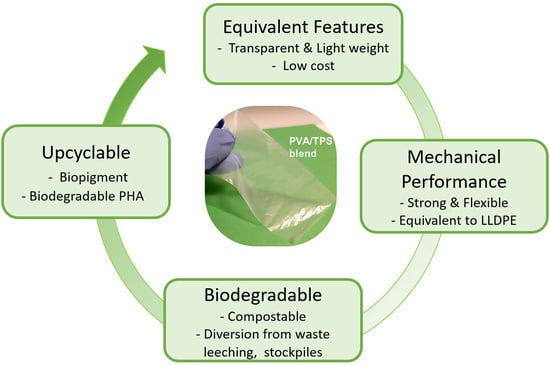
Upcycling Biodegradable PVA/Starch Film to a Bacterial Biopigment and Biopolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. ATR-Infrared Spectroscopy (ATR-FTIR)
2.3. Differential Scanning Calorimetry and Thermogravimetric Analysis (DSC/TG)
2.4. Atomic Force Microscopy (AFM) Analysis
2.5. Mechanical Properties of Films
2.6. Light Fastness
2.7. Study of Films Water-Contact Properties
2.7.1. Swelling Index (Q %)
2.7.2. Solubility (ML %)
2.7.3. Water Uptake (WU %)
2.8. Upcycling and Biodegradation Assessment
2.8.1. PVA/TPS Film as Substrate for Bacterial Growth
2.8.2. Model-Compost Degradation
3. Results and Discussion
3.1. Thermal and Mechanical Properties of PVA/TPS Films
3.2. Bacterial Upcycling and Biodegradation of PVA/TPS Material
3.2.1. PVA/TPS Films and PVA as Carbon Source for Bacterial Growth
3.2.2. PVA/TPS Degradation in Model Compost
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Rameshkumar, S.; Shaiju, P.; O’connor, K.E.; P., R.B. Bio-based and biodegradable polymers—State-of-the-art, challenges and emerging trends. Curr. Opin. Green Sus. Chem. 2020, 21, 75–81. [Google Scholar] [CrossRef]
- Blank, L.M.; Narancic, T.; Mampel, J.; Tiso, T.; O’connor, K. Biotechnological upcycling of plastic waste and other non-conventional feedstocks in a circular economy. Curr. Opin. Biotechnol. 2020, 62, 212–219. [Google Scholar] [CrossRef]
- Nikolaivits, E.; Pantelic, B.; Azeem, M.; Taxeidis, G.; Babu, R.; Topakas, E.; Brennan Fournet, M.; Nikodinovic-Runic, J. Progressing plastics circularity: A review of mechano-biocatalytic approaches for waste plastic (re)valorization. Front. Bioeng. Biotechnol. 2021, 9, 535. [Google Scholar] [CrossRef]
- Rommi, K.; Rahikainen, J.; Vartiainen, J.; Holopainen, U.; Lahtinen, P.; Honkapää, K.; Lantto, R. Potato peeling costreams as raw materials for biopolymer film preparation. J. Appl. Poly. Sci. 2016, 133, 42862. [Google Scholar] [CrossRef]
- Fahrngruber, B.; Eichelter, J.; Erhäusl, S.; Seidl, B.; Wimmer, R.; Mundigler, N. Potato-fiber modified thermoplastic starch: Effects of fiber content on material properties and compound characteristics. Eur. Polym. J. 2019, 111, 170–177. [Google Scholar] [CrossRef]
- Jane, J. Starch properties, modifications, and applications. J. Macromol. Sci. Part A 1995, 32, 751–757. [Google Scholar] [CrossRef]
- Tian, H.; Yan, J.; Rajulu, A.V.; Xiang, A.; Luo, X. Fabrication and properties of polyvinyl alcohol/starch blend films: Effect of composition and humidity. Int. J. Biol. Macromol. 2017, 96, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, S.; Milovanovic, J.; Mojicevic, M.; Bogojevic, S.S.; Nikodinovic-Runic, J. Understanding bioplastic materials–Current state and trends. J. Serb. Chem. Soc. 2020, 85, 1507–1538. [Google Scholar] [CrossRef]
- Afzal, A.; Khaliq, Z.; Ahmad, S.; Ahmad, F.; Noor, A.; Qadir, M.B. Development and characterization of biodegradable composite film. Environ. Technol. Innov. 2021, 23, 101664. [Google Scholar] [CrossRef]
- Mohd Asharuddin, S.; Othman, N.; Altowayti, W.A.H.; Abu Bakar, N.; Hassan, A. Recent advancement in starch modification and its application as water treatment agent. Environ. Technol. Innov. 2021, 23, 101637. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Ge, C.; Lansing, B.; Lewis, C.L. Thermoplastic starch and poly(vinyl alcohol) blends centered barrier film for food packaging applications. Food Packag. Shelf Life 2021, 27, 100610. [Google Scholar] [CrossRef]
