Structure of Polytetrafluoroethylene Modified by the Combined Action of γ-Radiation and High Temperatures
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Radiation Modification and Dosimetry
2.3. X-ray Computed Microtomography Measurements
2.4. X-ray Diffraction Pattern Measurements
2.5. Density Measurements
3. Results
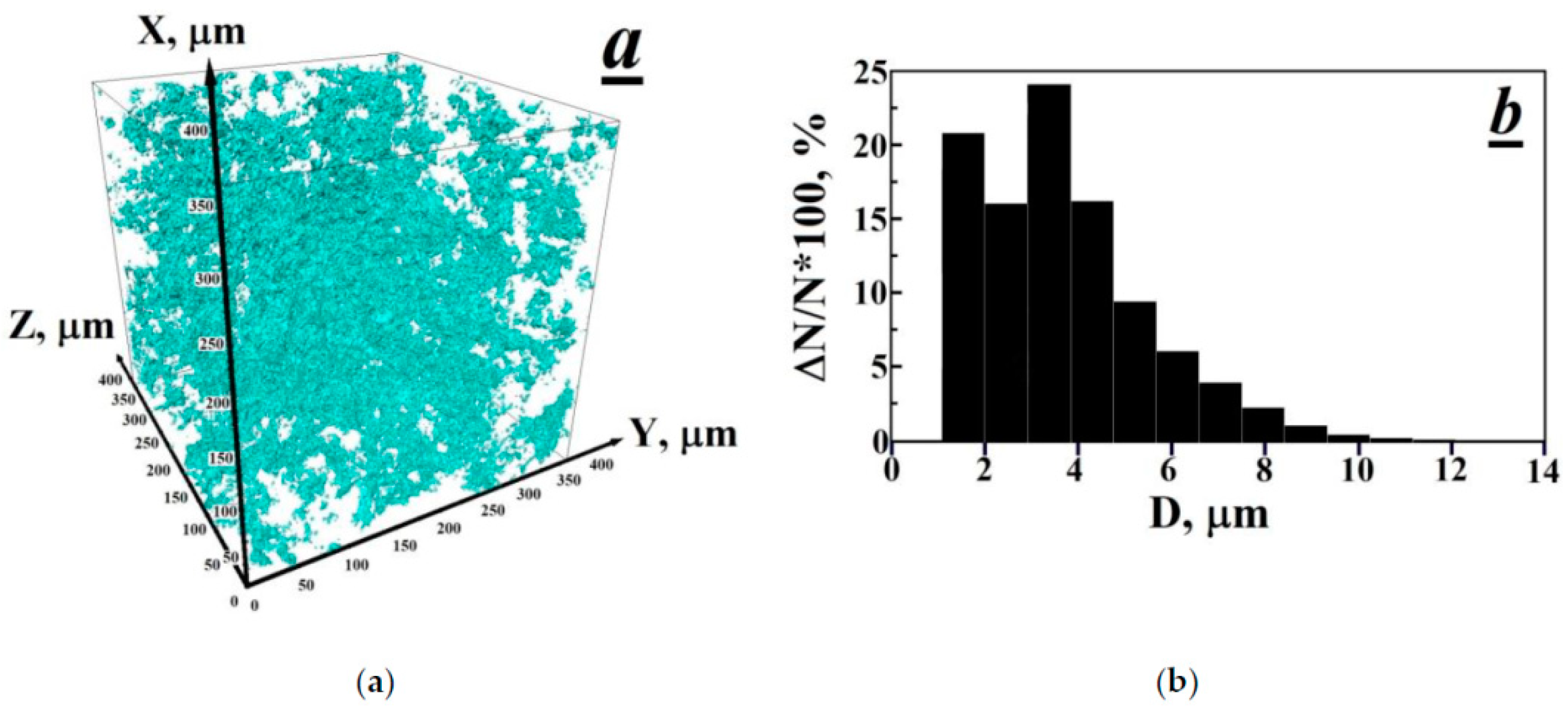
3.1. Observation of the Porous Structure and Its Disappearance after Thermal-Radiation Modification in Polytetrafluoroethylene
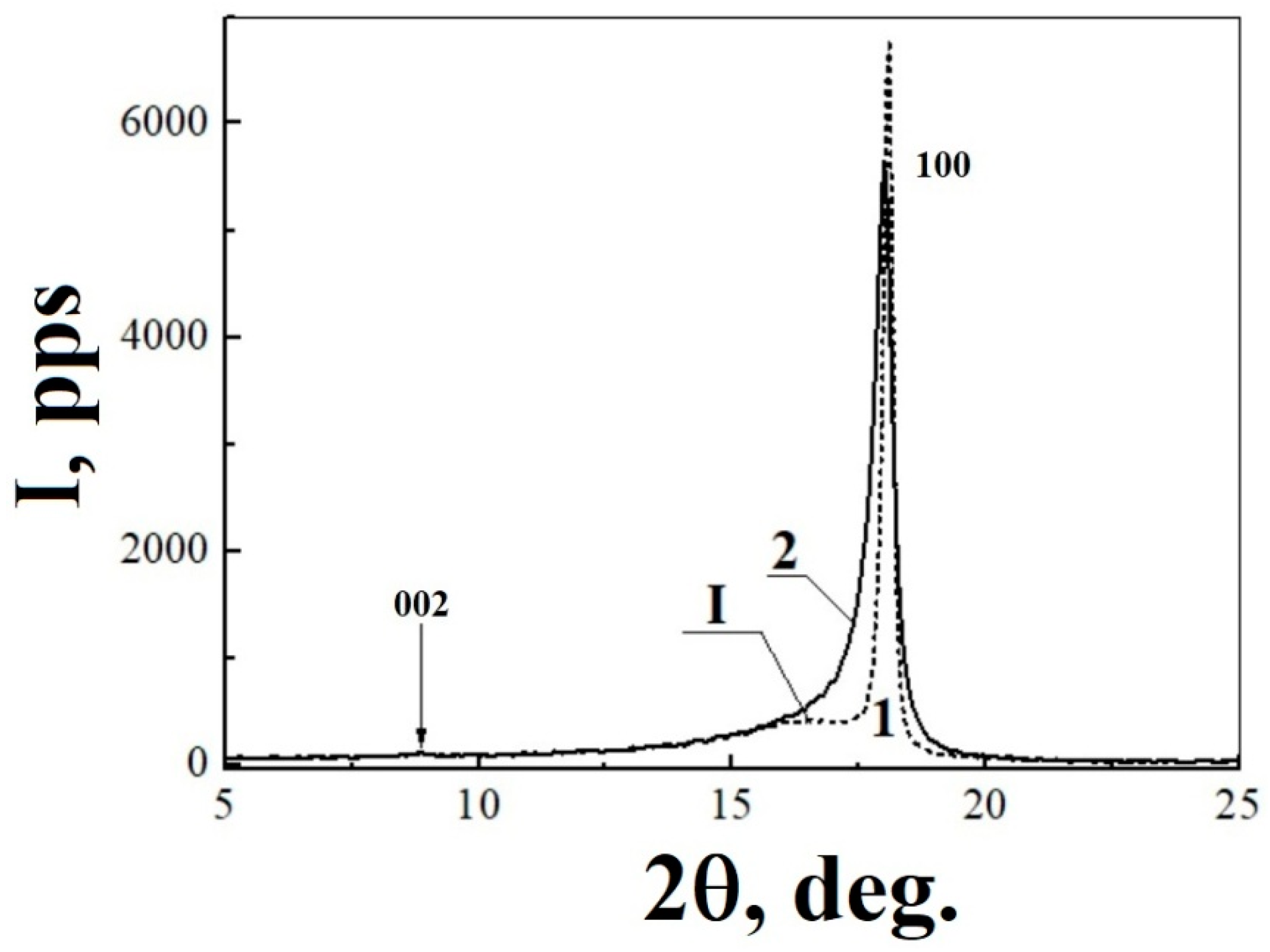
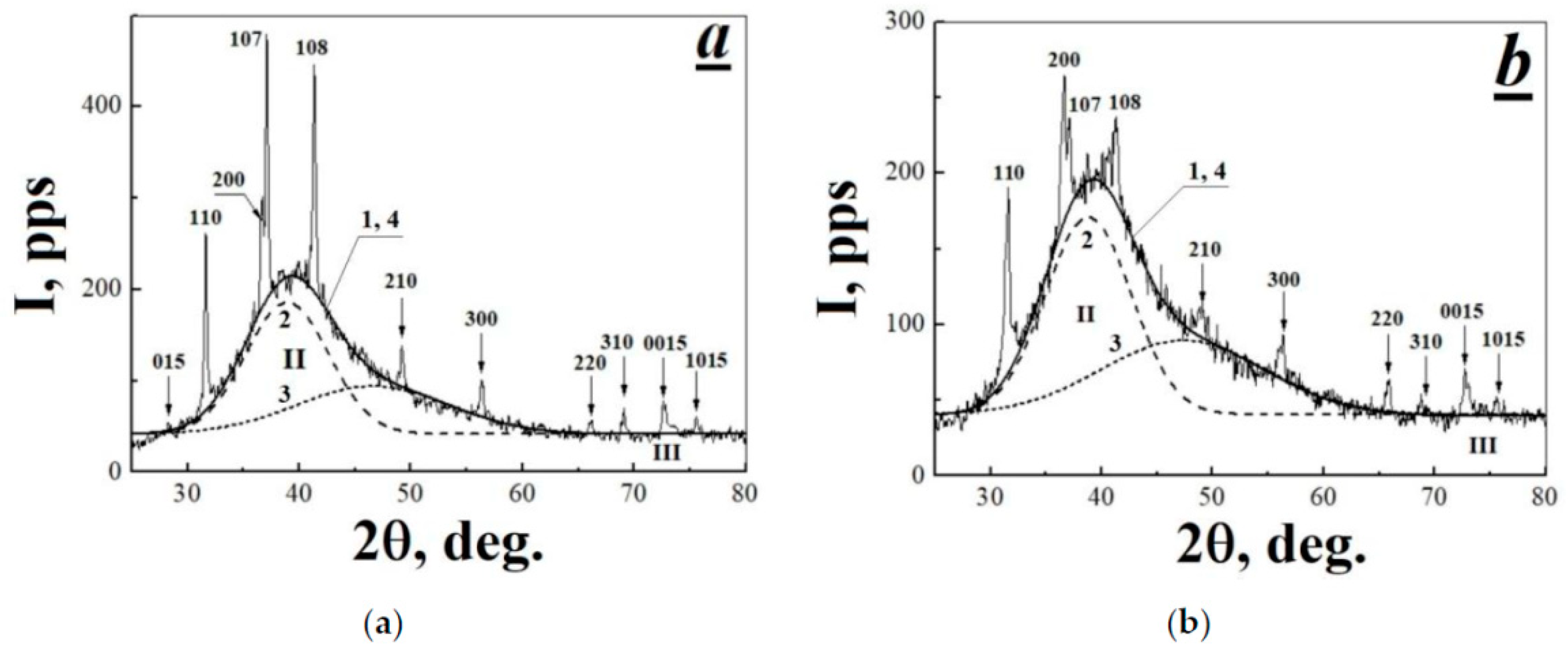
3.2. Characterization of X-ray Diffraction Patterns Registered for Initial and Thermal-Radiation Modified Polytetrafluoroethylene
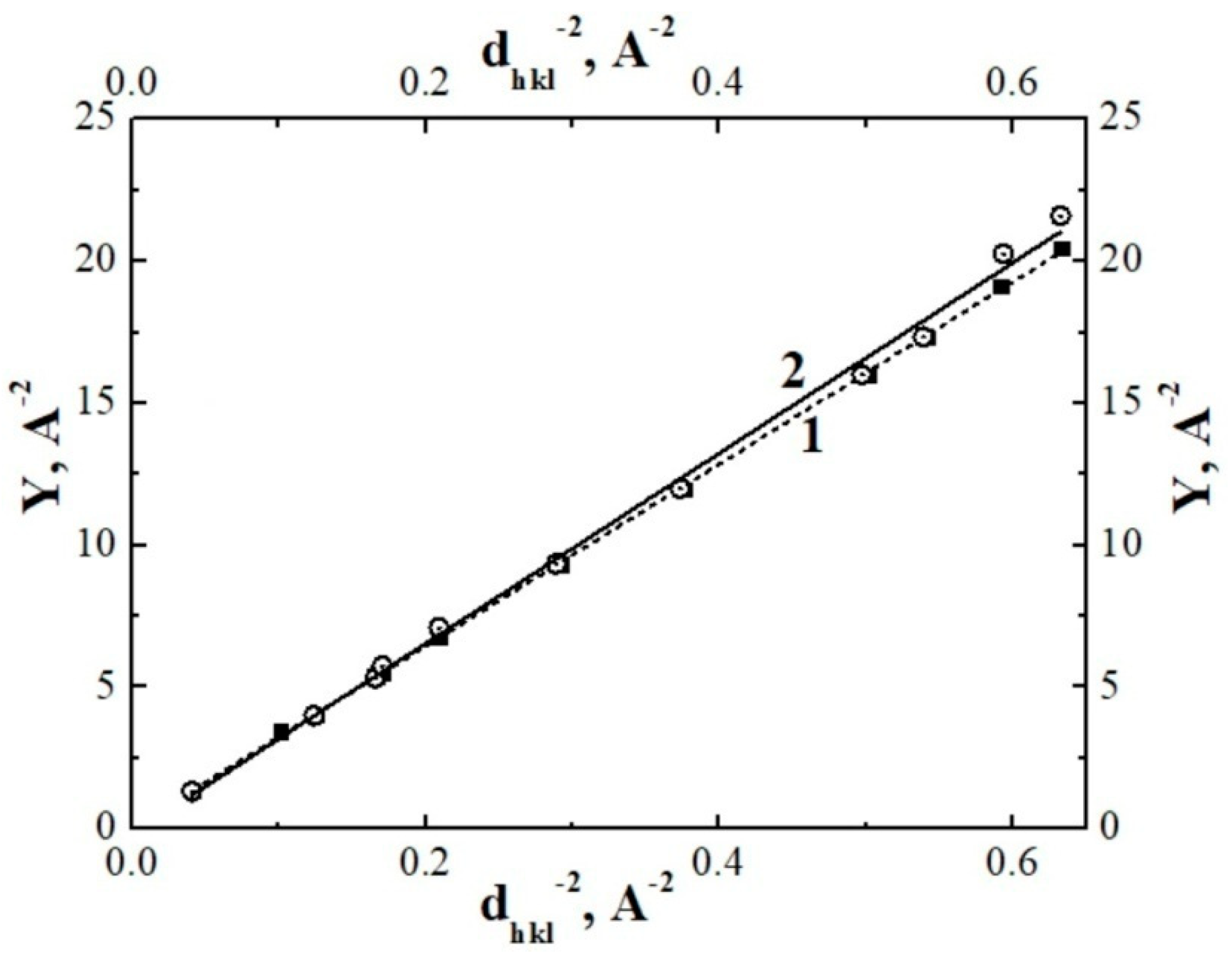
3.3. Determination of the Lattice Parameters for Initial and Thermal-Radiation Modified Polytetrafluoroethylene by Means of Hull-Davey Chart
3.4. Comparative Analysis of the Experimentally Established as Well as Obtained from X-ray Data Density Values of the Initial and Thermal-Radiation Modified Polytetrafluoroethylene
