Photoswitchable Zirconium MOF for Light-Driven Hydrogen Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Methods
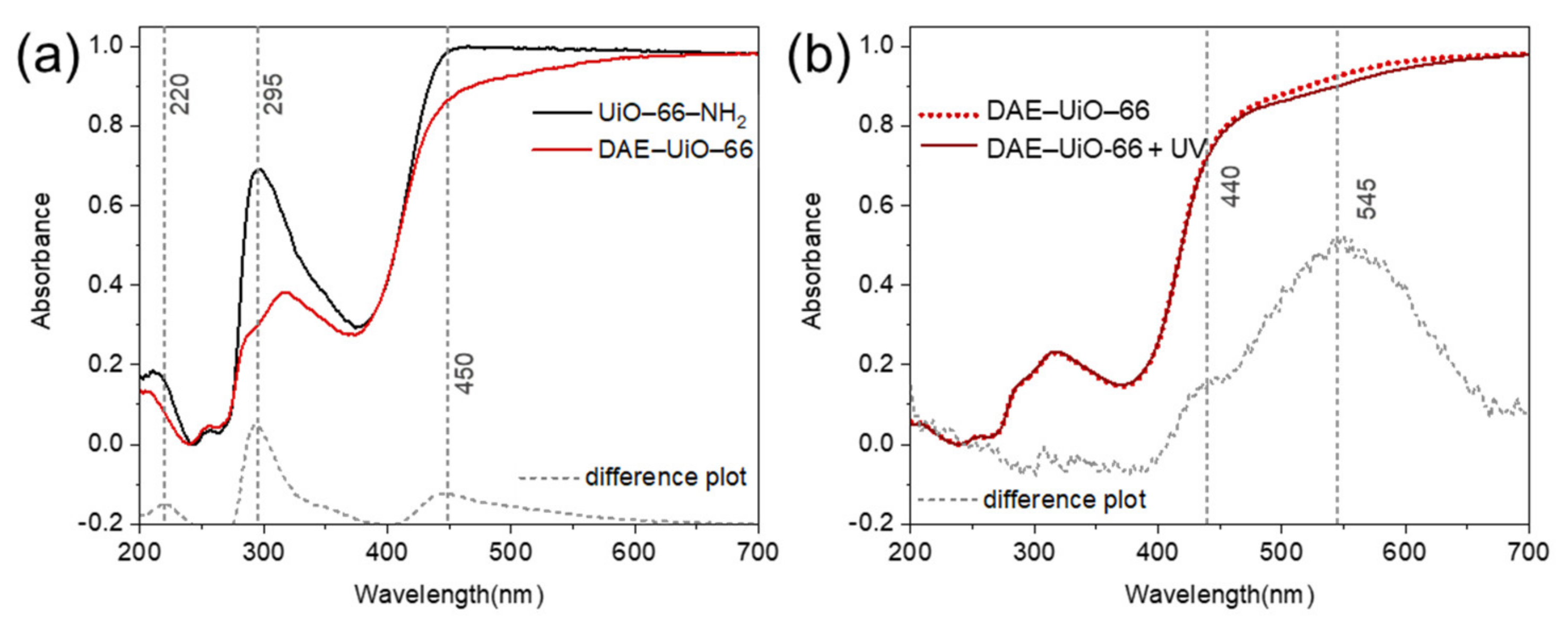
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortes, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butova, V.V.; Budnyk, A.P.; Charykov, K.M.; Vetlitsyna-Novikova, K.S.; Bugaev, A.L.; Guda, A.A.; Damin, A.; Chavan, S.M.; Oien-Odegaard, S.; Lillerud, K.P.; et al. Partial and Complete Substitution of the 1,4-Benzenedicarboxylate Linker in UiO-66 with 1,4-Naphthalenedicarboxylate: Synthesis, Characterization, and H-2-Adsorption Properties. Inorg. Chem. 2019, 58, 1607–1620. [Google Scholar] [CrossRef]
- Polyakov, V.A.; Butova, V.V.; Erofeeva, E.A.; Tereshchenko, A.A.; Soldatov, A.V. MW Synthesis of ZIF-7. The Effect of Solvent on Particle Size and Hydrogen Sorption Properties. Energies 2020, 13, 6306. [Google Scholar] [CrossRef]
- Gorban, I.E.; Soldatov, M.A.; Butova, V.V.; Medvedev, P.V.; Burachevskaya, O.A.; Belanova, A.; Zolotukhin, P.; Soldatov, A.V. L-Leucine Loading and Release in MIL-100 Nanoparticles. Int. J. Mol. Sci. 2020, 21, 9758. [Google Scholar] [CrossRef]
- Butova, V.V.; Burachevskaya, O.A.; Muratidi, M.A.; Surzhikova, I.I.; Zolotukhin, P.V.; Medvedev, P.V.; Gorban, I.E.; Kuzharov, A.A.; Soldatov, M.A. Loading of the Model Amino Acid Leucine in UiO-66 and UiO-66-NH2: Optimization of Metal-Organic Framework Carriers and Evaluation of Host-Guest Interactions. Inorg. Chem. 2021, 60, 5694–5703. [Google Scholar] [CrossRef]
- Butova, V.V.; Polyakov, V.A.; Budnyk, A.P.; Aboraia, A.M.; Bulanova, E.A.; Guda, A.A.; Reshetnikova, E.A.; Podkovyrina, Y.S.; Lamberti, C.; Soldatov, A.V. Zn/Co ZIF family: MW synthesis, characterization and stability upon halogen sorption. Polyhedron 2018, 154, 457–464. [Google Scholar] [CrossRef]
