Novel TiO2 Nanoparticles/Polysulfone Composite Hollow Microspheres for Photocatalytic Degradation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
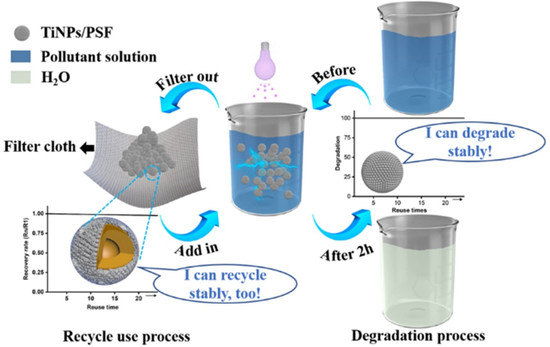
2.2. Preparation of TiNPs/PSF Composite Microspheres
2.3. Characterizations of TiNPs/PSF Composite Microspheres
2.4. Adsorption Performance of TiNPs/PSF Microsphere
2.5. Photocatalytic Activity Measurement of TiNPs/PSF Composite Microspheres
3. Results and Discussion
3.1. Characterization of TiNPs/PSF Microsphere
3.2. MB Removal by TiNPs/PSF Microsphere
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, T.; Sun, S.; Fu, G.; Hall, J.W.; Ni, Y.; He, L.; Yi, J.; Zhao, N.; Du, Y.; Pei, T.; et al. Pollution exacerbates China’s water scarcity and its regional inequality. Nat. Commun. 2020, 11, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zheng, Z.; Sun, F.; Miao, M.; Cui, M.-H.; Liu, H.; Zhang, H.; Zhang, C.; Hu, Z.; Liu, H. Valorization of citric acid production wastewater as alternative carbon source for biological nutrients removal: A pilot-scale case study. J. Clean. Prod. 2020, 258, 120576. [Google Scholar] [CrossRef]
- Parshetti, G.; Saratale, G.; Telke, A.; Govindwar, S. Biodegradation of hazardous triphenylmethane dye methyl violet by Rhizobium radiobacter (MTCC 8161). J. Basic Microbiol. 2009, 49 (Suppl. 1), S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Yun, J.; Seo, Y.; Byun, H.; Hou, J.; Kim, J.H. Mixed Dye Removal Efficiency of Electrospun Polyacrylonitrile-Graphene Oxide Composite Membranes. Polymers 2020, 12, 2009. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Saratale, G.D.; Saratale, R.G.; Cho, S.-K.; Ghodake, G.; Bharagava, R.N.; Park, Y.; Mulla, S.I.; Kim, D.-S.; Kadam, A.; Nair, S.; et al. Investigation of photocatalytic degradation of reactive textile dyes by Portulaca oleracea-functionalized silver nanocomposites and exploration of their antibacterial and antidiabetic potentials. J. Alloy. Compd. 2020, 833, 155083. [Google Scholar] [CrossRef]
- Baig, U.; Hawsawi, A.; Ansari, M.A.; Gondal, M.A.; Dastageer, M.A.; Falath, W.S. Synthesis, characterization and evaluation of visible light active cadmium sulfide-graphitic carbon nitride nanocomposite: A prospective solar light harvesting photo-catalyst for the deactivation of waterborne pathogen. J. Photochem. Photobiol. B Biol. 2020, 204, 111783. [Google Scholar] [CrossRef]
- Baig, U.; Hawsawi, A.; Gondal, M.A.; Dastageer, M.A. Pulsed laser based synthesis of polymeric-inorganic nanocomposites as efficient visible light active photo-catalysts for the degradation of organic pollutants in water. J. Photochem. Photobiol. A 2020, 390, 112266. [Google Scholar] [CrossRef]
- Baig, U.; Khan, A.; Gondal, M.A.; Dastageer, M.A.; Falath, W.S. Laser Induced Anchoring of Nickel Oxide Nanoparticles on Polymeric Graphitic Carbon Nitride Sheets Using Pulsed Laser Ablation for Efficient Water Splitting under Visible Light. Nanomaterials 2020, 10, 1098. [Google Scholar] [CrossRef]
- Baig, U.; Khan, A.; Gondal, M.A.; Dastageer, M.A.; Akhtar, S. Single-step synthesis of silicon carbide anchored graphitic carbon nitride nanocomposite photo-catalyst for efficient photoelectrochemical water splitting under visible-light irradiation. Colloid Surf. A 2021, 611, 125886. [Google Scholar] [CrossRef]