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oshima, A.; Ikeda, S.; Katoh, E.; Tabata, Y. Chemical structure and physical properties of radiation-induced crosslinking of polytetrafluoroethylene. Radiat. Phys. Chem. 2001, 62, 39–45. [Google Scholar] [CrossRef]
- Khatipov, S.A.; Serov, S.A.; Buznik, V.M. Radiation modification of polytetrafluoroethylene. In Opportunities for Fluoropolymer. Synthesis, Characterization, Processing, Simulation and Recycling. Progress in Fluorine Science; Bruno, A., Sergey, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–188. [Google Scholar] [CrossRef]
- Oshima, A.; Tabata, Y.; Ikeda, S.; Otsuhata, K.; Kudoh, H.; Seguchi, T. Radiation Induced Crosslinking of Polytetrafluoroethylene. In Proceedings of the JAERI-Conf 95-003, 6th Japan—China Bilateral Symposium on Radiation Chemistry, Waseda University, Tokyo, Japan, 6–11 November 1994; Hama, Y., Katsumura, Y., Kouchi, N., Makuuchi, K., Eds.; JAERI, Department of Technical Information: Tokai-mura, Japan, 1995; pp. 487–491. [Google Scholar]
- Sun, J.; Zhang, Y.; Zhong, X. Radiation crosslinking of polytetrafluoroethylene. Polymer 1994, 35, 2881–2883. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, M.; Zhao, Y.; Wu, G. Radiation resistance evaluation of cross-linked polytetrafluoroethylene by the investigation of friction and wear behavior. Radiat. Phys. Chem. 2011, 80, 496–500. [Google Scholar] [CrossRef]
- Konova, E.M.; Sakhno, Y.E.; Khatipov, S.A.; Klimenko, V.G.; Sychkova, S.T.; Sakhno, T.V. Mechanical and Optical Properties of Polytetrafluoroethylene Treated by γ-Irradiation near the Melting Point. Phys. Chem. Solid State 2011, 12, 1013–1017. [Google Scholar]
- Spadaro, G.; Alessi, S.; Dispenza, C. Ionizing radiation-induced crosslinking and degradation of polymers. In Applications of Ionizing Radiation in Materials Processing; Sun, Y., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; Volume 1, pp. 167–182. [Google Scholar]
- Geil, P.H.; Yang, J.; Williams, R.A.; Petersen, K.L.; Long, T.C.; Xu, P. Effect of molecular weight and melt time and temperature on the morphology of Poly(tetrafluoroethylene). Adv. Polym. Sci. 2005, 180, 89–159. [Google Scholar] [CrossRef]