- Butova, V.V.; Bulanova, E.A.; Polyakov, V.A.; Guda, A.A.; Aboraia, A.M.; Shapovalov, V.V.; Zahran, H.Y.; Yahia, I.S.; Soldatov, A.V. The effect of cobalt content in Zn/Co-ZIF-8 on iodine capping properties. Inorg. Chim. Acta 2019, 492, 18–22. [Google Scholar] [CrossRef]
- Butova, V.V.; Polyakov, V.A.; Erofeeva, E.A.; Yahia, I.S.; Zahran, H.Y.; Abd El-Rehim, A.F.; Aboraia, A.M.; Soldatov, A.V. Modification of ZIF-8 with triethylamine molecules for enhanced iodine and bromine adsorption. Inorg. Chim. Acta 2020, 509, 5. [Google Scholar] [CrossRef]
- Van Velthoven, N.; Henrion, M.; Dallenes, J.; Krajnc, A.; Bugaev, A.L.; Liu, P.; Bals, S.; Soldatov, A.; Mali, G.; De Vos, D.E. S,O-Functionalized Metal-Organic Frameworks as Heterogeneous Single-Site Catalysts for the Oxidative Alkenylation of Arenes via C- H activation. ACS Catal. 2020, 10, 5077–5085. [Google Scholar] [CrossRef]
- Braglia, L.; Borfecchia, E.; Maddalena, L.; Oien, S.; Lomachenko, K.A.; Bugaev, A.L.; Bordiga, S.; Soldatov, A.V.; Lillerud, K.P.; Lamberti, C. Exploring structure and reactivity of Cu sites in functionalized UiO-67 MOFs. Catal. Today 2017, 283, 89–103. [Google Scholar] [CrossRef]
- Gui, B.; Meng, Y.; Xie, Y.; Du, K.; Sue, A.C.H.; Wang, C. Immobilizing Organic-Based Molecular Switches into Metal-Organic Frameworks: A Promising Strategy for Switching in Solid State. Macromol. Rapid Commun. 2018, 39. [Google Scholar] [CrossRef]
