Improvements of Arboblend V2 Nature Characteristics through Depositing Thin Ceramic Layers
Abstract
:1. Introduction
2. Materials and Methods
- -
- Chromium Oxide Thermal Spray Powder, Amdry 6420 (Cr2O3): angular, blocky morphology, (10–105) µm;
- -
- Chromia–Silica composite powder, Metco 136F (Cr2O3-xSiO2-yTiO2): irregular or angular/blocky morphology, (9–110) µm, Cr2O3—Balance; SiO2—(3.0–4.5)%; TiO2 < 4.0%;
- -
- Zirconia–Titania–Yttria Composite Powder, Metco™ 143 (ZrO2 18TiO2 10Y2O3): spheroidal morphology; typically particle size between (3–40) μm;
- -
- The microindentation and scratch tests used the CETR UMT-2 microtribometer (Universal Materials Tester, CETR®, Campbell, SUA). Test conditions were as follows:
- -
- Scratch analysis: a blade with a tip of 0.4 mm (radius at the tip) was used, the samples were fixed on the table and during the test samples were pressed with a vertical force of 10 N NVIDIA blade, moving the table over a distance of 10 mm in 60 s, and the test speed was 0.167 mm/s. The software performed the automatic test and recorded the following parameters: vertical force Fz, horizontal force Fx, time and distance of movement in the horizontal direction Y (of the fixing mass of the sample).