- Roland, E.; Florin, R.E.; Parker, M.S.; Wall, L.A. The Mechanism of the Depolymerization of Polytetrafluoroethylene with Pyrolytic and Radiolytic Initiation. J. Res. Nat. Bur. Stand. A. Phys. Chem. 1966, 70, 115–131. [Google Scholar] [CrossRef]
- Huneau, B. Strain-Induced Crystallization of Natural Rubber: A Review of X-ray Diffraction Investigations. Rubber Chem. Technol. Am. Chem. Soc. 2011, 84, 425–452. [Google Scholar] [CrossRef] [Green Version]
- Smolyanskii, A.S.; Arsentyev, M.A.; Rashkovskii, A.Y.; Politova, E.D. Radiation-Induced Changes in the Degree of Crystallinity of Powdered Polytetrafluoroethylene. Crystallogr. Rep. 2019, 64, 553–558. [Google Scholar] [CrossRef]
- Kiryukhin, D.P.; Kichigina, G.A.; Allayarov, S.R.; Badamshina, E.R. Unique Research Facility “Gammatok-100”. High Energy Chem. 2019, 53, 228–237. [Google Scholar] [CrossRef]
- Available online: https://www.thermofisher.com/ru/ru/home/electron-microscopy/products/microct/heliscan-microct.html (accessed on 15 October 2021).