- Castellanos, S.; Kapteijn, F.; Gascon, J. Photoswitchable metal organic frameworks: Turn on the lights and close the windows. Crystengcomm 2016, 18, 4006–4012. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.L.; Hor, T.S.A.; Jin, G.X. Photodriven single-crystal-to-single-crystal transformation. Coord. Chem. Rev. 2017, 346, 112–122. [Google Scholar] [CrossRef]
- Rice, A.M.; Martin, C.R.; Galitskiy, V.A.; Berseneva, A.A.; Leith, G.A.; Shustova, N.B. Photophysics Modulation in Photoswitchable Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8790–8813. [Google Scholar] [CrossRef]
- Fan, C.B.; Liu, Z.Q.; Gong, L.L.; Zheng, A.M.; Zhang, L.; Yan, C.S.; Wu, H.Q.; Feng, X.F.; Luo, F. Photoswitching adsorption selectivity in a diarylethene-azobenzene MOF. Chem. Commun. 2017, 53, 763–766. [Google Scholar] [CrossRef]
- Hu, X.G.; Li, X.L.; Yang, S.I. Novel photochromic infinite coordination polymer particles derived from a diarylethene photoswitch. Chem. Commun. 2015, 51, 10636–10639. [Google Scholar] [CrossRef]
- Walton, I.M.; Cox, J.M.; Benson, C.A.; Patel, D.G.; Chen, Y.S.; Benedict, J.B. The role of atropisomers on the photo-reactivity and fatigue of diarylethene-based metal-organic frameworks. New J. Chem. 2016, 40, 101–106. [Google Scholar] [CrossRef]
- Li, Z.Q.; Wang, G.N.; Ye, Y.X.; Li, B.; Li, H.R.; Chen, B.L. Loading Photochromic Molecules into a Luminescent Metal-Organic Framework for Information Anticounterfeiting. Angew. Chem.-Int. Edit. 2019, 58, 18025–18031. [Google Scholar] [CrossRef]
- Schwartz, H.A.; Schaniel, D.; Ruschewitz, U. Tracking the light-induced isomerization processes and the photostability of spiropyrans embedded in the pores of crystalline nanoporous MOFsviaIR spectroscopy. Photochem. Photobiol. Sci. 2020, 19, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Fan, C.B.; Luo, M.B.; Wu, X.L.; Zhu, Y.; Pu, S.Z.; Xu, W.Y.; Guo, G.C. Photoswitching CO2 Capture and Release in a Photochromic Diarylethene Metal-Organic Framework. Angew. Chem.