- -
- Microindentation test: the Rockwell type indenter was used (cone with diamond tip having an angle of 120° and a radius at the peak of 200 microns), the samples were fixed on the table and during the test samples were pressed with a vertical force of 10 N (with steps/times described in work). Three samples of each type of powder were tested in order to be able to calculate the parameters statistically with the highest possible accuracy (hardness and Young’s modulus). The software performed the automatic test and recorded the following parameters: vertical force Fz, time and vertical travel distance C of the indenter (with capacitive sensor). Other process parameters were loading time—30 s; holding time—15 s; unloading time—30 s; and sensor of (0.2–20) N. Microindentation tests involved testing three samples for each type of ceramic powder in order to confirm experimental repeatability. The average values were obtained by calculating the arithmetic average, and the standard deviation highlights the variation in a set of numbers compared to the calculated average value.
3. Results and Discussion
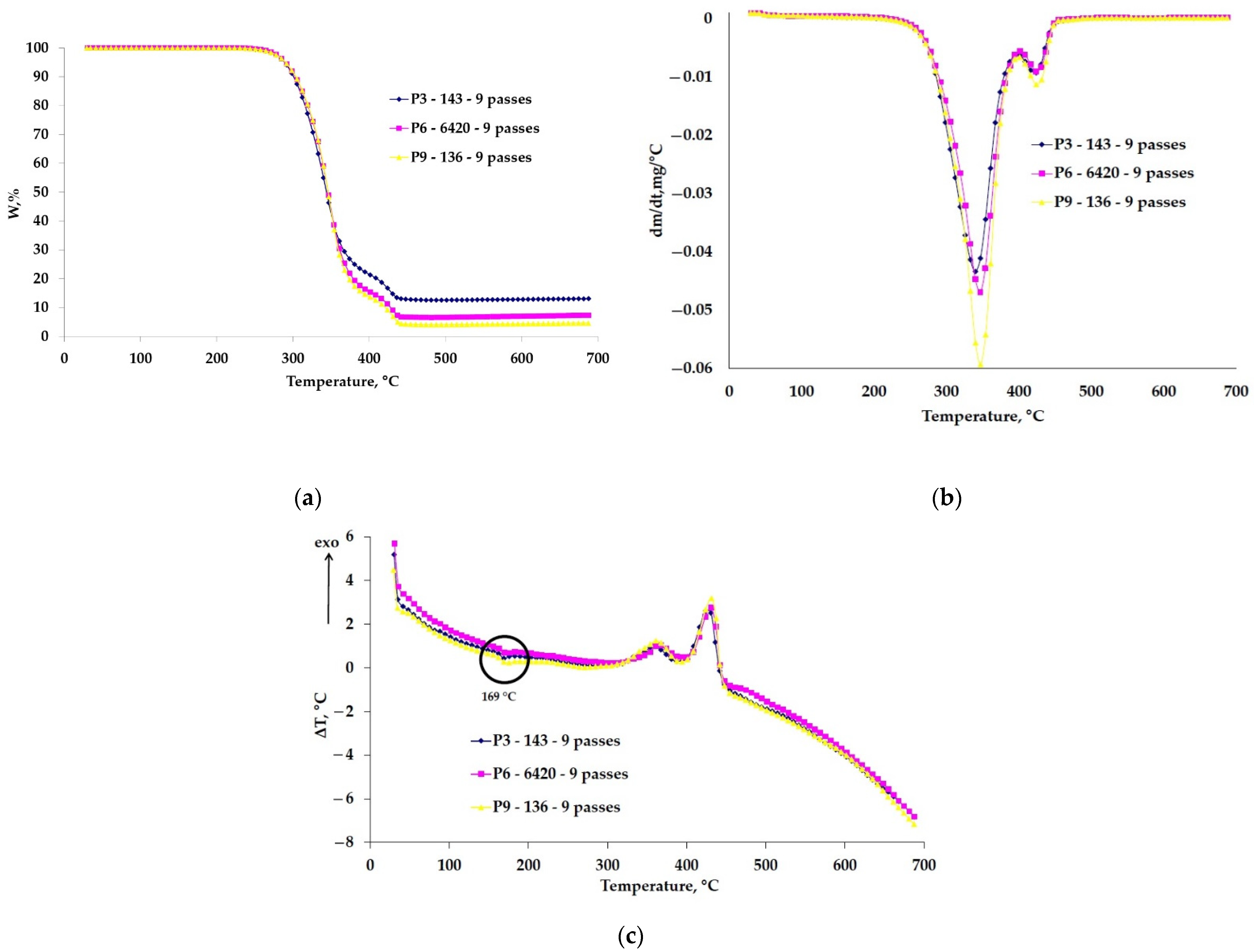
3.1. DSC Analyse
3.2. TG Analyses
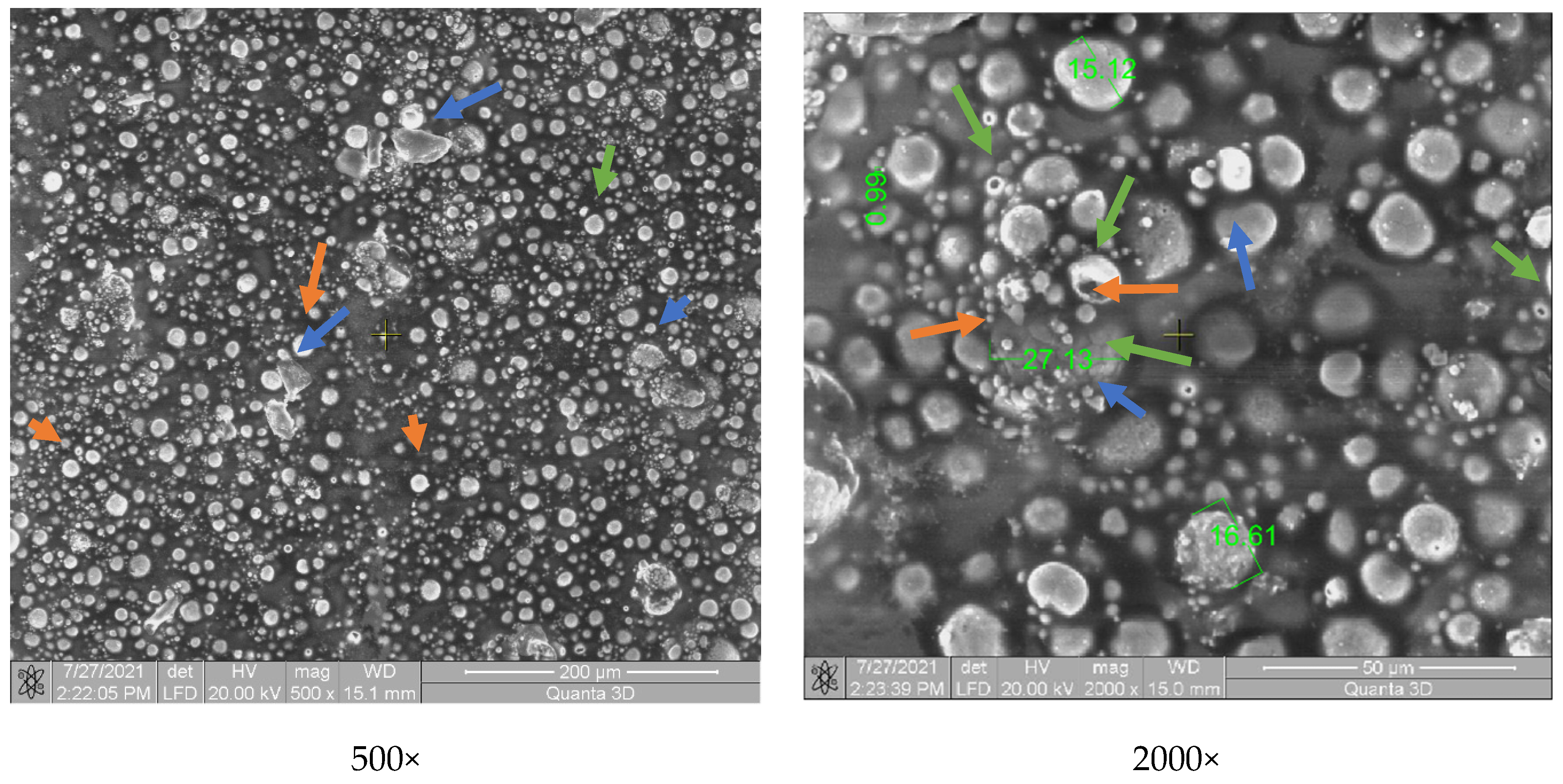
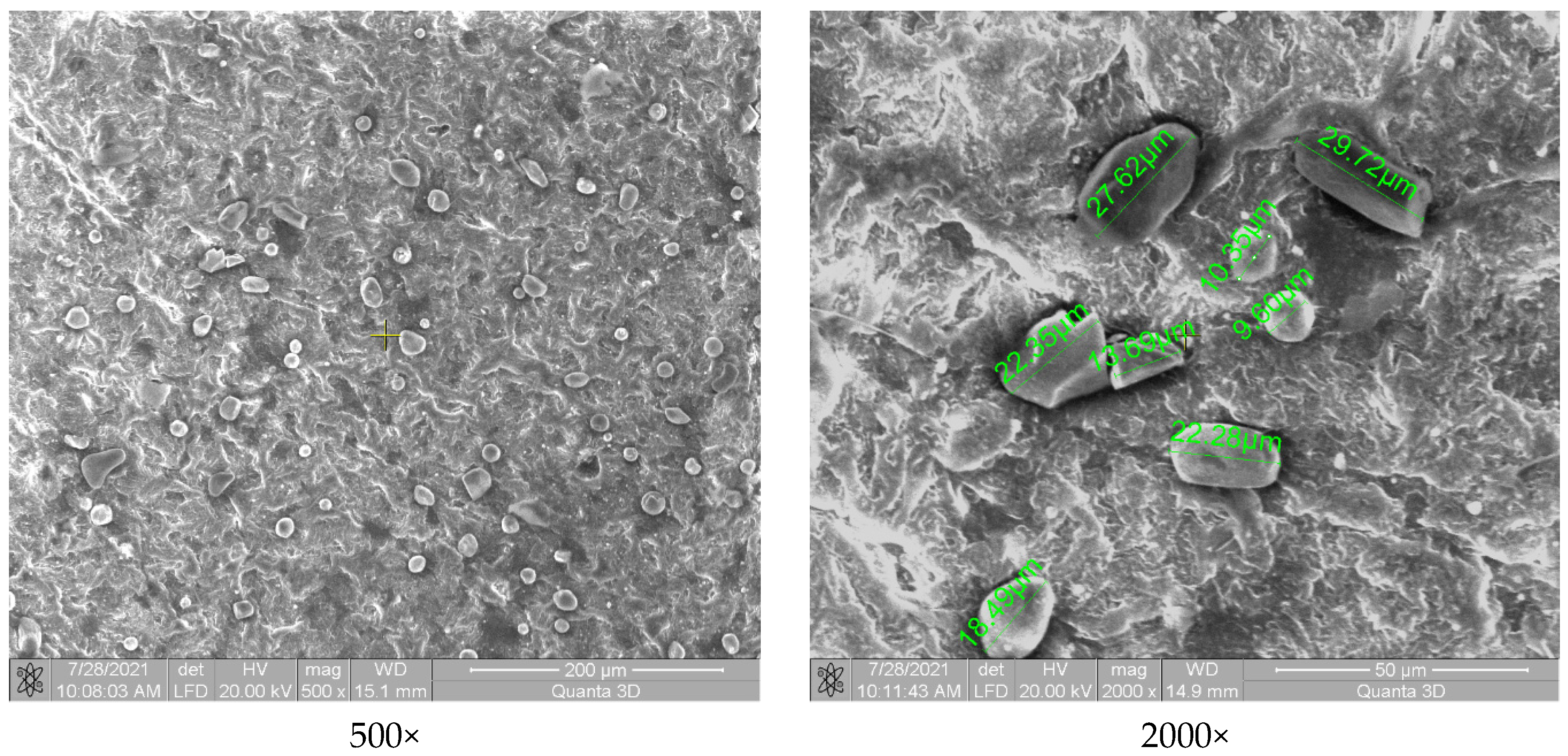
3.3. Surface and Structure Analysis of Coated Samples
3.3.1. SEM Analysis
3.3.2. EDX Analysis
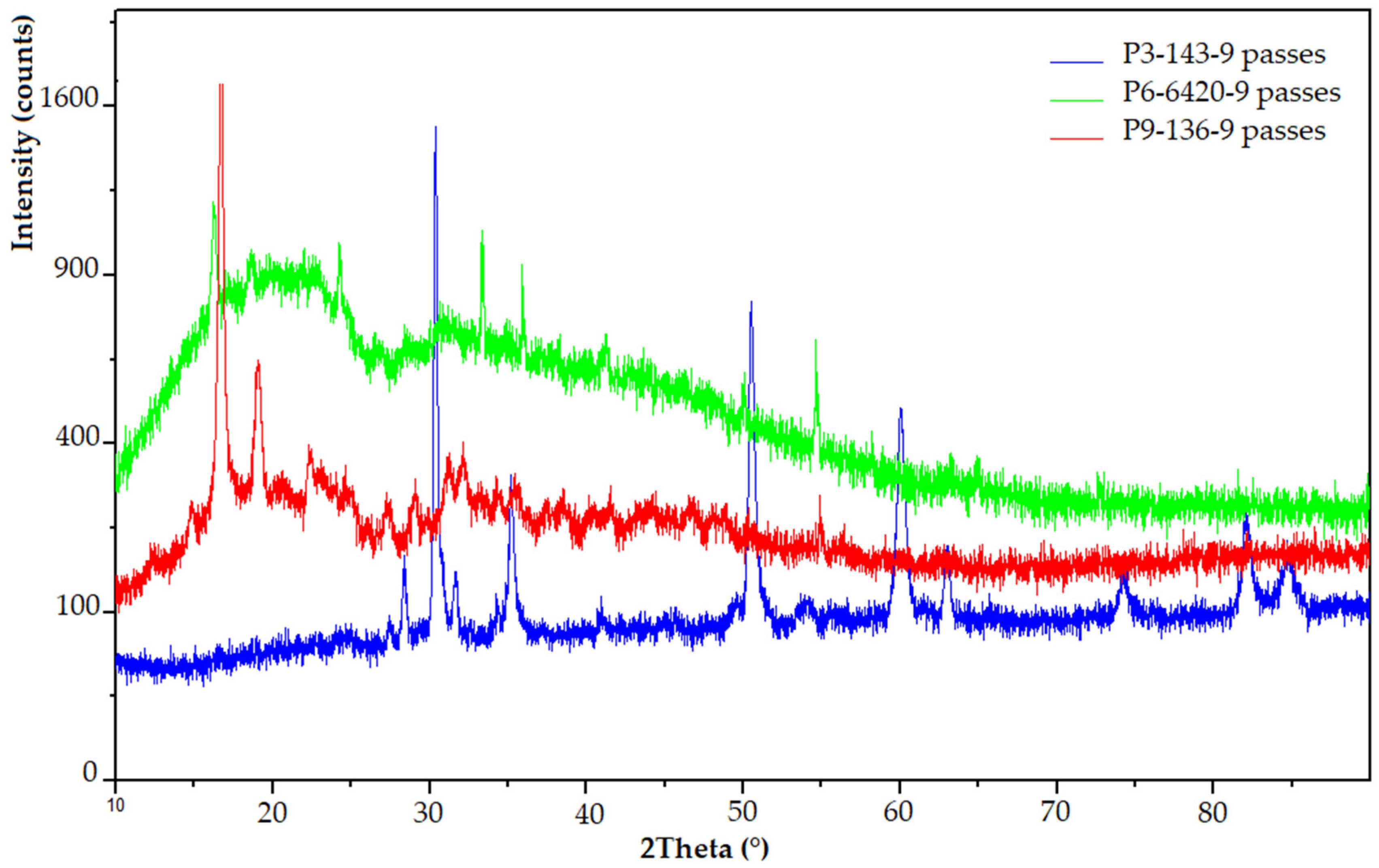
3.3.3. XRD Analysis
- The P3–143–9 passes sample (blue diffractogram) has a strong crystalline aspect due to the large amount of zirconium dioxide particles present in the structure of the ceramic powder. Thus, to the ZrO2 compound can be assigned the peaks from 2θ = 31.14°, 38.43°, 60°, 82° and 84.78° [46,52,53]. Titanium dioxide, as in the case of the P9–136–9 passes sample, is found at angles of 27.43°, 28.34°, 63.02° and 74.41° [54,55]. However, the intensity of the diffraction peaks is quite low, the highest being registered in the case of the 2θ = 28.34° angle. The low angle from 2-theta = 43°, was identified as the specific angle of Y2O3 crystallization [56].