- Buzug, T.M. Computed Tomography—From Photon Statistics to Modern Cone-Beam CT. In Springer Handbook of Medical Technology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 311–342. [Google Scholar] [CrossRef]
- Kak, A.C.; Slaney, M. Principles of Computerized Tomographic Imaging; Society of Industrial and Applied Mathematics: New York, NY, USA, 2001. [Google Scholar]
- Herman, G.T. Fundamentals of Computerized Tomography. Image Reconstruction from Projections; Springer: London, UK, 2009. [Google Scholar] [CrossRef]
- Kang, S.-J.L. Sintering, Densification, Grain Growth, and Microstructure; Elsevier Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Kitamura, T.; Okabe, S.; Tanigaki, M.; Kurumada, K.-I.; Oshima, M.; Kanazawa, S.-I. Morphology Change in Polytetrafluoroethylene (PTFE) Porous Membrane Caused by Heat Treatment. Polym. Eng. Sci. 2000, 40, 809–817. [Google Scholar] [CrossRef]
- Howard, E.G., Jr.; Moss, A.Z. Porous Polytetrafluoroethylene and Preparation. U.S. Patent US5721283A, 24 February 1998. [Google Scholar]
- Lowell, S.; Shields, J.E. Powder Surface Area and Porosity; Springer-Science+Business Media: Berlin/Heidelberg, Germany, 1991. [Google Scholar] [CrossRef]
- Matko, V. Porosity measurement using two stochastic signals. Sens. Actuators A 2004, 112, 101–106. [Google Scholar] [CrossRef]
- Appolonia, C.R.; Pottker, W.E. Non-destructive porosity profile measurement of amorphous materials by gamma-ray transmission. Appl. Radiat. Isotop. 2004, 61, 1133–1138. [Google Scholar] [CrossRef]
- Mang, J.T.; Hjelm, R.P.; Francois, E.G. Measurement of Porosity in a Composite High Explosive as a Function of Pressing Conditions by Ultra-Small-Angle Neutron Scattering with Contrast Variation. Propellants Explos. Pyrotech. 2010, 35, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.F.; de Sousa, M.J.L. The measurement of coal porosity with different gases. Int. J. Coal Geol. 2002, 48, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Stefanidou, M. Methods for porosity measurement in lime-based mortars. Constr. Build. Mater. 2010, 24, 2572–2578. [Google Scholar] [CrossRef]
- Cros, F.; Korb, J.-P.; Malier, L. Spectroscopic Mesopore Size Characterization and Diffusion Measurement in Closed Porosity by Xenon NMR. Langmuir 2000, 16, 10193–10197. [Google Scholar] [CrossRef]
- Talmon, Y.; Shtirberg, L.; Harneit, W.; Rogozhnikova, O.Y.; Tormyshev, V.; Blank, A. Molecular diffusion in porous media by PGSE ESR. Phys. Chem. Chem. Phys. 2010, 12, 5998–6007. [Google Scholar] [CrossRef]
- Wong, R.C.K.; Chau, K.T. Estimation of air void and aggregate spatial distributions in concrete under uniaxial compression using computer tomography scanning. Cem. Concr. Res. 2005, 35, 1566–1576. [Google Scholar] [CrossRef]
- Tadic, D.; Beckmann, F.; Donath, T.; Epple, M. Comparison of different methods for the preparation of porous bone substitution materials and structural investigations by synchrotron µ-computer tomography. Mater. Werkst. 2004, 35, 240–244. [Google Scholar] [CrossRef]
- Bloom, M.; Russell, M.J.; Kustau, A.; Sukumaran, B. Measurement of Porosity in Granular Particle Distributions Using Adaptive Thresholding. IEEE Trans. Instr. Meas. 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Ketchama, R.A.; Iturrino, G.J. Nondestructive high-resolution visualization and measurement of anisotropic effective porosity in complex lithologies using high-resolution X-ray computed tomography. J. Hydrol. 2005, 302, 92–106. [Google Scholar] [CrossRef]
- Dehl, R.E.; Grant, W.G.; Cassel, J.M. NBSIR 81-2459. Characterization of Porosity in Porous Polymeric Implant Materials. In Annual Report for Period October 1980–September 1981; US Department of Commerce National Bureau of Standards Dental and Medical Materials. Polymer Science and Standards Division: Washington, DC, USA, 1982. [Google Scholar]
- Jiang, C.; Cai, S.; Mao, L.; Wang, Z. Effect of Porosity on Dynamic Mechanical Properties and Impact Response Characteristics of High Aluminum Content PTFE/Al Energetic Materials. Materials 2020, 13, 140. [Google Scholar] [CrossRef] [Green Version]