-Int. Edit. 2014, 53, 9298–9301. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.L.; Feng, X.F.; Luo, F. Novel azo-Metal-Organic Framework Showing a 10-Connected bct Net, Breathing Behavior, and Unique Photoswitching Behavior toward CO2. Inorg. Chem. 2015, 54, 11587–11589. [Google Scholar] [CrossRef] [PubMed]
- Lyndon, R.; Konstas, K.; Evans, R.A.; Keddie, D.J.; Hill, M.R.; Ladewig, B.P. Tunable Photodynamic Switching of DArE@PAF-1 for Carbon Capture. Adv. Funct. Mater. 2015, 25, 4405–4411. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.H.; Hill, M.R.; Babarao, R.; Medhekar, N.V. CO2 Adsorption in Azobenzene Functionalized Stimuli Responsive Metal-Organic Frameworks. J. Phys. Chem. C 2016, 120, 16658–16667. [Google Scholar] [CrossRef]
- Cox, J.M.; Walton, I.M.; Benedict, J.B. On the design of atropisomer-separable photochromic diarylethene-based metal-organic framework linkers. J. Mater. Chem. C 2016, 4, 4028–4033. [Google Scholar] [CrossRef]
- Fan, C.B.; Gong, L.L.; Huang, L.; Luo, F.; Krishna, R.; Yi, X.F.; Zheng, A.M.; Zhang, L.; Pu, S.Z.; Feng, X.F.; et al. Significant Enhancement of C2H2/C2H4 Separation by a Photochromic Diarylethene Unit: A Temperature- and Light-Responsive Separation Switch. Angew. Chem.-Int. Edit. 2017, 56, 7900–7906. [Google Scholar] [CrossRef]
- Furlong, B.J.; Katz, M.J. Bistable Dithienylethene-Based Metal-Organic Framework Illustrating Optically Induced Changes in Chemical Separations. J. Am. Chem. Soc. 2017, 139, 13280–13283. [Google Scholar] [CrossRef]