- Saratale, R.G.; Ghodake, G.S.; Shinde, S.K.; Cho, S.K.; Saratale, G.D.; Pugazhendhi, A.; Bharagava, R.N. Photocatalytic activity of CuO/Cu(OH)2 nanostructures in the degradation of Reactive Green 19A and textile effluent, phytotoxicity studies and their biogenic properties (antibacterial and anticancer). J. Environ. Manag. 2018, 223, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Sansotera, M.; Kheyli, S.G.M.; Baggioli, A.; Bianchi, C.L.; Pedeferri, M.P.; Diamanti, M.V.; Navarrini, W. Absorption and photocatalytic degradation of VOCs by perfluorinated ionomeric coating with TiO2 nanopowders for air purification. Chem. Eng. J. 2019, 361, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, X.; Chen, Q.; Xu, H.; Dai, M.; Zhang, M.; Wang, W.; Song, H. Microstructural modification of hollow TiO2 nanospheres and their photocatalytic performance. Appl. Surf. Sci. 2021, 535, 147641. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Liu, Y.; Feng, T.; Xi, S.; Wang, X.; Xue, C.; Qian, J.; Li, G. A symbiotic hetero-nanocomposite that stabilizes unprecedented CaCl2-type TiO2 for enhanced solar-driven hydrogen evolution reaction. Chem. Sci. 2019, 10, 8323–8330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Zhang, X.; Ma, L.; Xiang, C.; Li, L. TiO2-Doped Chitosan Microspheres Supported on Cellulose Acetate Fibers for Adsorption and Photocatalytic Degradation of Methyl Orange. Polymers 2019, 11, 1293. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Liu, Y.Y.; Lin, R.H.; Yen, F.S. Photocatalytic degradation of p-phenylenediamine with TiO2-coated magnetic PMMA microspheres in an aqueous solution. J. Hazard. Mater. 2009, 163, 973–981. [Google Scholar] [CrossRef]
- Bai, W.; Yao, R.; Tian, X.; Guan, M.; Lai, N.; Chen, Q.; Xu, Y.; Lin, J. Sunlight highly photoactive TiO2 @poly- p -phenylene composite microspheres for malachite green degradation. J. Taiwan Inst. Chem. E 2018, 87, 112–116. [Google Scholar] [CrossRef]
- Baig, U.; Gondal, M.A.; Ilyas, A.M.; Sanagi, M.M. Band gap engineered polymeric-inorganic nanocomposite catalysts: Synthesis, isothermal stability, photocatalytic activity and photovoltaic performance. J. Mater. Sci. Technol. 2017, 33, 547–557. [Google Scholar] [CrossRef]
- Neghi, N.; Kumar, M.; Burkhalov, D. Synthesis and application of stable, reusable TiO2 polymeric composites for photocatalytic removal of metronidazole: Removal kinetics and density functional analysis. Chem. Eng. J. 2019, 359, 963–975. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Zhang, S.; Wang, Y.; Liu, B.; Ren, Y. Core-Shell Structured Phenolic Polymer@TiO2 Nanosphere with Enhanced Visible-Light Photocatalytic Efficiency. Nanomaterials 2020, 10, 467. [Google Scholar] [CrossRef] [Green Version]
- Gar Alalm, M.; Samy, M.; Ookawara, S.; Ohno, T. Immobilization of S-TiO2 on reusable aluminum plates by polysiloxane for photocatalytic degradation of 2,4-dichlorophenol in water. J. Water Process. Eng. 2018, 26, 329–335. [Google Scholar] [CrossRef]
- Magalhães, F.; Moura, F.C.C.; Lago, R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organic contaminants. Desalination 2011, 276, 266–271. [Google Scholar] [CrossRef]
- Srikanth, B.; Goutham, R.; Badri Narayan, R.; Ramprasath, A.; Gopinath, K.P.; Sankaranarayanan, A.R. Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment. J. Environ. Manag. 2017, 200, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ding, M.; Chen, W.; Li, Y.; Wang, K. Nitrogen–doped GO/TiO2 nanocomposite ultrafiltration membranes for improved photocatalytic performance. Sep. Purif. Technol. 2018, 195, 70–82. [Google Scholar] [CrossRef]
- Mozia, S.; Darowna, D.; Wróbel, R.; Morawski, A.W. A study on the stability of polyethersulfone ultrafiltration membranes in a photocatalytic membrane reactor. J. Membr. Sci. 2015, 495, 176–186. [Google Scholar] [CrossRef]