- Aizawa, T.; Wakui, Y. Correlation between the Porosity and Permeability of a Polymer Filter Fabricated via CO2-Assisted Polymer Compression. Membranes 2020, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Matsuda, Y.; Tasaka, S. Fabrication of a porous structure of poly(tetrafluoroethylene) from a mixture with fumaric acid. Polym. J. 2010, 42, 509–513. [Google Scholar] [CrossRef]
- Xia, Z.; Ma, S.; Qiu, X.; Wu, Y.; Wang, F. Influence of porosity on the stability of charge and piezoelectricity for porous polytetrafluoroethylene film electrets. J. Electrostatics 2003, 59, 57–69. [Google Scholar] [CrossRef]
- Khatipov, S.A.; Kabanov, S.P.; Konova, E.M.; Ivanov, S.A. Change in the Porosity of Polytetrafluoroethylene during Radiation Modification above the Melting Temperature. Polym. Sci. Ser. A 2012, 54, 644–650. [Google Scholar] [CrossRef]
- Khatipov, S.A.; Kabanov, S.P.; Konova, E.M.; Ivanov, S.A.; Serov, S.A. Effect of PTFE irradiation above the melting point on its porosity. Radiat. Phys. Chem. 2012, 81, 273–277. [Google Scholar] [CrossRef]
- Bunn, C.W.; Cobbold, A.J.; Palmer, R.P. The Fine Structure of Polytetrafluoroethylene. J. Polym. Sci. 1958, 28, 363–376. [Google Scholar] [CrossRef]
- Mirkin, S.I. Handbook of X-ray Analysis of Polycrystalline Materials; Consultants Bureau: New York, NY, USA, 1964. [Google Scholar]
- Suryanarayana, C.; Norton, M.G. X-ray Diffraction. A Practical Approach; Springer Science + Business Media, LLC: New York, NY, USA, 1998. [Google Scholar] [CrossRef] [Green Version]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Cohen, M., Ed.; Addison-Wesley Publishing Company, Inc.: Reading, MA, USA; Menlo Park, CA, USA; London, UK; Amsterdam, The Netherlands; Don Mills, ON, Canada; Sydney, Australia, 1978. [Google Scholar]
- Jenkins, R.; Fawcett, T.; Smith, D.; Visser, J.; Morris, M.; Frevel, L. JCPDS—International Centre for Diffraction Data Sample Preparation Methods in X-ray Powder Diffraction. Powder Diffr. 1986, 1, 51–63. [Google Scholar] [CrossRef]
- Lebedev, Y.A.; Korolev, Y.M.; Polikarpov, V.M.; Ignat’eva, L.N.; Antipov, E.M. X-ray Powder Diffraction Study of Polytetrafluoroethylene. Crystallogr. Rep. 2010, 55, 609–614. [Google Scholar] [CrossRef]
- Bouznik, V.M.; Kirik, S.D.; Solovyov, L.A.; Tsvetnikov, A.K. A crystal structure of ultra-dispersed form of polytetrafluoroethylene based on X-ray powder diffraction data. Powder Diffr. 2004, 19, 219–224. [Google Scholar] [CrossRef]
- Kalistratova, L.F.; Mashkov, Y.K.; Egorova, V.A. Calculation of X-ray Density of Amorphous–Crystalline Polymer Taking into Account Degree of Ordering of Amorphous Phase. Inorg. Mater. Appl. Res. 2018, 9, 687–692. [Google Scholar] [CrossRef]
- Lobo, B. Iodine Doping and Heat Treatment Studies on Some Polymers Using Positron Annihilation Spectroscopy and Thermal Analysis. Ph.D. Thesis, Mangalore University, Mangalore, Karnataka, 1999. [Google Scholar] [CrossRef]
- Sedighiamiri, A.; van Erp, T.B.; Peters, G.W.M.; Govaert, L.E.; van Dommelen, J.A.W. Micromechanical Modeling of the Elastic Properties of Semicrystalline Polymers: A Three-Phase Approach. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 2173–2184. [Google Scholar] [CrossRef]
- Picciochi, R.; Wang, Y.; Alves, N.M.; Manj, J.F. Glass transition of semi-crystalline PLLA with different morphologies as studied by dynamic mechanical analysis. Colloid Polym. Sci. 2007, 285, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Negrov, D.A.; Eremin, E.N. Structuring peculiarities of polytetrafluoroethylene modified with boron nitride when activated with ultrasonic exposure. Procedia Eng. 2016, 152, 570–575. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.; Meng, Q.B. Microporous polymers: Synthesis, characterization, and applications. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; Wiley & Sons, Ltd.: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Geil, P.H. Polymer Single Crystals; Interscience (Wiley): New York, NY, USA, 1963. [Google Scholar] [CrossRef]