- Srivastava, K.R.; Dixit, S.; Pal, D.B.; Mishra, P.K.; Srivastava, P.; Srivastava, N.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F. Effect of nanocellulose on mechanical and barrier properties of PVA–banana pseudostem fiber composite films. Environ. Technol. Innov. 2021, 21, 101312. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Das, A.; Mahanwar, P.A.; Gadekar, P.T. Starch based bio-plastics: The future of sustainable packaging. Open Polymer Chem. 2018, 8, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and applicability of starch-based films: An eco-friendly approach. Foods 2021, 10, 2181. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Techno-economic assessment of a sustainable and cost-effective bioprocess for large scale production of polyhydroxybutyrate. Chemosphere 2021, 284, 131371. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Hamada, N.; Watanabe, Y. Studies on the poly (vinyl alcohol) degrading enzyme. Part VI. Degradation mechanism of poly (vinyl alcohol) by successive reactions of secondary alcohol oxidase and. BETA.-diketone hydrolase from Pseudomonas sp. Agric. Biol. Chem. 1986, 50, 989–996. [Google Scholar] [CrossRef]
- Suzuki, T. Degradation of poly (vinyl alcohol) by microorganisms. Appl. Polym. Symp. 1979, 35, 431–437. [Google Scholar]
- Shimao, M.; Fujita, I.; Kato, N.; Sakazawa, C. Enhancement of pyrroloquinoline quinone production and polyvinyl alcohol degradation in mixed continuous cultures of Pseudomonas putida VM15A and Pseudomonas sp. strain VM15C with mixed carbon sources. Appl. Environ. Microbiol. 1985, 49, 1389. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Deng, Y.; Chen, P.; Duan, M.; Lin, X.; Zhang, Y. Biodegradation analysis of polyvinyl alcohol during the compost burial course. J. Basic Microbiol. 2019, 59, 368–374. [Google Scholar] [CrossRef]
- Mori, T.; Sakimoto, M.; Kagi, T.; Sakai, T. Isolation and characterization of a strain of Bacillus megaterium that degrades poly (vinyl alcohol). Biosci. Biotechnol. Biochem. 1996, 60, 330–332. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the biological degradation of polymers in various environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef] [PubMed]
- Vikman, M.; Itävaara, M.; Poutanen, K. Biodegradation of starch-based materials. J. Macromol. Sci. A 1995, 32, 863–866. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Díaz, D.A.; Amaya, E. Biodegradability and mechanical properties of starch films from Andean crops. Int. J. Biol. Macromol. 2011, 48, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-H.; Wee, Y.-J.; Byun, H.-S.; Yoon, S.-D. Biodegradability of chemically modified starch (RS4)/PVA blend films: Part 2. J. Polym. Environ. 2008, 16, 12–18. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Biores. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Mcadam, B.; Brennan Fournet, M.; Mcdonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Tang, R.; Weng, C.; Peng, X.; Han, Y. Metabolic engineering of Cupriavidus necator H16 for improved chemoautotrophic growth and PHB production under oxygen-limiting conditions. Metab. Eng. 2020, 61, 11–23. [Google Scholar] [CrossRef]
- Kim, B.S. Production of poly (3-hydroxybutyrate) from inexpensive substrates. Enzyme Microb. Tech. 2000, 27, 774–777. [Google Scholar] [CrossRef]
- Haas, R.; Jin, B.; Zepf, F.T. Production of poly (3-hydroxybutyrate) from waste potato starch. Biosci. Biotechnol. Biochem. 2008, 72, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Dufossé, L.; Renuka Devi, P. Bacterial Pigments: Sustainable Compounds with Market Potential for Pharma and Food Industry. Front. Sus. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Stankovic, N.; Senerovic, L.; Ilic-Tomic, T.; Vasiljevic, B.; Nikodinovic-Runic, J. Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Appl. Microbiol. Biotechnol. 2014, 98, 3841–3858. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Garg, S.; Bajpai, S. Thermal decomposition kinetics and properties of grafted barley husk reinforced PVA/starch composite films for packaging applications. Carbohyd. Polym. 2020, 240, 116225. [Google Scholar] [CrossRef]