- Williams, D.E.; Rietman, J.A.; Maier, J.M.; Tan, R.; Greytak, A.B.; Smith, M.D.; Krause, J.A.; Shustova, N.B. Energy Transfer on Demand: Photoswitch-Directed Behavior of Metal-Porphyrin Frameworks. J. Am. Chem. Soc. 2014, 136, 11886–11889. [Google Scholar] [CrossRef]
- Park, J.; Feng, D.W.; Yuan, S.; Zhou, H.C. Photochromic Metal-Organic Frameworks: Reversible Control of Singlet Oxygen Generation. Angew. Chem.-Int. Edit. 2015, 54, 430–435. [Google Scholar] [CrossRef]
- Chakarova, K.; Strauss, I.; Mihaylov, M.; Drenchev, N.; Hadjiivanov, K. Evolution of acid and basic sites in UiO-66 and UiO-66-NH2 metal-organic frameworks: FTIR study by probe molecules. Microporous Mesoporous Mater. 2019, 281, 110–122. [Google Scholar] [CrossRef]
- Klet, R.C.; Liu, Y.Y.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Evaluation of Bronsted acidity and proton topology in Zr- and Hf-based metal-organic frameworks using potentiometric acid-base titration. J. Mater. Chem. A 2016, 4, 1479–1485. [Google Scholar] [CrossRef]
- Butova, V.V.; Aboraia, A.M.; Solayman, M.; Yahia, I.S.; Zahran, H.Y.; Abd El-Rehim, A.F.; Algarni, H.; Khabiri, G.; Soldatov, A.V. The joint effect of naphthalene-system and defects on dye removal by UiO-66 derivatives. Microporous Mesoporous Mater. 2021, 325, 111314. [Google Scholar] [CrossRef]
- Strauss, I.; Chakarova, K.; Mundstock, A.; Mihaylov, M.; Hadjiivanov, K.; Guschanski, N.; Caro, J. UiO-66 and UiO-66-NH2 based sensors: Dielectric and FTIR investigations on the effect of CO2 adsorption. Microporous Mesoporous Mater. 2020, 302, 110227. [Google Scholar] [CrossRef]