- Khatipov, S.A.; Serov, S.A.; Sadovskaya, N.V.; Konova, E.M. Morphology of polytetrafluoroethylene before and after irradiation. Radiat. Phys. Chem. 2012, 81, 256–263. [Google Scholar] [CrossRef]
- Gao, J.; Ni, Z.; Liu, Y. The investigation of the structural change and the wetting behavior of electron beam irradiated PTFE film. e-Polymers 2016, 16, 111–115. [Google Scholar] [CrossRef]
- Paajanen, A.; Vaari, J.; Verho, T. Crystallization of cross-linked polyethylene by molecular dynamics simulation. Polymer 2019, 171, 80–86. [Google Scholar] [CrossRef]
- Starkweather, H.W., Jr. The density of amorphous Polytetrafluoroethylene. J. Polym. Sci. Polym. Phys. Ed. 1982, 20, 2159–2161. [Google Scholar] [CrossRef]
- Feng, B.; Fang, X.; Wang, H.-X.; Dong, W.; Li, Y.-C. The Effect of Crystallinity on Compressive Properties of Al-PTFE. Polymers 2016, 8, 356. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, R.T.; Davidson, T. Kinetics of the 20 °C Phase Transformation in Polytetrafluoroethylene. J. Polym. Sci. Polym. Phys. Ed. 1972, 10, 2209–2222. [Google Scholar] [CrossRef]
- Egorov, V.M.; Yakushev, P.N.; Arsent’ev, M.A.; Smolyanskii, A.S. The Effect of Gamma Irradiation on Phase Transitions in Polytetrafluoroethylene Doped with Silicon Dioxide of Plant Origin. Phys. Solid State 2020, 62, 1506–1511. [Google Scholar] [CrossRef]
- Bol’bit, N.M.; Duflot, V.R.; Dobrov, I.V.; Lomonosova, N.V.; Plotnikov, V.G. Method for Producing Wear-Resistant Polytetrafluoroethylene. Russian Federation Patent No. 2207351 C2, 22 December 2000. [Google Scholar]
- Xu, Y.; Tsai, Y.; Zheng, D.W.; Tua, K.N.; Ong, C.W.; Choy, C.L.; Zhao, B.; Liu, Q.-Z.; Brongo, M. Measurement of mechanical properties for dense and porous polymer films having a low dielectric constant. J. Appl. Phys. 2000, 88, 5744–5750. [Google Scholar] [CrossRef] [Green Version]
- Coekelbergs, M.; Crucq, A.; Frennet, A. Radiation Catalysis. Adv. Catalysis. Acad. Press 1962, 13, 55–136. [Google Scholar] [CrossRef]
- Swallow, A.J. Radiation Chemistry of Organic Compounds; Pergamon Press: Oxford, UK; London, UK; New York, NY, USA; Paris, France, 1960. [Google Scholar]
- Wojna’rovits, L. Radiation Chemistry Chapter 23. In Handbook of Nuclear Chemistry. Attila Vértes, Sándor Nagy, Zoltán Klencsár, Rezso; Lovas, G., Rösch, F., Eds.; Springer Science+Business Media B.V.: Berlin/Heidelberg, Germany, 2011; pp. 1267–1331. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Whittaker, A.K. Radiation chemistry of polymers. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Briskman, B.A.; Tlebaev, K.B. Radiation Effects on Thermal Properties of Polymers. II. Polytetrafluoroethylene. High Perform. Polym. 2008, 20, 86–114. [Google Scholar] [CrossRef]
- Machi, S.; Silverman, J. Bubble Formation in Radiation-Induced Grafting of Styrene to Polyethylene. J. Polym. Sci. Part A-1 1969, 7, 2737–2740. [Google Scholar] [CrossRef]
- Grogan, J.M.; Schneider, N.M.; Ross, F.M.; Bau, H.H. Bubble and Pattern Formation in Liquid Induced by an Electron Beam. Nano Lett. 2014, 14, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, K.; Singh, B.; Kashyap, Y.S.; Shukla, M.; Manjunath, B.S.; Gadkari, S.C. Gamma-irradiation-induced micro-structural variations in flame-retardant polyurethane foam using synchrotron X-ray micro-tomography. J. Synchr. Radiat. 2019, 26, 1797–1807. [Google Scholar] [CrossRef]
- Hollenberg, G.W.; Mastel, B.; Basmajian, J.A. Effect of Irradiation Temperature on the Growth of Helium Bubbles in Boron Carbide. J. Am. Ceram. Soc. 1979, 63, 376–380. [Google Scholar] [CrossRef]
- Kaletta, D. The growth of gas bubbles in solids under irradiation at elevated temperatures around 0.5 TM. Radiat. Eff. 1983, 78, 245–259. [Google Scholar] [CrossRef]
- Gross, B.; Faria, R.M.; Ferreira, G.F.L. Radiation-induced conductivity in Teflon irradiated by X-rays. J. Appl. Phys. 1981, 52, 571. [Google Scholar] [CrossRef]