- Costa, N.N.; De Faria Lopes, L.; Ferreira, D.F.; De Prado, E.M.L.; Severi, J.A.; Resende, J.A.; De Paula Careta, F.; Ferreira, M.C.P.; Carreira, L.G.; De Souza, S.O.L. Polymeric films containing pomegranate peel extract based on PVA/starch/PAA blends for use as wound dressing: In vitro analysis and physicochemical evaluation. Mat. Sci. Eng. C 2020, 109, 110643. [Google Scholar] [CrossRef]
- Flores, S.K.; Costa, D.; Yamashita, F.; Gerschenson, L.N.; Grossmann, M.V. Mixture design for evaluation of potassium sorbate and xanthan gum effect on properties of tapioca starch films obtained by extrusion. Mat. Sci. Eng. C 2010, 30, 196–202. [Google Scholar] [CrossRef]
- Anglès, M.N.; Dufresne, A. Plasticized starch/tunicin whiskers nanocomposites. 1. Structural analysis. Macromolecules 2000, 33, 8344–8353. [Google Scholar] [CrossRef]
- Stankovic, N.; Radulovic, V.; Petkovic, M.; Vuckovic, I.; Jadranin, M.; Vasiljevic, B.; Nikodinovic-Runic, J. Streptomyces sp. JS520 produces exceptionally high quantities of undecylprodigiosin with antibacterial, antioxidative, and UV-protective properties. Appl. Microbiol. Biotechnol. 2012, 96, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nature Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, H.G.; Kaltwasser, H.; Gottschalk, G. A submersion method for culture of hydrogen-oxidizing bacteria: Growth physiological studies. Arch. Mikrobiol. 1961, 38, 209–222. [Google Scholar] [CrossRef]
- Juengert, J.R.; Bresan, S.; Jendrossek, D. Determination of polyhydroxybutyrate (PHB) content in Ralstonia eutropha using gas chromatography and Nile Red staining. Bio-Protocol 2018, 8, e2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponjavic, M.; Nikolic, M.S.; Nikodinovic-Runic, J.; Jeremic, S.; Stevanovic, S.; Djonlagic, J. Degradation behaviour of PCL/PEO/PCL and PCL/PEO block copolymers under controlled hydrolytic, enzymatic and composting conditions. Polym. Test. 2017, 57, 67–77. [Google Scholar] [CrossRef]
- Jose, J.; Al-Harthi, M.A.; Alma’adeed, M.a.A.; Bhadra Dakua, J.; De, S.K. Effect of graphene loading on thermomechanical properties of poly (vinyl alcohol)/starch blend. J. Appl. Poly. Sci. 2015, 132, 41827. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Liu, H.; Chen, L.; Li, L. In situ thermal decomposition of starch with constant moisture in a sealed system. Poly. Deg. Stabil. 2008, 93, 260–262. [Google Scholar] [CrossRef]
- Guerrini, L.M.; De Oliveira, M.P.; Branciforti, M.C.; Custódio, T.A.; Bretas, R.E. Thermal and structural characterization of nanofibers of poly (vinyl alcohol) produced by electrospinning. J. Appl. Poly. Sci. 2009, 112, 1680–1687. [Google Scholar] [CrossRef]
- Cassu, S.N.; Felisberti, M.I. Poly (vinyl alcohol) and poly (vinyl pyrrolidone) blends: Miscibility, microheterogeneity and free volume change. Polymer 1997, 38, 3907–3911. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Z.; Zhao, T.; Liu, M.; Hu, M.; Li, J. Synthesis, characterization, and swelling behaviors of salt-sensitive maize bran–poly (acrylic acid) superabsorbent hydrogel. J. Agri. Food Chem. 2014, 62, 8867–8874. [Google Scholar] [CrossRef]
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mat. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Musa, B.H.; Hameed, N.J. Study of the mechanical properties of polyvinyl alcohol/starch blends. Mat. Today Proceed. 2020, 20, 439–442. [Google Scholar] [CrossRef]
- Guohua, Z.; Ya, L.; Cuilan, F.; Min, Z.; Caiqiong, Z.; Zongdao, C. Water resistance, mechanical properties and biodegradability of methylated-cornstarch/poly (vinyl alcohol) blend film. Poly. Deg. Stabil. 2006, 91, 703–711. [Google Scholar] [CrossRef]