- The P9–136–9 passes sample (red diffractogram) shows diffraction maxima associated with the presence of the polymer matrix, which has in its structure polylactic acid (16.73°) and lignin or natural fibers (19.04°) [53,57]. Diffraction angles corresponding to the coating with ceramic micropowder are also visible: The specific peaks to Cr2O3 crystallization at 2-theta angles of low intensity are 30.35°, 31.70°, 35.16°, 50.48° and 54.12° [47,48,49,58]. For SiO2 microspheres, a peak located at about 2θ = 22.5 is observed [59]. No other diffraction peaks can be detected for this compound. According to the literature [54,55], the diffraction angles that can be attributed to the titanium dioxide (TiO2) present are at 27.33° and 32.13° in the case of Metco 136F micropowder.
3.4. Scratch Analysis
3.5. Microindentation Test
4. Conclusions
- -
- A slight increase in the melting point, from 172 °C (base material [35,61]) up to 174 °C, varying depending on the thickness of the deposited layer. The thickness value is closely influenced by the microparticles size that constitute the ceramic powder. Thus, the larger the size of the microparticles, the higher the thermal resistance of the coated material is.
- -
- No significant changes were visible both in terms of temperature range and the amount of mass lost during the thermal degradation, and the differences were also attributed in this situation to the size of the ceramic microparticles.
- -
- Regarding the coatings’ uniformity, the SEM surface analysis indicated a good and uniform incorporation of microparticles in the case of composite powder based on zirconium oxide. The other two ceramic micropowders in contact with the polymer matrix did not reveal a good adhesion due to the lower working temperature than the melting point (2435 °C).
- -
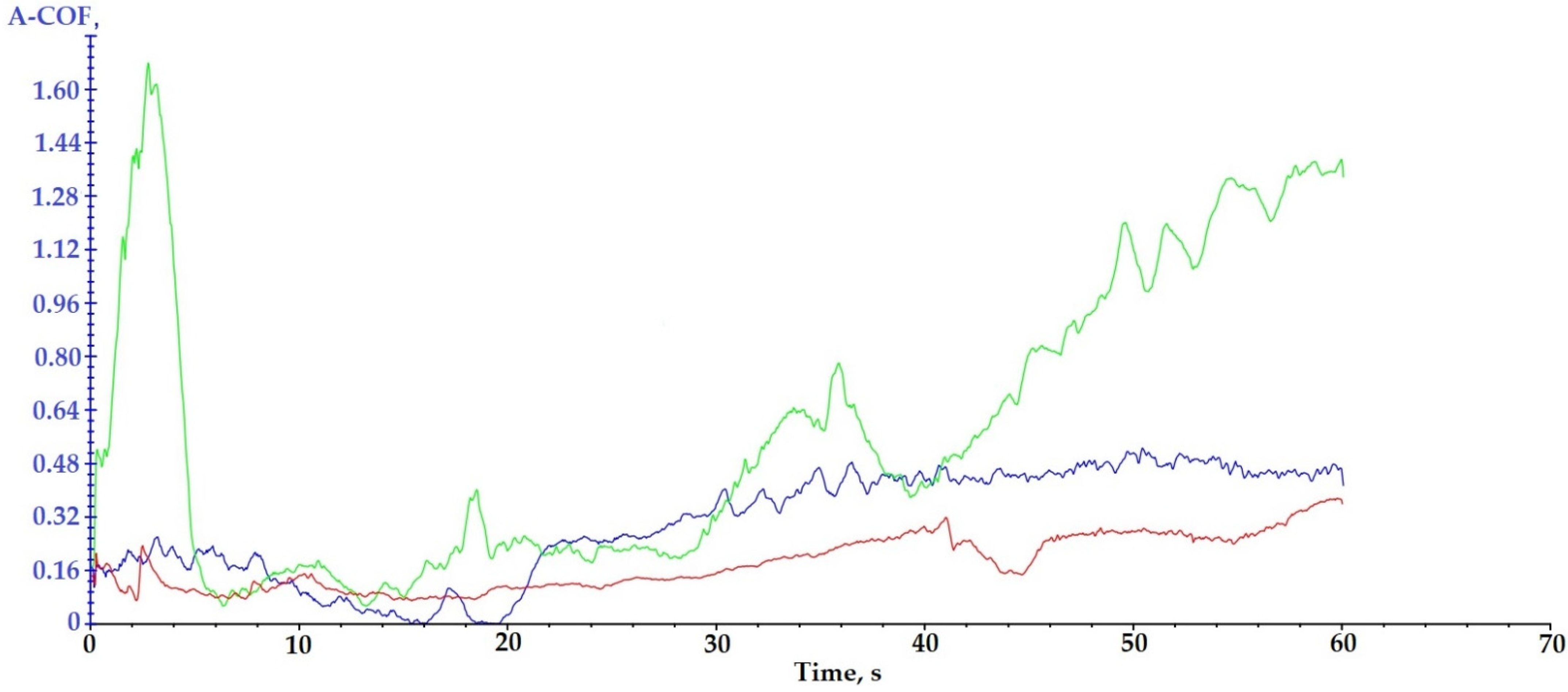
- XRD and EDX analyses highlighted the presence of microceramic layers. Their crystalline/semi-crystalline structure confers hardness; thus, they are suitable for applications that require this feature. The coating with Cr2O3-xSiO2-yTiO2 (sample 9) formed the hardest layer (0.17 ± 0.01 GPa), which was demonstrated during the microindentation test. Analyzing the obtained results from the SEM and scratch analyses point of view, it can be concluded that the deposition was not uniform due to the fact that the adhesion between the microparticles of chromium oxide, silicon oxide and titanium oxide is lower than in the case of the other two samples, the average value of the apparent friction coefficient (A-COF) being 0.18 ± 0.08.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| TPS | Titanium Plasma Spraying |
| APS | Atmospheric Plasma Spray |
| PDMS | Polydimethylsiloxane |
| PDC | biodegradable multiblock copolymer |
| TFX | Polyetherurethane |
| PLA | polylactic acid |
| NLPM | normal litre per minute |
| DC | Direct Current |
| DSC | Differential scanning calorimetry |
| RT | room temperature |
| TG | Thermogravimetric curves |
| DTG | derived thermogravimetric curves |
| DTA | differential thermal analyzes |
| Tonset | starting temperature |
| Tpeak | middle temperature |
| Tend | finish temperature |
| SEM | Scanning Electron Microscopy |
| LFD | Large Field Detector |
| EDX | Energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffraction analysis |
| W% | percentage mass loss at each stage |
| A-COF | apparent friction coefficient |
| Y | testing distance (mm) |
References
- Lu, Z.; Myoung, S.W.; Kim, E.H.; Lee, J.; Hand Jung, Y.G. Microstructure Evolution and thermal durability with coating thickness in APS thermal barrier coatings. Mater. Today Proc. 2014, 1, 35–43. [Google Scholar] [CrossRef]
