A Novel Self-Assembled Graphene-Based Flame Retardant: Synthesis and Flame Retardant Performance in PLA
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PMrG
2.3. Preparation of PMrG/PLA Composites
2.4. Characterization
3. Results and Discussion
3.1. Fabrication of PMrG
3.2. Fabrication of PMrG/PLA Composite
3.3. Flame-Resistant Effect of PMrG on PLA
3.4. Mechanism for the Flame Retardant Effect of PMrG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weng, Y.X. Review of study of synthesis, production, process and application of PLA. China Plast. Ind. 2007, 35, 60–73. [Google Scholar]
- Song, Y.P.; Wang, D.Y.; Wang, X.L.; Lin, L.; Wang, Y.Z. A method for simultaneously improving the flame retardancy and toughness of PLA. Polym. Adv. Technol. 2011, 22, 2295–2301. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly (lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Chalotra, N.; Singh, A. Rapid Growth and Development of 3D Printing. Int. J. Eng. Sci. 2016, 113, 6305–6319. [Google Scholar]
- Chen, Y.; Wang, W.; Qiu, Y.; Li, L.; Qian, L.; Fei, X. Terminal group effects of phosphazene-triazinebi-group flameretardant additives in flame retardant polylactic acid composites. Polym. Degrad. Stab. 2017, 140, 166–175. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.X.; Wang, W.J.; Gu, X.Y.; Li, H.F.; Li, J.H.; Sun, J. Intercalation of phosphotungstic acid into layered double hydroxides by reconstruction method and its application in intumescent flame retardant poly (lactic acid) composites. Polym. Degrad. Stab. 2018, 147, 142–150. [Google Scholar] [CrossRef]
- Tawiah, B.; Yu, B.; Wei, R.; Yuen, R.K.; Chen, W.; Xin, J.H.; Fei, B. Simultaneous fire safety enhancement and mechanical reinforcement of poly (lacticacid) biocomposite with hexapheny (nitrilotris(ethane-2,1-diyl)) tris (phosphormi-date). J. Hazard. Mater. 2019, 380, 120856. [Google Scholar] [CrossRef]