- Massey, L.K. Film Properties of Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Yan, C.; Li, H.; Zhang, X.; Zhu, Y.; Fan, X.; Yu, L. Preparation and properties of continuous glass fiber reinforced anionic polyamide-6 thermoplastic composites. Mater. Design 2013, 46, 688–695. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of starch composition and molecular weight on physicochemical properties of biodegradable films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, Z.W.; Dong, Y. Biodegradable and water resistant poly(vinyl) alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Garg, S.; Bajpai, S. Fabrication and characteristics of poly (vinyl alcohol)-starch-cellulosic material based biodegradable composite film for packaging application. Mat. Today Proc. 2020, 21, 1577–1582. [Google Scholar] [CrossRef]
- Nikodinovic-Runic, J.; Guzik, M.; Kenny, S.T.; Babu, R.; Werker, A.; Ke, O.C. Carbon-rich wastes as feedstocks for biodegradable polymer (polyhydroxyalkanoate) production using bacteria. Adv. Appl. Microbiol. 2013, 84, 139–200. [Google Scholar] [PubMed]
- Sohn, Y.J.; Kim, H.T.; Baritugo, K.A.; Jo, S.Y.; Song, H.M.; Park, S.Y.; Park, S.K.; Pyo, J.; Cha, H.G.; Kim, H. Recent advances in sustainable plastic upcycling and biopolymers. Biotechnol. J. 2020, 15, 1900489. [Google Scholar] [CrossRef]
- Tiso, T.; Narancic, T.; Wei, R.; Pollet, E.; Beagan, N.; Schröder, K.; Honak, A.; Jiang, M.; Kenny, S.T.; Wierckx, N. Towards bio-upcycling of polyethylene terephthalate. Metab. Eng. 2021, 66, 167–178. [Google Scholar] [CrossRef]
- Yip, C.H.; Yarkoni, O.; Ajioka, J.; Wan, K.L.; Nathan, S. Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl. Microbiol. Biotechnol. 2019, 103, 1667–1680. [Google Scholar] [CrossRef] [Green Version]
- Merck. Undecylprodigiosin hydrochloride. Available online: https://www.sigmaaldrich.com/rs/en/product/sigma/sml1576 (accessed on 23 August 2021).
- Luti, K.J.K. Mixture design of experiments for the optimization of carbon source for promoting undecylprodigiosin and actinorhodin production. J. Pure Appl. Microbiol. 2018, 12, 1783–1794. [Google Scholar] [CrossRef]
- Luti, K.; Younis, R. An induction of undecylprodigiosin production from Streptomyces coelicolor by elicitation with microbial cells using solid state fermentation. Iraqi J. Sci. 2014, 55, 553–1562. [Google Scholar]
- Nguyen, T.-H.; Wang, S.-L.; Nguyen, D.-N.; Nguyen, A.-D.; Nguyen, T.-H.; Doan, M.-D.; Ngo, V.-A.; Doan, C.-T.; Kuo, Y.-H.; Nguyen, V.-B. Bioprocessing of marine chitinous wastes for the production of bioactive prodigiosin. Molecules 2021, 26, 3138. [Google Scholar] [CrossRef]
- Volodina, E.; Raberg, M.; Steinbüchel, A. Engineering the heterotrophic carbon sources utilization range of Ralstonia eutropha H16 for applications in biotechnology. Crit. Rev. Biotechnol. 2016, 36, 978–991. [Google Scholar] [CrossRef]
- Song, Y.; Matsumoto, K.I.; Tanaka, T.; Kondo, A.; Taguchi, S. Single-step production of polyhydroxybutyrate from starch by using α-amylase cell-surface displaying system of Corynebacterium glutamicum. J. Biosci. Bioeng. 2013, 115, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.K.; Shim, Y.-H.; Jeon, J.-M.; Brigham, C.J.; Kim, Y.-H.; Kim, H.-J.; Seo, H.-M.; Lee, J.-H.; Kim, J.-H.; Yi, D.-H.; et al. Starch based polyhydroxybutyrate production in engineered Escherichia coli. Bioprocess Biosyst. Eng. 2015, 38, 1479–1484. [Google Scholar] [CrossRef]
- Konsula, Z.; Liakopoulou-Kyriakides, M. Hydrolysis of starches by the action of an α-amylase from Bacillus subtilis. Process Biochem. 2004, 39, 1745–1749. [Google Scholar] [CrossRef]