- Matikainen, V.; Bolelli, G.; Koivuluoto, H.; Sassatelli, P.; Lusvarghi, L.; Vuoristo, P. Sliding wear behaviour of HVOF and HVAF sprayed Cr3C2-based coatings. Wear 2017, 388–389, 57–71. [Google Scholar] [CrossRef]
- Suarez, M.; Bellayer, S.; Traisnel, M.; Gonzalez, W.; Chicot, D.; Lesage, J.; Puchi-Cabrera, E.S.; Staia, M.H. Corrosion behavior of Cr3C2–NiCr vacuum plasma sprayed coatings. Surf. Coat. Technol. 2008, 202, 4566–4571. [Google Scholar] [CrossRef]
- Wypych, A.; Siwak, P.; Andrzejewski, D.; Jakubowicz, J. Titanium Plasma-sprayed coatings on polymers for hard tissue applications. Materials 2018, 11, 2536. [Google Scholar] [CrossRef] [Green Version]
- Gariboldi, E.; Rovatti, L.; Lecis, N.; Mondora, L.; Mondora, G.A. Tribological and mechanical behaviour of Cr3C2–NiCr thermally sprayed coatings after prolonged aging. Surf. Coat. Technol. 2016, 305, 83–92. [Google Scholar] [CrossRef]
- Tsui, Y.C.; Clyne, T.W. An analytical model for predicting residual stresses in progressively deposited coatings Part 1: Planar geometry. Thin Solid Films 1997, 306, 23–33. [Google Scholar] [CrossRef]
- Thermal Spray Materials Guide. Available online: https://www.oerlikon.com/ecomaXL/files/oerlikon_BRO-0001.17_TS_MaterialGuide_EN.pdf (accessed on 4 August 2020).
- Channagiri Mohankumar Praveen, K.; Avinash, L.; Manjunath Patel Gowdru, C.; Pimenov, D.Y.; Khaled, G. Electrodeposition based preparation of Zn–Ni alloy and Zn–Ni–WC nano-composite coatings for corrosion-resistant applications. Coatings 2021, 11, 712. [Google Scholar] [CrossRef]
- Shkirskiy, V.; Uebel, M.; Maltseva, A.; Lefèvre, G.; Volovitch, P.; Rohwerder, M. Cathodic driven coating delamination suppressed by inhibition of cation migration along Zn polymer interface in atmospheric CO2. npj Mater. Degrad. 2019, 3, 2. [Google Scholar] [CrossRef]
- Aung, M.M.; Wong, J.L.; Hong, N.L. Improvement of anticorrosion coating properties in bio-based polymer epoxy acrylate incorporated with nano zinc oxide particles. Ind. Eng. Chem. Res. 2020, 59, 1753–1763. [Google Scholar] [CrossRef]
- Rajkovic, A.; Uyttendaele, M.; Deley, W.; Van Soom, A.; Rijsselaere, T.; Debevere, J. Dynamics of boar semen motility inhibition as a semi-quantitative measurement of Bacillus cereus emetic toxin (Cereulide). J. Microbiol. Methods 2006, 65, 525–534. [Google Scholar] [CrossRef]
- Divine, S.; Chun-Wei, Y.; Ian, L. Mechanical durability of engineered superhydrophobic surfaces for anti-corrosion. Coatings 2018, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Ulaeto, S.; Rajan, R.; Pancrecious, J.; Rajan, T.; Pai, B. Developments in smart anticorrosive coatings with multifunctional characteristics. Prog. Org. Coat. 2017, 111, 294–314. [Google Scholar] [CrossRef]
- Asri, N.; Husaini, T.; Sulong, A.; Majlan, E.; Daud, W. Coating of stainless steel and titanium bipolar plates for anticorrosion in PEMFC: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Fengjuan, X.; Cheng, Q.; Mingyang, G.; Junzhong, W.; Xinrui, Y.; Hongli, L.; Lin, Y. Anticorrosive durability of zinc-based waterborne coatings enhanced by highly dispersed and conductive polyaniline/graphene oxide composite. Prog. Org. Coat. 2018, 125, 79–88. [Google Scholar] [CrossRef]
- Sorensen, P.; Kiil, S.; Dam-Johansen, K.; Weinell, C. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Shchukin, D.; Yasakau, K.; Möhwald, H.; Ferreira, M. Anticorrosion coatings with self-healing effect based on nanocontainers impregnated with corrosion inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Plyushch, A.; Macutkevic, J.; Banys, J.; Kuzhir, P.; Kalanda, N.; Petrov, A.; Silvestre, C.; Uimin, M.A.; Yermakov, A.Y.; Shenderova, O. Carbon-coated nickel nanoparticles: Effect on the magnetic and electric properties of composite materials. Coatings 2018, 8, 165. [Google Scholar] [CrossRef] [Green Version]