- Zhao, X.; Guerrero, F.R.; Llorca, J.; Wang, D.Y. New Superefficiently flame-retardant bioplastic poly(lactic acid): Flammability, thermal decomposition behavior and tensile properties. ACS Sustain. Chem. Eng. 2016, 4, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Laufer, G.; Kirkland, C.; Morgan, B.; Jaime, C. Intumescent multilayer nanocoating, made with renewable polyelectrolytes for flame-retardant cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef]
- Cheng, X.W.; Guan, J.P.; Tang, R.C.; Liu, K.Q. Phytic acid as a bio-based phosphorus flame retardant for poly(lactic acid) nonwoven fabric. J. Clean. Prod. 2016, 124, 114–119. [Google Scholar] [CrossRef]
- Jin, X.D.; Gu, X.Y.; Chen, C.; Tang, W.F.; Li, H.F.; Liu, X.D.; Serge, B.; Zhang, Z.W.; Sun, J.; Zhang, S. The fire performance of polylactic acid containing a novel intumescent flame retardant and intercalated layered double hydroxides. J. Mater. Sci. 2017, 52, 12235–12250. [Google Scholar] [CrossRef]
- Jin, X.D.; Cui, S.P.; Sun, S.B.; Gu, X.Y.; Li, H.F.; Liu, X.D.; Tang, W.F.; Sun, J.; Serge, B.; Zhang, S. The preparation of a bio-polyelectrolytes based core-shell structure and its application in flame retardant polylactic acid composites. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105485. [Google Scholar] [CrossRef]
- Yang, W.; Tawiah, B.; Yu, C.; Qian, Y.F.; Wang, L.L.; Hu, E.Z.; Yu, B.; Lu, H.D.; Wang, X.; Hu, Y.; et al. Manufacturing, mechanical and flame retardant properties of poly(lactic acid) biocomposites based on calcium magnesium phytate and carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2018, 110, 227–236. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, Y.; Cai, W.; Zhang, D.C.; Yao, C.X.; Hu, Y.; Hu, W.C. Construction of multifunctional boron nitride nanosheet towards reducing toxic volatiles (CO and HCN) generation and fire hazard of thermoplastic polyurethane. J. Hazard. Mater. 