442.6 | −13.2 | ||
| 75 | 2131.5 | 0.674 | 1982.4 | −7.0 | ||
| 100 | 1470 | 0.529 | 1554.0 | 5.7 | ||
| 125 | 1347.2 | 0.419 | 1231.6 | −8.6 | ||
| 150 | 700 | 0.264 | 777.4 | 11.1 | ||
| 175 | - | 0.144 | 422.9 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korolev, A.; Mishnev, M.; Zherebtsov, D.; Vatin, N.I.; Karelina, M. Polymers under Load and Heating Deformability: Modelling and Predicting. Polymers 2021, 13, 428. https://doi.org/10.3390/polym13030428
Korolev A, Mishnev M, Zherebtsov D, Vatin NI, Karelina M. Polymers under Load and Heating Deformability: Modelling and Predicting. Polymers. 2021; 13(3):428. https://doi.org/10.3390/polym13030428
Chicago/Turabian StyleKorolev, Alexander, Maxim Mishnev, Dmitry Zherebtsov, Nikolai Ivanovich Vatin, and Maria Karelina. 2021. "Polymers under Load and Heating Deformability: Modelling and Predicting" Polymers 13, no. 3: 428. https://doi.org/10.3390/polym13030428