- Ausanio, G.; Hison, C.L.; Iannotti, V.; Lanotte, L.; Lanotte, L. Magneto-piezoresistance in elastomagnetic composites. J. Appl. Phys. 2011, 110, 063903. [Google Scholar] [CrossRef]
- Dumitrescu, M.; Lisa Iordan, A.R.; Tudorache, F.; Petrila, I.; Borhana, A.I.; Palamaru, M.N.; Mihailescu, C.; Leontie, L.; Munteanu, C. Ni ferrite highly organized as humidity sensors. Mater. Chem. Phys. 2015, 156, 170–179. [Google Scholar] [CrossRef]
- Mohr, R.; Kratz, K.; Weigel, T.; Lucka-Gabor, M.; Moneke, M.; Lendlein, A. Initiation of shape-memory effect by inductive heating of magnetic nanoparticles in thermoplastic polymers. Proc. Nat. Acad. Sci. USA 2006, 103, 3540–3545. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.M. Electromagnetic activation of shape memory polymer networks containing magnetic nanoparticles. Macromol. Rapid Commun. 2006, 27, 1168–1172. [Google Scholar] [CrossRef]
- Baljinder, K.; Forkan, S.; Piyanuch, L.; Myler, P. Thermal protection of carbon fiber-reinforced composites by ceramic particles. Coatings 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Santos, T.A. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Freitas, P.; Ferreira, R.; Cardoso, S.; Cardoso, F. Magnetoresistive sensors. J. Phys. Condens. Matter 2007, 19, 165221. [Google Scholar] [CrossRef]
- Mocanu, Z.; Airimioaei, M.; Ciomaga, C.; Curecheriu, L.; Tudorache, F.; Tascu, S.; Iordan, A.; Palamaru, N.; Mitoseriu, L. Investigation of the functional properties of MgxNi1-xFe2O4 ceramics. J. Mater. Sci. 2014, 49, 3276–3286. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef] [Green Version]
- Berry, C.C.; Curtis, A.S. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Geng, D.; Cui, W.; Yang, F.; Xie, Z.; Kang, D.; Zhang, Z. (Fe, Ni)/C nanocapsules for electromagnetic-wave-absorber in the whole Ku-band. Carbon 2009, 47, 470–474. [Google Scholar] [CrossRef]
- Wu, N.; Liu, X.; Zhao, C.; Cui, C.; Xia, A. Effects of particle size on the magnetic and microwave absorption properties of carbon-coated nickel nanocapsules. J. Alloys Compd. 2016, 656, 628–634. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, R.; Zhao, X.; Wang, X.; Zhang, X.; Qin, G. Dependence of gigahertz microwave absorption on the mass fraction of Co@C nanocapsules in composite. J. Alloys Compd. 2017, 724, 1023–1029. [Google Scholar] [CrossRef]
- TECNARO–The Biopolymer Company. Available online: https://www.tecnaro.de/en/ (accessed on 20 August 2020).
- Nedelcu, D.; Marguta, A.; Mazurchevici, S.; Munteanu, C.; Istrate, B. Micro-structural and morphological analyses of coated ‘liquid wood’ samples by ceramic particles. Mater. Res. Express 2019, 6, 085326. [Google Scholar] [CrossRef]
- Mazurchevici, S.-N.; Mazurchevici, A.-D.; Nedelcu, D. Dynamical mechanical and thermal analyses of biodegradable raw materials for additive manufacturing. Materials 2020, 13, 1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazurchevici, S.-N.; Motaș, J.G.; Diaconu, M.; Lisa, G.; Lohan, N.M.; Glod, M.; Nedelcu, D. Nanocomposite biopolymer arboblend V2 nature AgNPs. Polymers 2021, 13, 2932. [Google Scholar] [CrossRef]
- Broitman, E.; Nedelcu, D.; Mazurchevici, S.; Glenat, H.; Grillo, S. Tribological and nanomechanical behavior of liquid wood. J. Tribol. 2019, 141, 022001. [Google Scholar] [CrossRef]
- Nedelcu, D.; Santo, L.; Santo, A.G.; Plavanescu Mazurchevici, S. Mechanical behaviour evaluation of arboform material samples by bending deflection test. Mater. Plast. 2015, 52, 423–426. [Google Scholar]
- Oerlikon Metco–Materials and Surface Solutions. Available online: https://www.oerlikon.com/metco/en/ (accessed on 12 June 2020).