2019, 362, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Tawiah, B.; Noor, N.; Sun, J.; Zhang, S.; Richard, K.; Yuen, B.; Fei, B. A facile and sustainable approach for simultaneously flame retarded, UV protective and reinforced poly(lactic acid) composites using fully bio-based complexing couples. Compos. Part B Eng. 2016, 215, 108833. [Google Scholar] [CrossRef]
- Yang, Y.X.; Haurie, L.; Zhang, J.; Zhang, X.Q.; Wang, R.; Wang, D.Y. Effect of bio-based phytate (PA-THAM) on the flame retardant and mechanical properties of polylactide (PLA). Express Polym. Lett. 2020, 8, 705–716. [Google Scholar] [CrossRef]
- Chen, Y.J.; Li, A.; Yang, D.D.; Liu, T.Y.; Li, X.W.; Tang, J.; Jiang, C.L. Study on the Interaction between low-viscosity high-permeability pregrouting sealing material and coal and its application. Adv. Polym. Technol. 2020, 12, 1217285. [Google Scholar] [CrossRef]
- Yu, H.H.; Xu, X.H.; Xia, Y.F.; Pan, M.Z.; Zarshad, N.; Pang, B.; Rahman, A.U.; Mu, M.; Ni, H.M. Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin. e-Polymers 2020, 20, 303–316. [Google Scholar] [CrossRef]
- Salasinska, K.; Mizera, K.; Celinski, M.; Kozikowski, P.; Borucka, M.; Gajek, A. Thermal properties and fire behavior of polyethylene with a mixture of copper phosphate and melamine phosphate as a novel flame retardant. Fire Saf. J. 2020, 115, 103137. [Google Scholar] [CrossRef]
- Sun, C.B.; Mu, H.D.; Chen, F.; Fu, Q. Preparation of polylactide composite with excellent flame retardance and improved mechanical properties. Chin. J. Polym. Sci. 2018, 36, 1385–1393. [Google Scholar] [CrossRef]
- Wang, P.J.; Liao, D.J.; Hu, X.P.; Pan, N.; Li, W.X.; Wang, D.Y.; Yao, Y. Facile fabrication of biobased P-N-C-containing nano-layered hybrid: Preparation, growth mechanism and its efficient fire retardancy in epoxy. Polym. Degrad. Stab. 2019, 159, 153–162. [Google Scholar] [CrossRef]