- Tran, H.T.M.; Cheirsilp, B.; Hodgson, B.; Umsakul, K. Potential use of Bacillus subtilis in a co-culture with Clostridium butylicum for acetone–butanol–ethanol production from cassava starch. Biochem. Eng. J. 2010, 48, 260–267. [Google Scholar] [CrossRef]
- Jayasekara, R.; Harding, I.; Bowater, I.; Christie, G.B.; Lonergan, G.T. Biodegradation by composting of surface modified starch and PVA blended films. J. Polym. Environ. 2003, 11, 49–56. [Google Scholar] [CrossRef]
- Thakur, K.; Rajhans, A.; Kandasubramanian, B. Starch/PVA hydrogels for oil/water separation. Environ. Sci. Pollut. Res. 2019, 26, 32013–32028. [Google Scholar] [CrossRef] [PubMed]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohyd. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Pirzada, T.; Arvidson, S.A.; Saquing, C.D.; Shah, S.S.; Khan, S.A. Hybrid silica–PVA nanofibers via sol–gel electrospinning. Langmuir 2012, 28, 5834–5844. [Google Scholar] [CrossRef]
- Lima, E.; Raphael, E.; Sentanin, F.; Rodrigues, L.; Ferreira, R.; Carlos, L.; Silva, M.; Pawlicka, A. Photoluminescent polymer electrolyte based on agar and containing europium picrate for electrochemical devices. Mat. Sci. Eng. B 2012, 177, 488–493. [Google Scholar] [CrossRef]
- Guan, Y.; Qian, L.; Xiao, H.; Zheng, A. Preparation of novel antimicrobial-modified starch and its adsorption on cellulose fibers: Part I. Optimization of synthetic conditions and antimicrobial activities. Cellulose 2008, 15, 609–618. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]









| Cast | Extruded: Longitudinal | Extruded: Transverse | |
|---|---|---|---|
| σu (MPa) | 37.2 ± 2.2 | 38.6 ± 4.6 | 22.2 ± 4.3 |
| εu (%) | 197 ± 70.0 | 136 ± 17.0 | 325 ± 73.0 |
| E (GPa) | 249.9 ± 94.7 | 60.3 ± 20.4 | 52.9 ± 14.1 |
| Property | PVA/TPS | LDPE | HDPE | LLDPE | PVA | TPS |
|---|---|---|---|---|---|---|
| σu (MPa) | 22–39 | 36–57 | 38–44 | 36–60 | 22–30 | 4–8 |
| εu (%) | 136–325 | 300–500 | 600–860 | 450–850 | 99–112 | 35–100 |
| E (MPa) | 53–250 | 190–520 | 827–1069 | 204–275 | 64–176 | 116–294 |
| MSM + PVA/TPS Film 10 g/L | MSM + PVA/TPS Film 20 g/L | |||
|---|---|---|---|---|
| Strain | Biomass, mg/mL | Conversion, % | Biomass, mg/mL | Conversion, % |
| Ralstonia eutropha H16 | 0.80 ± 0.08 | 8 | 2.75 ± 0.05 | 13.7 |
| Streptomyces sp. JS520 | 1.20 ± 0.09 | 12 | 4.40 ± 0.08 | 22.0 |
| Bacillus subtilis ATCC6633 | 1.45 ± 0.04 | 14.5 | 3.95 ± 0.04 | 19.7 |
| Compost | Day 3 | Day 10 | Day 30 | Weight Loss, % |
|---|---|---|---|---|
| Nontreated | 31 ± 2 mg | 22 ± 1 mg | 22 ± 1 mg | 42 |
| Heat-pretreated | 29 ± 1 mg | 25 ± 2 mg | 20 ± 1 mg | 47 |
| Bioaugmented | 23 ± 1 mg | 22 ± 1 mg | 19 ± 1 mg | 50 |
| Sample * | RMS, nm 3 Days | RMS, nm 10 Days | RMS, nm 30 Days |
|---|---|---|---|
| PVA/starch film nontreated | 104.7 | 100.3 | 98.8 |
| PVA/starch film heat-pretreated | 195.5 | 91.5 | 77.4 |
| PVA/starch film bioaugmented | 130.8 | 150.7 | 66.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantelic, B.; Ponjavic, M.; Jankovic, V.; Aleksic, I.; Stevanovic, S.; Murray, J.; Fournet, M.B.; Nikodinovic-Runic, J. Upcycling Biodegradable PVA/Starch Film to a Bacterial Biopigment and Biopolymer. Polymers 2021, 13, 3692. https://doi.org/10.3390/polym13213692
Pantelic B, Ponjavic M, Jankovic V, Aleksic I, Stevanovic S, Murray J, Fournet MB, Nikodinovic-Runic J. Upcycling Biodegradable PVA/Starch Film to a Bacterial Biopigment and Biopolymer. Polymers. 2021; 13(21):3692. https://doi.org/10.3390/polym13213692
Chicago/Turabian StylePantelic, Brana, Marijana Ponjavic, Vukasin Jankovic, Ivana Aleksic, Sanja Stevanovic, James Murray, Margaret Brennan Fournet, and Jasmina Nikodinovic-Runic. 2021. "Upcycling Biodegradable PVA/Starch Film to a Bacterial Biopigment and Biopolymer" Polymers 13, no. 21: 3692. https://doi.org/10.3390/polym13213692