- Shepelenko, E.N.; Makarova, N.I.; Karamov, O.G.; Dubonosov, A.D.; Podshibakin, V.A.; Metelitsa, A.V.; Bren’, V.A.; Minkin, V.I. Synthesis and Photochromic Properties of Asymmetric Dihetarylethenes Based on 5-methoxy-1,2-dimethylindole and 5-(4-bromophenyl)-2-methylthiophene. Chem. Heterocycl. Compd. 2014, 50, 932–940. [Google Scholar] [CrossRef]
- Yang, X.L.; Ding, C.; Guan, R.F.; Zhang, W.H.; Feng, Y.; Xie, M.H. Selective dual detection of H2S and Cu2+ by a post-modified MOF sensor following a tandem process. J. Hazard. Mater. 2021, 403, 123698. [Google Scholar] [CrossRef]
- Du, Z.Y.; Li, B.L.; Jiang, C.; Sun, R.P.; Chen, S.W. Sorption of U(VI) on Schiff-base functionalized metal-organic frameworks UiO-66-NH2. J. Radioanal. Nucl. Chem. 2021, 327, 811–819. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallog. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Morris, W.; Doonan, C.J.; Yaghi, O.M. Postsynthetic Modification of a Metal–Organic Framework for Stabilization of a Hemiaminal and Ammonia Uptake. Inorg. Chem. 2011, 50, 6853–6855. [Google Scholar] [CrossRef]
- De Jong, J.J.D.; Browne, W.R.; Walko, M.; Lucas, L.N.; Barrett, L.J.; McGarvey, J.J.; van Esch, J.H.; Feringa, B.L. Raman scattering and FT-IR spectroscopic studies on dithienylethene switches—Towards non-destructive optical readout. Org. Biomol. Chem. 2006, 4, 2387–2392. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.T.; Sato, H.; Wu, P.Y.; Jeon, H.J.; Matsuda, R.; Kitagawa, S. Flexible interlocked porous frameworks allow quantitative photoisomerization in a crystalline solid. Nat. Commun. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Hou, I.C.-Y.; Berger, F.; Narita, A.; Müllen, K.; Hecht, S. Proton-Gated Ring-Closure of a Negative Photochromic Azulene-Based Diarylethene. Angew. Chem. Int. Ed. 2020, 59, 18532–18536. [Google Scholar] [CrossRef]