- Hu, X.P.; Guo, Y.Y.; Chen, L.; Wang, X.L.; Li, L.J.; Wang, Y.Z. A novel polymeric intumescent flame retardant: Synthesis, thermal degradation mechanism and application in ABS copolymer. Polym. Degrad. Stab. 2012, 97, 1772–1778. [Google Scholar] [CrossRef]
- Zhou, R.; Li, W.J.; Mu, J.J.; Ding, Y.M.; Jiang, J.C. Synergistic effects of aluminum diethylphosphinate and melamine on improving the flame retardancy of phenolic resin. Materials 2020, 13, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, B.H.; Song, L.; Liu, K.M.; Hu, Y. Solid acid-reduced graphene oxide nanohybrid for enhancing thermal stability, mechanical property and flame retardancy of polypropylene. Polym. Soc. Chem. 2015, 5, 41307–41316. [Google Scholar] [CrossRef]
- Hu, W.Z.; Yu, B.; Jiang, S.D.; Song, L.; Hu, Y.; Wang, B.B. Hyper-branched polymer grafting graphene oxide as an effective flame retardant and smoke suppressant for polystyrene. J. Hazard. Mater. 2015, 300, 58–66. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, Y.S.; Liu, P.J.; Xu, C.A.; Liu, Y.; Wang, Q. The preparation and application of a graphene-based hybrid flame retardant containing a long-chain phosphaphenanthrene. Sci. Rep. 2017, 7, 8759. [Google Scholar] [CrossRef]
- Bao, C.L.; Song, L.; Xing, W.Y.; Yuan, B.H.; Charles, A.; Huang, J.L.; Guo, Y.Q.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088–6096. [Google Scholar] [CrossRef]
- Song, P.J.; Fang, Z.P.; Ran, S.Y.; Fang, F.; Wang, H. Improved flame resistance and thermo-mechanical properties of epoxy resin nanocomposites from functionalized graphene oxide via self-assembly in water. Compos. Part B Eng. 2019, 165, 406–416. [Google Scholar]