- Fischer, K.; Gläser, R.; Schulze, A. Nanoneedle and nanotubular titanium dioxide—PES mixed matrix membrane for photocatalysis. Appl. Catal. B Environ. 2014, 160-161, 456–464. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N,Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Li, Z.; Zhu, Y.; Wang, H. Polysulfone/SiO2 Hybrid Shell Microcapsules Synthesized by the Combination of Pickering Emulsification and the Solvent Evaporation Technique and Their Application in Self-Lubricating Composites. Langmuir 2017, 33, 14149–14155. [Google Scholar] [CrossRef]
- Gao, J.; Song, X.; Huang, X.; Wang, L.; Li, B.; Xue, H. Facile preparation of polymer microspheres and fibers with a hollow core and porous shell for oil adsorption and oil/water separation. Appl. Surf. Sci. 2018, 439, 394–404. [Google Scholar] [CrossRef]
- He, M.; Fan, X.; Yang, Z.; Zhang, R.; Liu, Y.; Fan, L.; Zhang, Q.; Su, Y.; Jiang, Z. Antifouling high-flux membranes via surface segregation and phase separation controlled by the synergy of hydrophobic and hydrogen bond interactions. J. Membr. Sci. 2016, 520, 814–822. [Google Scholar] [CrossRef]
- Mei, S.; Xiao, C.; Hu, X. Preparation of porous PVC membrane via a phase inversion method from PVC/DMAc/water/additives. J. Appl. Polym. Sci. 2011, 120, 557–562. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Y.; Wang, T. Preparation of porous TiO2/Ag heterostructure films with enhanced photocatalytic activity. Chem. Eng. J. 2015, 270, 418–427. [Google Scholar] [CrossRef]
- Hir, Z.A.M.; Moradihamedani, P.; Abdullah, A.H.; Mohamed, M.A. Immobilization of TiO2 into polyethersulfone matrix as hybrid film photocatalyst for effective degradation of methyl orange dye. Mat. Sci. Semicond. Process. 2017, 57, 157–165. [Google Scholar] [CrossRef]
- Swain, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Gas permeation and selectivity characteristics of PSf based nanocomposite membranes. Polymer 2019, 180, 121692. [Google Scholar] [CrossRef]
- Kandziolka, M.V.; Kidder, M.K.; Gill, L.; Wu, Z.; Savara, A. Aromatic-hydroxyl interaction of an alpha-aryl ether lignin model-compound on SBA-15, present at pyrolysis temperatures. Phys. Chem. Chem. Phys. 2014, 16, 24188–24193. [Google Scholar] [CrossRef]
- Chi, M.; Sun, X.; Sujan, A.; Davis, Z.; Tatarchuk, B.J. A quantitative XPS examination of UV induced surface modification of TiO2 sorbents for the increased saturation capacity of sulfur heterocycles. Fuel 2019, 238, 454–461. [Google Scholar] [CrossRef]
- Muhulet, A.; Tuncel, C.; Miculescu, F.; Pandele, A.M.; Bobirica, C.; Orbeci, C.; Bobirica, L.; Palla-Papavlu, A.; Voicu, S.I. Synthesis and characterization of polysulfone–TiO2 decorated MWCNT composite membranes by sonochemical method. Appl. Phys. A Mater. 2020, 126. [Google Scholar] [CrossRef]
- Lavanya, C.; Geetha Balakrishna, R. Naturally derived polysaccharides-modified PSF membranes: A potency in enriching the antifouling nature of membranes. Sep. Purif. Technol. 2020, 230, 115887. [Google Scholar] [CrossRef]
- Zhu, L.; Song, H.; Wang, G.; Zeng, Z.; Xue, Q. Symmetrical polysulfone/poly(acrylic acid) porous membranes with uniform wormlike morphology and pH responsibility: Preparation, characterization and application in water purification. J. Membr. Sci. 2018, 549, 515–522. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wu, X.; Wang, M.; Wang, X.; Zhang, J.; Zhang, J.; Xu, Q. Hollow poly(cyclotriphosphazene-co-phloroglucinol) microspheres: An effective and selective adsorbent for the removal of cationic dyes from aqueous solution. Chem. Eng. J. 2015, 281, 42–52. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M. Effective photocatalytic degradation of sulfamethazine by CNTs/LaVO4 in suspension and dip coating modes. Sep. Purif. Technol. 2020, 235, 116138. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Mengting, Z.; Fu, D.; Yeap, S.K.; Othman, M.H.D.; Avtar, R.; Ouyang, T. Functionalizing TiO2 with graphene oxide for enhancing photocatalytic degradation of methylene blue (MB) in contaminated wastewater. J. Environ. Manag. 2020, 270, 110871. [Google Scholar] [CrossRef] [PubMed]