- Kobatake, S.; Terakawa, Y. Acid-induced photochromic system switching of diarylethene derivatives between P- and T-types. Chem. Commun. 2007, 17, 1698–1700. [Google Scholar] [CrossRef]


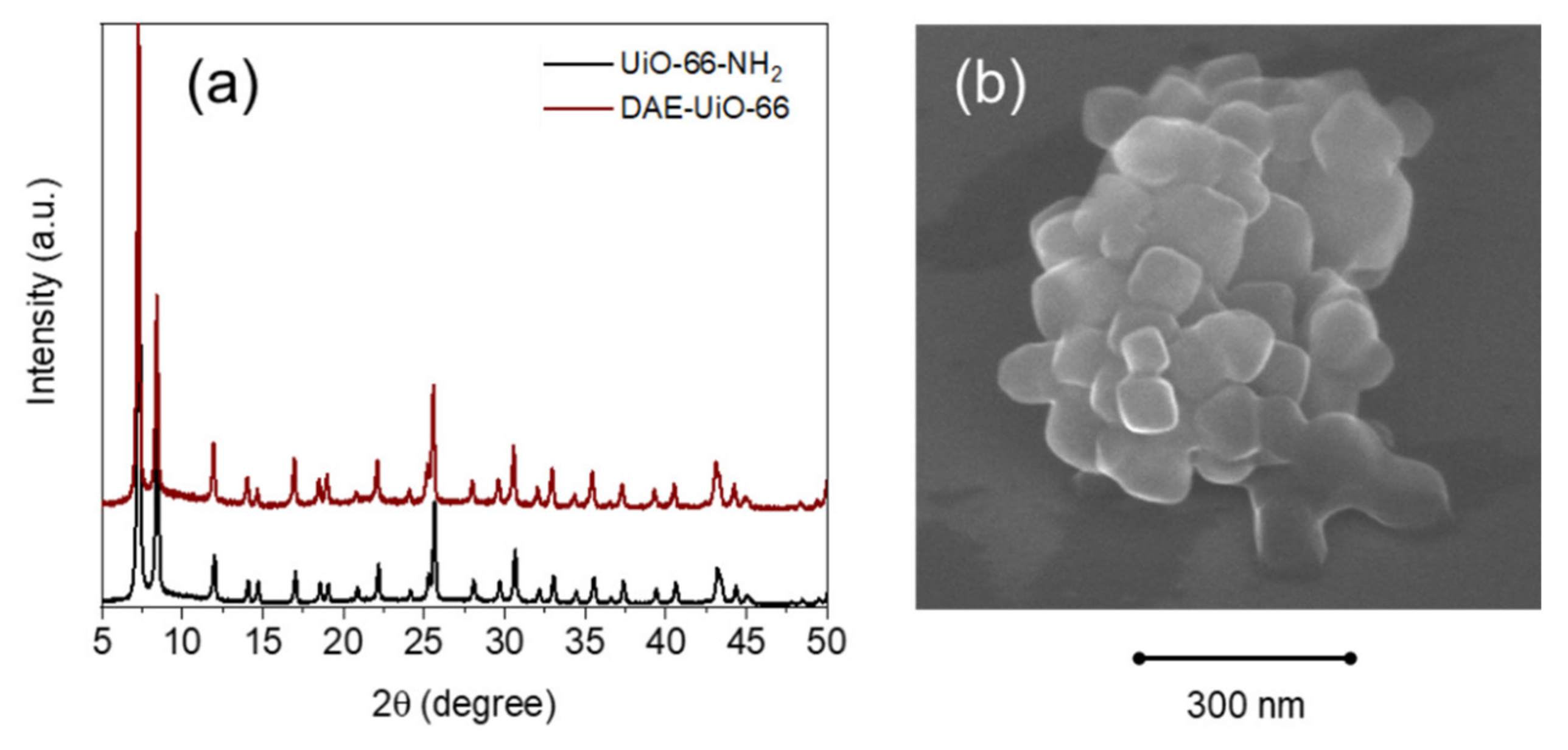
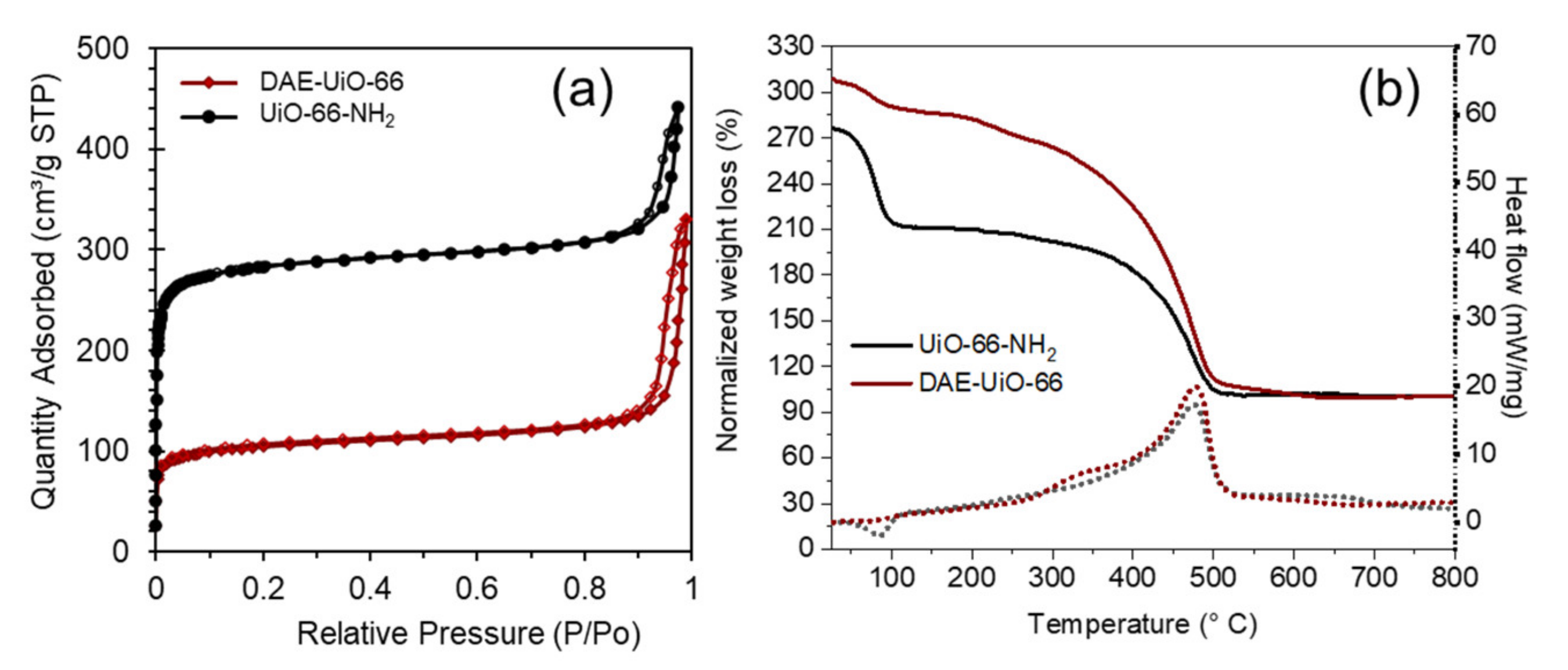
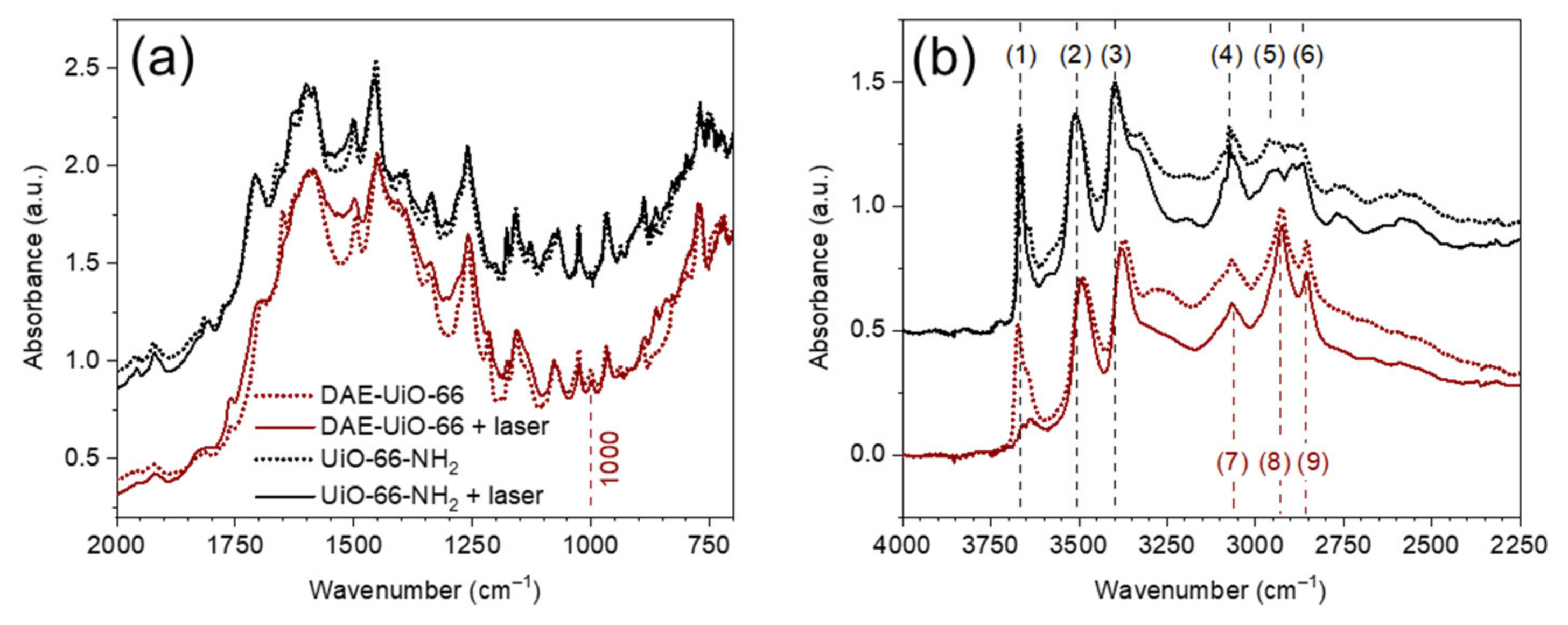


| Samples’ Designation | Unit Cell Parameters | Nitrogen Adsorption | TGA | XRF | ||||
|---|---|---|---|---|---|---|---|---|
| a, Å | V, Å3 | SSA, m2/g | Weight Loss, % | Molecular Weight | DAE Content | Zr:S:Cl | DAE Content | |
| UiO-66-NH2 | 20.794(5) | 8991(4) | 1111 | 51.3 | 1519 | - | 6:0:3 | - |
| DAE-UiO-66 | 20.836(6) | 9046(5) | 398 | 62.4 | 1945 | 9% | 6:0.5:0.3 | 8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butova, V.V.; Burachevskaya, O.A.; Podshibyakin, V.A.; Shepelenko, E.N.; Tereshchenko, A.A.; Shapovalova, S.O.; Il’in, O.I.; Bren’, V.A.; Soldatov, A.V. Photoswitchable Zirconium MOF for Light-Driven Hydrogen Storage. Polymers 2021, 13, 4052. https://doi.org/10.3390/polym13224052
Butova VV, Burachevskaya OA, Podshibyakin VA, Shepelenko EN, Tereshchenko AA, Shapovalova SO, Il’in OI, Bren’ VA, Soldatov AV. Photoswitchable Zirconium MOF for Light-Driven Hydrogen Storage. Polymers. 2021; 13(22):4052. https://doi.org/10.3390/polym13224052
Chicago/Turabian StyleButova, Vera V., Olga A. Burachevskaya, Vitaly A. Podshibyakin, Evgenii N. Shepelenko, Andrei A. Tereshchenko, Svetlana O. Shapovalova, Oleg I. Il’in, Vladimir A. Bren’, and Alexander V. Soldatov. 2021. "Photoswitchable Zirconium MOF for Light-Driven Hydrogen Storage" Polymers 13, no. 22: 4052. https://doi.org/10.3390/polym13224052