- Yuan, B.H.; Sheng, H.B.; Mu, X.W.; Song, L.; Tai, Q.L.; Shi, Y.Q.; Kim, M.L.; Hu, Y. Enhanced flame retardancy of polypropylene by melamine-modified graphene oxide. J. Mater. Sci. 2015, 50, 5389–5401. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhang, J.T.; Qu, L.T.; Shi, G.Q.; Liu, J.Y.; Chen, J.F.; Dai, L.M. N, P-codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reaction. Angew. Chem. 2016, 128, 2270–2274. [Google Scholar] [CrossRef]
- Wei, L.F.; Wang, R.; Zhu, Z.G.; Wang, W.Q.; Wu, H.G. Functionalization of PET with phosphazene grafted graphene oxide for synthesis, flammability, and mechanism. Materials 2021, 14, 1470. [Google Scholar]
- Xie, R.J.; Qu, B.J. Expandable graphite systems for halogen-free flame retarding of polyolefins. II. structures of intumescent char and flame-retardant mechanism. J. Appl. Polym. Sci. 2001, 80, 1190–1197. [Google Scholar] [CrossRef]


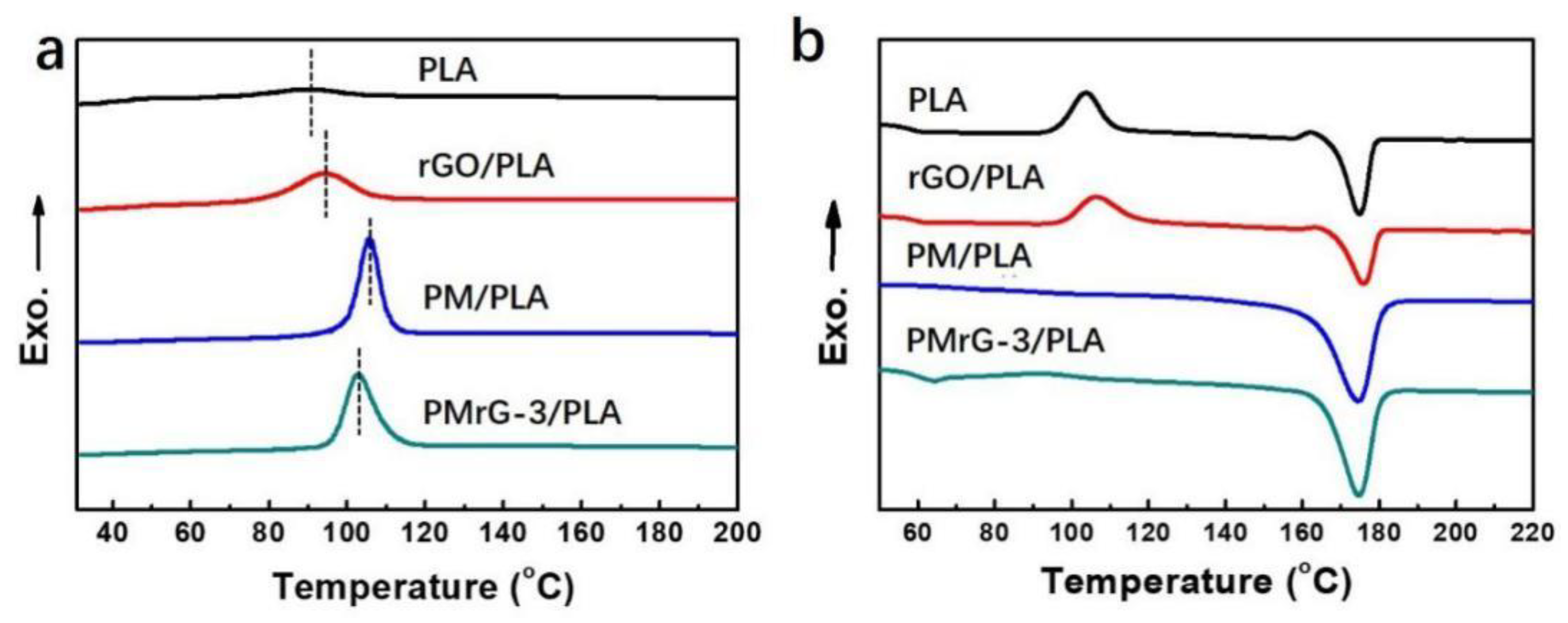

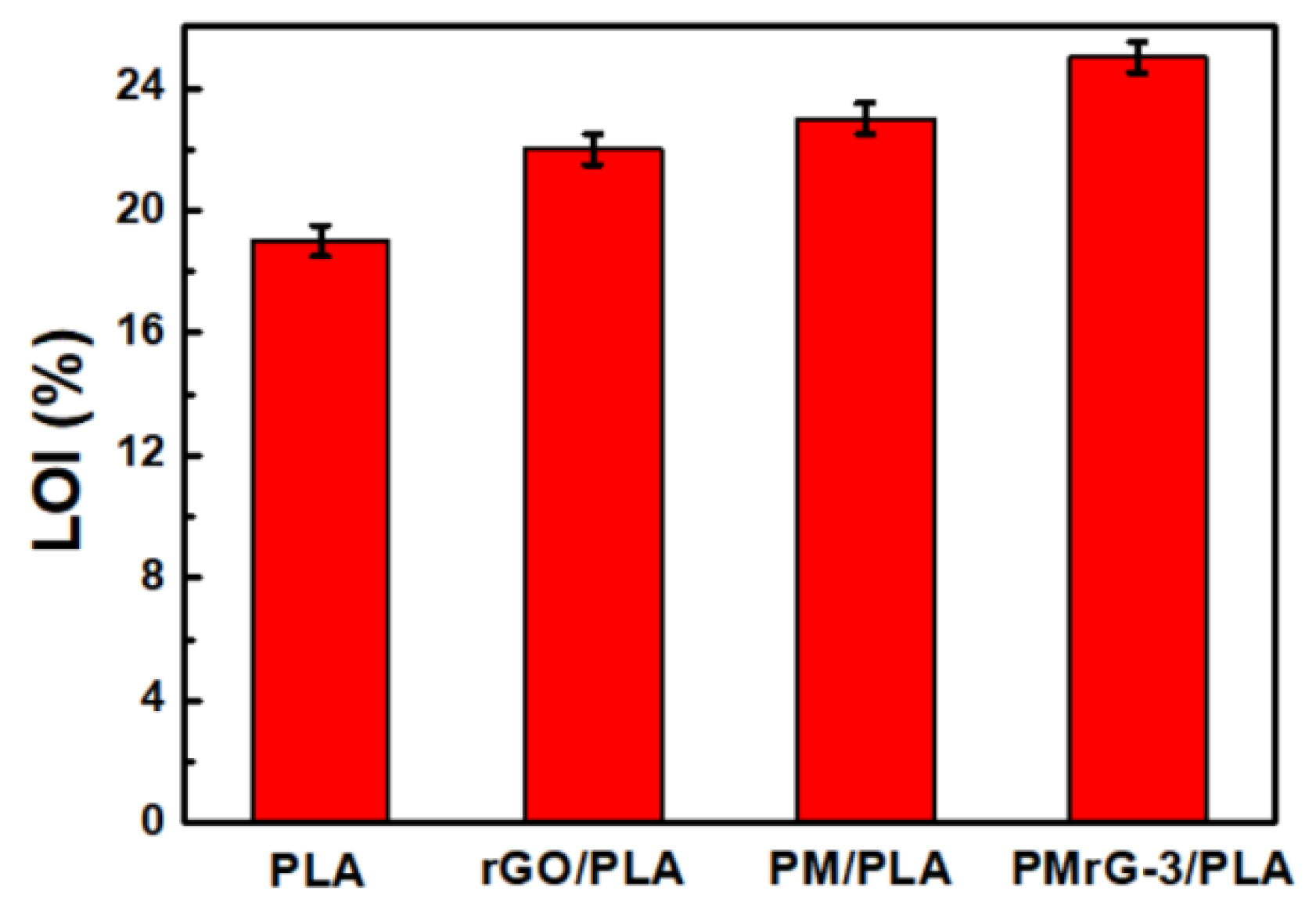




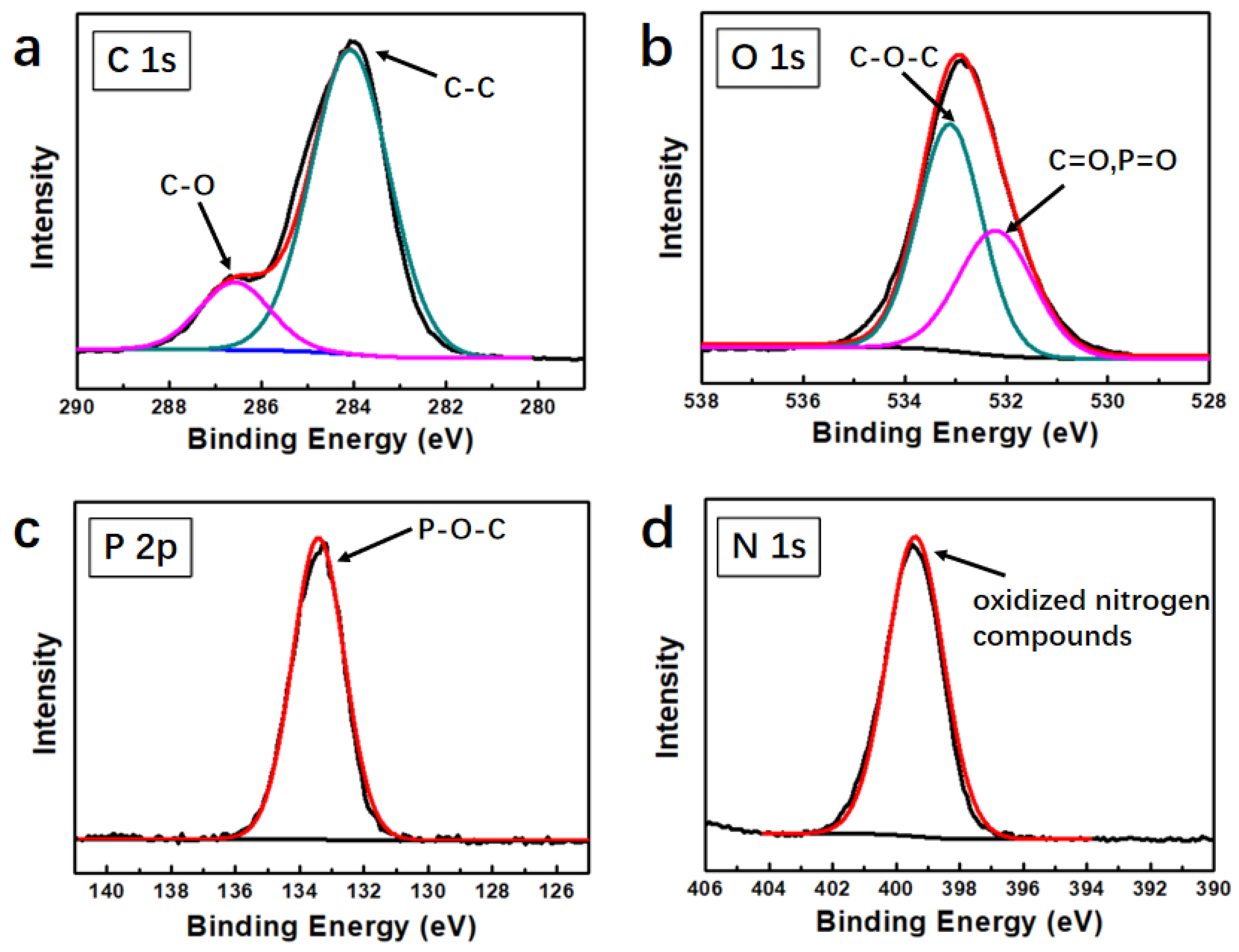

| Sample | TTI (s) | Phrr (Kw/m2) | THR (MJ/m2) | TSR (m2/m2) | Char Residue (%) |
|---|---|---|---|---|---|
| PLA | 74 | 426.6 | 58.8 | 52.7 | 0.3 |
| rGO/PLA | 43 | 309.1 | 58.5 | 411.7 | 4.8 |
| PM/PLA | 49 | 456.6 | 43.2 | 331.3 | 6.7 |
| PMrG-3/PLA | 59 | 276.1 | 46.5 | 191.5 | 15.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Wu, H.; Yang, F.; Yang, J.; Wang, R.; Zhu, Z. A Novel Self-Assembled Graphene-Based Flame Retardant: Synthesis and Flame Retardant Performance in PLA. Polymers 2021, 13, 4216. https://doi.org/10.3390/polym13234216
Yang P, Wu H, Yang F, Yang J, Wang R, Zhu Z. A Novel Self-Assembled Graphene-Based Flame Retardant: Synthesis and Flame Retardant Performance in PLA. Polymers. 2021; 13(23):4216. https://doi.org/10.3390/polym13234216
Chicago/Turabian StyleYang, Peixin, Hanguang Wu, Feifei Yang, Jie Yang, Rui Wang, and Zhiguo Zhu. 2021. "A Novel Self-Assembled Graphene-Based Flame Retardant: Synthesis and Flame Retardant Performance in PLA" Polymers 13, no. 23: 4216. https://doi.org/10.3390/polym13234216