| NUM | TiNPs/PSF-x (x Means the Weight Ratio) | Actual Effective Content | Size (μm) | Porosity (%) | Density (g cm−3) | |
|---|---|---|---|---|---|---|
| TiO2 (wt.%.) | PSF (wt.%.) | |||||
| 1 | 0.08 | 7.0 | 93.0 | 43.75 | 35.2 | 0.846 |
| 2 | 0.24 | 16.0 | 84.0 | 43.18 | 32.6 | 0.943 |
| 3 | 0.40 | 22.5 | 77.5 | 38.64 | 30.7 | 1.022 |
| 4 | 0.56 | 28.5 | 71.5 | 47.73 | 27.5 | 1.126 |
| 5 | 0.72 | 33.5 | 66.5 | 46.18 | 25.0 | 1.220 |
| 6 | 0.88 | 35.4 | 64.6 | 42.72 | 24.1 | 1.254 |
| Kinetic Model and Parameter | First-Order | Second-Order | Diffusion | |||||
|---|---|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 (min) | R2 | qe (mg g−1) | k2 (min) | R2 | K3 | R2 | |
| PSF | 0.650 | 0.215 | 0.9759 | 0.726 | 0.443 | 0.8791 | 0.157 | 0.7953 |
| TiO2 | 0.780 | 0.125 | 0.8867 | 1.374 | 0.030 | 0.1418 | 0.295 | 0.9876 |
| TiNPs/PSF-0.08 | 0.720 | 0.228 | 0.9757 | 0.799 | 0.447 | 0.9020 | 0.168 | 0.8084 |
| TiNPs/PSF-0.24 | 0.770 | 0.220 | 0.9836 | 0.853 | 0.410 | 0.9005 | 0.181 | 0.8187 |
| TiNPs/PSF-0.40 | 0.770 | 0.221 | 0.9848 | 0.843 | 0.435 | 0.9128 | 0.176 | 0.8402 |
| TiNPs/PSF-0.56 | 0.790 | 0.222 | 0.9775 | 0.856 | 0.456 | 0.9232 | 0.174 | 0.8406 |
| TiNPs/PSF-0.72 | 0.760 | 0.219 | 0.9718 | 0.831 | 0.436 | 0.8903 | 0.173 | 0.8223 |
| TiNPs/PSF-0.88 | 0.740 | 0.232 | 0.9724 | 0.827 | 0.415 | 0.9084 | 0.176 | 0.7850 |
| Type of Photocatalysts | Catalyst Loading (wt.%) | TiO2 Concentration (mg mL−1) | MB Removal Results (%) 120 min |
|---|---|---|---|
| TiNPs/PSF-0.08 | 7.0 | 0.175 | 94.7 |
| TiNPs/PSF-0.24 | 16.0 | 0.400 | 98.9 |
| TiNPs/PSF-0.40 | 22.5 | 0.563 | 100.0 |
| TiNPs/PSF-0.56 | 28.5 | 0.713 | 100.0 |
| TiNPs/PSF-0.72 | 33.5 | 0.838 | 100.0 |
| TiNPs/PSF-0.88 | 35.4 | 0.885 | 100.0 |
| Composite Catalyst | Target Contaminant | Light Source | Cycle-Index | Degradation Degree (%) | Reference | ||
|---|---|---|---|---|---|---|---|
| Sample | TiO2 Concentration (mg mL−1) | Kind | Concentration (mg L–1) | ||||
| LDPE-TiO2 | 2.48 | methylene blue | 50 | 15 W (254 nm) | 3 | 100.0 | [17] |
| PMMA-TiO2 | 2.50 | phenylenediamine | 10 | 15 W (254 nm) | 5 | none | [18] |
| PPP-TiO2 | 1.00 | methylene green | 15 | 125 W (254 nm) | 5 | 86.6 | [19] |
| TiO2/PSF | membrane | methylene blue | 50 | 300 W (254 nm) | 5 | 98.0 | [40] |
| TiNPs/PSF-0.40 | 0.56 | methylene blue | 10 | 250 W (254 nm) | 20 | 96.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, Q.; Dai, F.; Gu, Y.; Qian, G.; Chen, C.; Yu, Y. Novel TiO2 Nanoparticles/Polysulfone Composite Hollow Microspheres for Photocatalytic Degradation. Polymers 2021, 13, 336. https://doi.org/10.3390/polym13030336
Zhang S, Wang Q, Dai F, Gu Y, Qian G, Chen C, Yu Y. Novel TiO2 Nanoparticles/Polysulfone Composite Hollow Microspheres for Photocatalytic Degradation. Polymers. 2021; 13(3):336. https://doi.org/10.3390/polym13030336
Chicago/Turabian StyleZhang, Shangying, Qi Wang, Fengna Dai, Yangyang Gu, Guangtao Qian, Chunhai Chen, and Youhai Yu. 2021. "Novel TiO2 Nanoparticles/Polysulfone Composite Hollow Microspheres for Photocatalytic Degradation" Polymers 13, no. 3: 336. https://doi.org/10.3390/polym13030336