- Wagner, M. Thermal analysis in practice, fundamental aspects. In Thermal Analysis in Practice; Wagner, M., Ed.; Hanser: München, Germany, 2018; pp. 1–9. ISBN 9781569906439. [Google Scholar]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cell Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Cai, Y.H.; Xie, Y.C.; Tang, Y.; Zhao, L.S. Thermal decomposition kinetics of Poly(L-lactic acid) after heat treatment. Open Mater. Sci. J. 2015, 9, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Mofokeng, J.P.; Luyt, A.S.; Tabi, T.; Kovacs, J. Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices. J. Thermoplast. Compos. Mater. 2011, 25, 927–948. [Google Scholar] [CrossRef]
- Prieur, B.; Meub, M.; Wittemann, M.; Klein, R.; Bellayer, S.; Fontaine, G.; Bourbigot, S. Phosphorylation of lignin: Characteri-zation and investigation of the thermal decomposition. RSC Adv. 2017, 7, 16866–16877. [Google Scholar] [CrossRef] [Green Version]
- Chien, Y.C.; Liang, C.; Liu, S.H.; Yang, S.H. Combustion kinetics and emission characteristics of polycyclic aromatic hydrocarbons from polylactic acid combustion. J. Air Waste Manag. Assoc. 2012, 60, 849–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sameni, J.; Krigstin, S.; Rosa, D.d.S.; Leao, A.; Sain, M. Thermal characteristics of lignin residue from industrial processes. BioResources 2014, 9, 725–737. [Google Scholar] [CrossRef] [Green Version]
- Istrate, B.; Rau, J.V.; Munteanu, C.; Antoniac, I.V.; Saceleanu, S. Properties and in vitro assessment of ZrO2-based coatings obtained by atmospheric plasma jet spraying on biodegradable Mg-Ca and Mg-Ca-Zr alloys. Ceram. Int. 2020, 46, 15897–15906. [Google Scholar] [CrossRef]
- Fatemeh, T.; Alireza, S.; Hossein, M. Synthesis of Cr2O3/TiO2 nanocomposite and its application as the blocking layer in solar cells. J. Environ. Anal. Chem. 2018, 5, 1000231. [Google Scholar] [CrossRef]
- Bhagyashri, K.; Mahesh, N.; Garadkar, K.M.; Rahul, B.M.; Kiran Kumar, K.S.; Balu, D.A.; Shivaji, T. Ionic liquid assisted synthesis of chromium oxide (Cr2O3) nanoparticles and their application in glucose sensing. J. Mater. Sci. Mater. Electron. 2019, 30, 13984–13993. [Google Scholar] [CrossRef]
- Sone, B.T.; Manikandan, E.; Gurib-Fakim, A.; Maaza, M. Single-phase α-Cr2O3 nanoparticles’ green synthesis using Callistemon viminalis’ red flower extract. Green Chem. Lett. Rev. 2016, 9, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Benwood, C.; Anstey, A.; Andrzejewski, J.; Misra, M.; Mohanty, A.K. Improving the impact strength and heat resistance of 3d printed models: Structure, property, and processing correlationships during fused deposition modeling (FDM) of Poly(Lactic Acid). ACS Omega 2018, 3, 4400–4411. [Google Scholar] [CrossRef]
- Teixeira, E.d.M.; Campos, A.d.; Marconcini, J.M.; Bondancia, T.J.; Wood, D.; Klamczynski, A.; Mattosoa, L.H.C.; Glenn, G.M. Starch/fiber/poly(lactic acid) foam and compressed foam composites. RSC Adv. 2014, 4, 6616–6623. [Google Scholar] [CrossRef]
- Wu, C.-S. Analysis of mechanical, thermal, and morphological behavior of polycaprolactone/wood flour blends. J. Appl. Polym. Sci. 2004, 94, 1000–1006. [Google Scholar] [CrossRef] [Green Version]
- Onkar, M.; Savita, R. Monoclinic zirconium oxide nanostructures having tunable band gap synthesized under extremely non-equilibrium plasma conditions. Proceedings 2019, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Sagadevan, S.; Jiban, P.; Isha, D. Hydrothermal synthesis of zirconium oxide nanoparticles and its characterization. J. Mater. Sci. Mater. Electron. 2016, 27, 5622–5627. [Google Scholar] [CrossRef]
- Suning, L.; Qian, W.; Tao, C.; Zhihua, Z.; Ying, W.; Jiajun, F. Study on cerium-doped nano-TiO2 coatings for corrosion protection of 316 L stainless steel. Nanoscale Res. Lett. 2012, 7, 227. [Google Scholar] [CrossRef] [Green Version]
- Vorrada, L.; Natnapin, J.; Thirawich, C.; Achanai, B. The photocatalytic reduction of hexavalent chromiumby controllable mesoporous anatase TiO2 nanoparticles. Adv. Mater. Sci. Eng. 2014, 2014, 348427. [Google Scholar] [CrossRef] [Green Version]
- Tamrakar, R.K.; Upadhyay, K.; Bisen, D.P. Gamma ray induced thermoluminescence studies of yttrium (III) oxide nanopowders doped with gadolinium. J. Radiat. Res. Appl. Sci. 2014, 7, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Mohanty, S.; Nayak, S.K. Preparation and characterization of lignin nanofibre by electrospinnig technique. Int. J. Sci. Eng. Appl. Sci. 2015, 1, 184–190, ISSN:2395-3470. [Google Scholar]