- Tyutnev, A.P.; Saenko, V.S.; Zhadov, A.D.; Abrameshin, D.A. Theoretical Analysis of the Radiation-Induced Conductivity in Polymers Exposed to Pulsed and Continuous Electron Beams. Polymers 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlesby, A. Atomic Radiation and Polymers; Pergamon Press: Oxford, UK, 1960. [Google Scholar]


| Miller Indexes | Initial Polytetrafluoroethylene | Thermal-Radiation-Modified Polytetrafluoroethylene | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| h | k | l | 2θmax, deg. | I, pps | I/Imax, % | dhkl, Å | 2θ, deg. | I, pps | I/Imax, % | dhkl, Å |
| 0 | 0 | 2 | 8.848 | 17.637 | 0.283 | 9.986 | 8.803 | 40.79 | 0.727 | 10.037 |
| 1 | 0 | 0 | 18.076 | 6229.521 | 100 | 4.904 | 17.955 | 5607.94 | 100 | 4.936 |
| 0 | 1 | 5 | 28.330 | 10.889 | 0.175 | 3.148 | - | - | - | - |
| 1 | 1 | 0 | 31.619 | 178.654 | 2.868 | 2.827 | 31.515 | 110.45 | 1.970 | 2.837 |
| 2 | 0 | 0 | 36.655 | 123.719 | 1.986 | 2.450 | 36.569 | 100.18 | 1.786 | 2.455 |
| 1 | 0 | 7 | 37.091 | 279.397 | 4.485 | 2.422 | 37.110 | 70.18 | 1.251 | 2.421 |
| 1 | 0 | 8 | 41.345 | 227.647 | 3.654 | 2.182 | 41.286 | 51.29 | 0.915 | 2.185 |
| 2 | 1 | 0 | 49.208 | 40.559 | 0.651 | 1.850 | 48.932 | 26.23 | 0.468 | 1.860 |
| 3 | 0 | 0 | 56.348 | 39.484 | 0.634 | 1.631 | 56.175 | 25.69 | 0.458 | 1.636 |
| 2 | 2 | 0 | 66.131 | 15.177 | 0.244 | 1.412 | 65.820 | 20.51 | 0.366 | 1.418 |
| 3 | 1 | 0 | 69.080 | 24.203 | 0.389 | 1.359 | 68.887 | 31.84 | 0.568 | 1.362 |
| 0 | 0 | 15 | 72.693 | 33.577 | 0.539 | 1.230 | 72.821 | 15.43 | 0.275 | 1.298 |
| 1 | 0 | 15 | 75.614 | 16.701 | 0.268 | 1.257 | 75.572 | 9.28 | 0.165 | 1.257 |
| Description of Characteristics, Dimension | Initial Polytetrafluoroethylene | Thermal-Radiation-Modified Polytetrafluoroethylene |
|---|---|---|
| Xc, % | 65.04 | 77.02 |
| L, Å | 348.588 | 91.138 |
| c/a * | 3.432 | 3.329 |
| a, Å | 5.664 ± 0.377 | 5.792 ± 0.784 |
| c, Å | 19.429 ± 1.293 | 19.299 ± 2.612 |
| V, Å3 | 539.778 | 560.673 |
| ρc, g/cm3 | 2.307 | 2.221 |
| ρtheor, g/cm3 | 2.217 | 2.182 |
| ρexp, g/cm3 | 2.205 | 2.180 |
| No. | Δ(2θ)halo, deg. | 2θmax, deg. | A, deg.·pps | b, deg. | H, pps |
|---|---|---|---|---|---|
| Initial polytetrafluoroethylene | |||||
| I | 10–25 | 16.670 ± 0.059 | 1546.042 ± 62.703 | 3.729 | 389.496 |
| IIa | 25–70 | 38.905 ± 0.054 | 1356.051 ± 115.925 | 8.747 | 145.638 |
| IIб | 25–70 | 46.603 ± 1.003 | 894.570 ± 131.306 | 15.989 | 52.561 |
| III | 72–75 | 73.418 ± 0.105 | 10.020 ± 2.498 | 0.914 | 10.304 |
| Thermal-radiation-modified polytetrafluoroethylene | |||||
| I | 10–25 | 16.680 ± 0.043 | 1626.748 ± 45.961 | 3.404 | 448.911 |
| IIa | 25–70 | 38.807 ± 0.045 | 1258.245 ± 81.325 | 9.075 | 130.246 |
| IIб | 25–70 | 47.350 ± 0.778 | 919.392 ± 96.847 | 17.545 | 49.229 |
| III | 72–75 | 74.135 ± 0.145 | 9.001 ± 2.368 | 1.408 | 6.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolyanskii, A.S.; Politova, E.D.; Koshkina, O.A.; Arsentyev, M.A.; Kusch, P.P.; Moskvitin, L.V.; Slesarenko, S.V.; Kiryukhin, D.P.; Trakhtenberg, L.I. Structure of Polytetrafluoroethylene Modified by the Combined Action of γ-Radiation and High Temperatures. Polymers 2021, 13, 3678. https://doi.org/10.3390/polym13213678
Smolyanskii AS, Politova ED, Koshkina OA, Arsentyev MA, Kusch PP, Moskvitin LV, Slesarenko SV, Kiryukhin DP, Trakhtenberg LI. Structure of Polytetrafluoroethylene Modified by the Combined Action of γ-Radiation and High Temperatures. Polymers. 2021; 13(21):3678. https://doi.org/10.3390/polym13213678
Chicago/Turabian StyleSmolyanskii, Alexander Sergeevich, Ekaterina Dmitrievna Politova, Ol’ga Alekseevna Koshkina, Mikhail Aleksandrovich Arsentyev, Pavel Prokof’evich Kusch, Lev Vladimirovich Moskvitin, Sergei Vital’evich Slesarenko, Dmitrii Pavlovich Kiryukhin, and Leonid Izrailevich Trakhtenberg. 2021. "Structure of Polytetrafluoroethylene Modified by the Combined Action of γ-Radiation and High Temperatures" Polymers 13, no. 21: 3678. https://doi.org/10.3390/polym13213678