- Adnan, A.; Shahid, A.; Murtaza, S.; Shahid, M.R.; Shahzad, N.; Saadat, A.S. Structural and magnetic phase transition of sol–gel-synthesized Cr2O3 and MnCr2O4 nanoparticles. J. Sol-Gel Sci. Technol. 2016, 80, 96–102. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Santos, P.L.; Bonacin, J.A.; Passos, R.R.; Aparecido Pocrifka, L. Rice husk reuse in the preparation of SnO2/SiO2 nanocomposite. Mater. Res. 2015, 18, 639–643. [Google Scholar] [CrossRef]
- Nedelcu, D.; Comaneci, R. Microstructure, mechanical properties and technology of samples obtained by injection from arboblend. Indian J. Eng. Mater. Sci. 2014, 21, 272–276. [Google Scholar]






| Powder | Gun Type | N2 | H2 | Electric | 9MP Powder Dispenser | Spray Distance (mm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure (Bar) | Gas Flow (NLPM) | Pressure (Bar) | Gas Flow (NLPM) | DC (A) | DC (V) | Carrier Gas Flow (NLPM) | Air Pressure (Bar) | Amount (g/min) | |||
| ZrO2 18TiO2 10Y2O3 | 9MB | 3.4 | 44 | 3.4 | 6.6 | 400 | 70–80 | 5.3 | 1.4 | 144 | 137 |
| Cr2O3 | 3.6 | 39 | 3.6 | 6.6 | 400 | 70–80 | 5.1 | 1.4 | 126 | 145 | |
| Cr2O3-xSiO2-yTiO2 | 3.7 | 42 | 3.7 | 6.6 | 400 | 70–80 | 5.1 | 1.4 | 132 | 145 | |
| No.crt. | Sample Number | Powder Type | Number of Passes |
|---|---|---|---|
| 1 | 1 | 143 | 5 |
| 2 | 2 | 143 | 7 |
| 3 | 3 | 143 | 9 |
| 4 | 4 | 6420 | 5 |
| 5 | 5 | 6420 | 7 |
| 6 | 6 | 6420 | 9 |
| 7 | 7 | 136 | 5 |
| 8 | 8 | 136 | 7 |
| 9 | 9 | 136 | 9 |
| Sample | Transformation | Tonset [°C] | Tpeak [°C] | Tend [°C] | ΔH/m [kJ/kg] |
|---|---|---|---|---|---|
| P3–143–9 passes | Ist | 62.0 | 64.7 | 68.5 | −8.81 |
| IInd | 81.9 | 87.2 | 93.0 | 19.36 | |
| IIIrd | 162.7 | 169.3 | 175.3 | −40.1 | |
| P6–6420–9 passes | Ist | 62.1 | 65.3 | 71.2 | −4.29 |
| IInd | 81.4 | 87.2 | 94.2 | 7.16 | |
| IIIrd | 164.0 | 173.7 | 185.3 | −38.14 | |
| P9–136–9 passes | Ist | 61.8 | 65.8 | 71.8 | −5.57 |
| IInd | 81.7 | 88.7 | 95.6 | 8.66 | |
| IIIrd | 166.0 | 171.4 | 178.0 | −48.38 |
| Sample | Stage | Tonset [°C] | Tpeak [°C] | Tend [°C] | W [%] | DTA Characteristic | Residue [%] |
|---|---|---|---|---|---|---|---|
| P3–143–9 passes | I | 289 | 341 | 369 | 84.98 | exo | 3.81 |
| II | 413 | 423 | 436 | 11.21 | exo | ||
| P6–6420–9 passes | I | 282 | 346 | 373 | 84.61 | exo | 6.60 |
| II | 413 | 426 | 438 | 8.79 | exo | ||
| P9–136–9 passes | I | 281 | 347 | 367 | 88.06 | exo | 1.68 |
| II | 415 | 426 | 438 | 10.26 | exo |
| Sample | A-COF Medium Value | A-COF Maximum | Time of A-COF Maximum[s] |
|---|---|---|---|
| P3–143–9 passes | 0.29 ± 0.16 | 0.53 | 50 |
| P6–6420–9 passes | 0.56 ± 0.42 | 1.62/1.37 | 3.0/60 |
| P9–136–9 passes | 0.18 ± 0.08 | 0.37 | 60 |
| Sample | Test | Max Load (N) | Max Depth (µm) | Young’s Modulus (GPa) | Micro Hardness (GPa) |
|---|---|---|---|---|---|
| P3–143–9 passes | 1 | 8.99 | 73.55 | 1.52 | 0.11 |
| 2 | 8.7 | 72.78 | 1.69 | 0.11 | |
| 3 | 8.98 | 73.02 | 1.48 | 0.11 | |
| Average | 8.98 ± 0.01 | 73.12 ± 0.4 | 1.56 ± 0.11 | 0.11 ± 0.00 | |
| P6–6420–9 passes | 1 | 8.99 | 66.64 | 1.69 | 0.13 |
| 2 | 8.98 | 71.70 | 1.91 | 0.11 | |
| 3 | 8.98 | 71.02 | 2.53 | 0.10 | |
| Average | 8.99 ± 0.01 | 69.79 ± 2.75 | 2.04 ± 0.44 | 0.12 ± 0.01 | |
| P9–136–9 passes | 1 | 8.99 | 53.77 | 2.92 | 0.16 |
| 2 | 8.99 | 53.08 | 3 | 0.16 | |
| 3 | 8.97 | 50.42 | 2.9 | 0.17 | |
| Average | 8.99 ± 0.01 | 52.42 ± 1.77 | 2.94 ± 0.05 | 0.17 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurchevici, S.-N.; Marguta, A.; Istrate, B.; Benchea, M.; Boca, M.; Nedelcu, D. Improvements of Arboblend V2 Nature Characteristics through Depositing Thin Ceramic Layers. Polymers 2021, 13, 3765. https://doi.org/10.3390/polym13213765
Mazurchevici S-N, Marguta A, Istrate B, Benchea M, Boca M, Nedelcu D. Improvements of Arboblend V2 Nature Characteristics through Depositing Thin Ceramic Layers. Polymers. 2021; 13(21):3765. https://doi.org/10.3390/polym13213765
Chicago/Turabian StyleMazurchevici, Simona-Nicoleta, Alina Marguta, Bogdan Istrate, Marcelin Benchea, Mihai Boca, and Dumitru Nedelcu. 2021. "Improvements of Arboblend V2 Nature Characteristics through Depositing Thin Ceramic Layers" Polymers 13, no. 21: 3765. https://doi.org/10.3390/polym13213765









