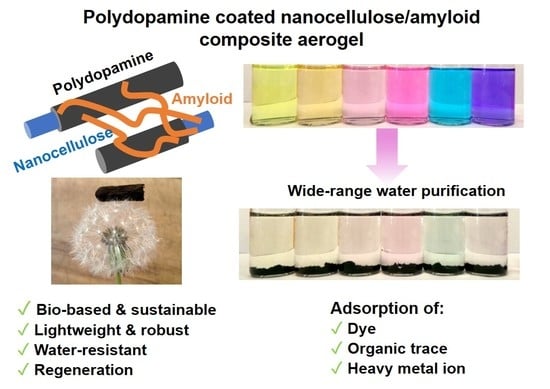
Bio-Based and Robust Polydopamine Coated Nanocellulose/Amyloid Composite Aerogel for Fast and Wide-Spectrum Water Purification
Abstract
:1. Introduction
2. Materials and Methods
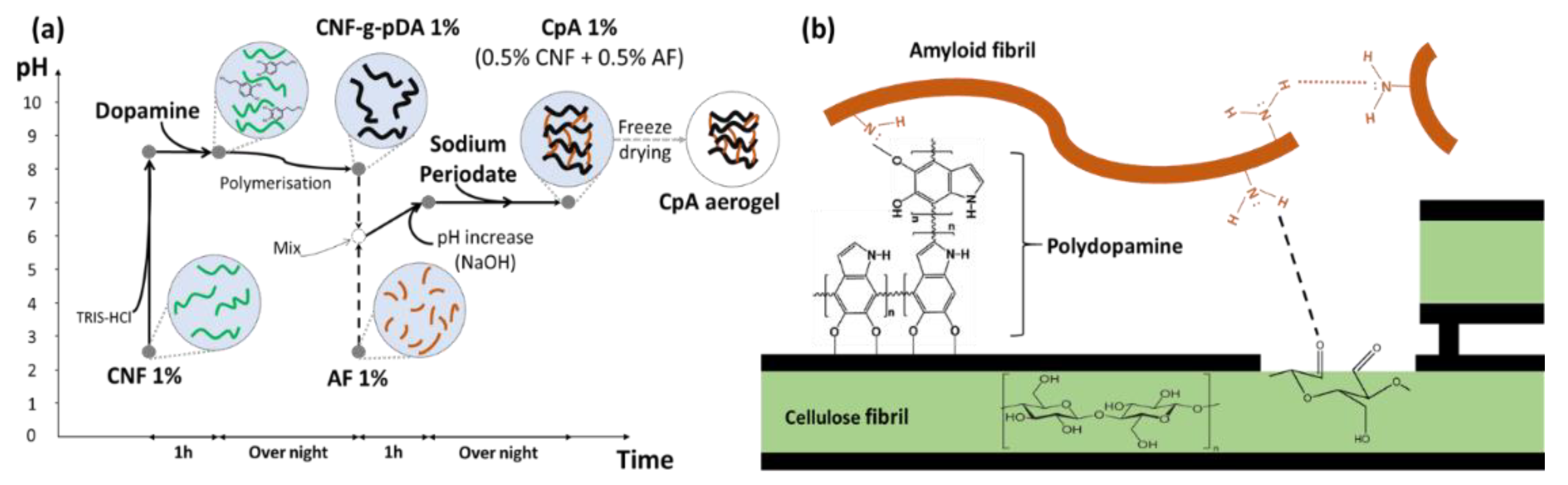
2.1. Preparation of CpA Aerogel
2.2. Characterisation of CpA Aerogel
2.3. Adsorption Experiments
2.3.1. Organic Trace Adsorption
2.3.2. Dye Adsorption
2.3.3. Heavy Metal Ion Adsorption
2.3.4. Adsorption Kinetics
2.3.5. Adsorption Mechanism
3. Results
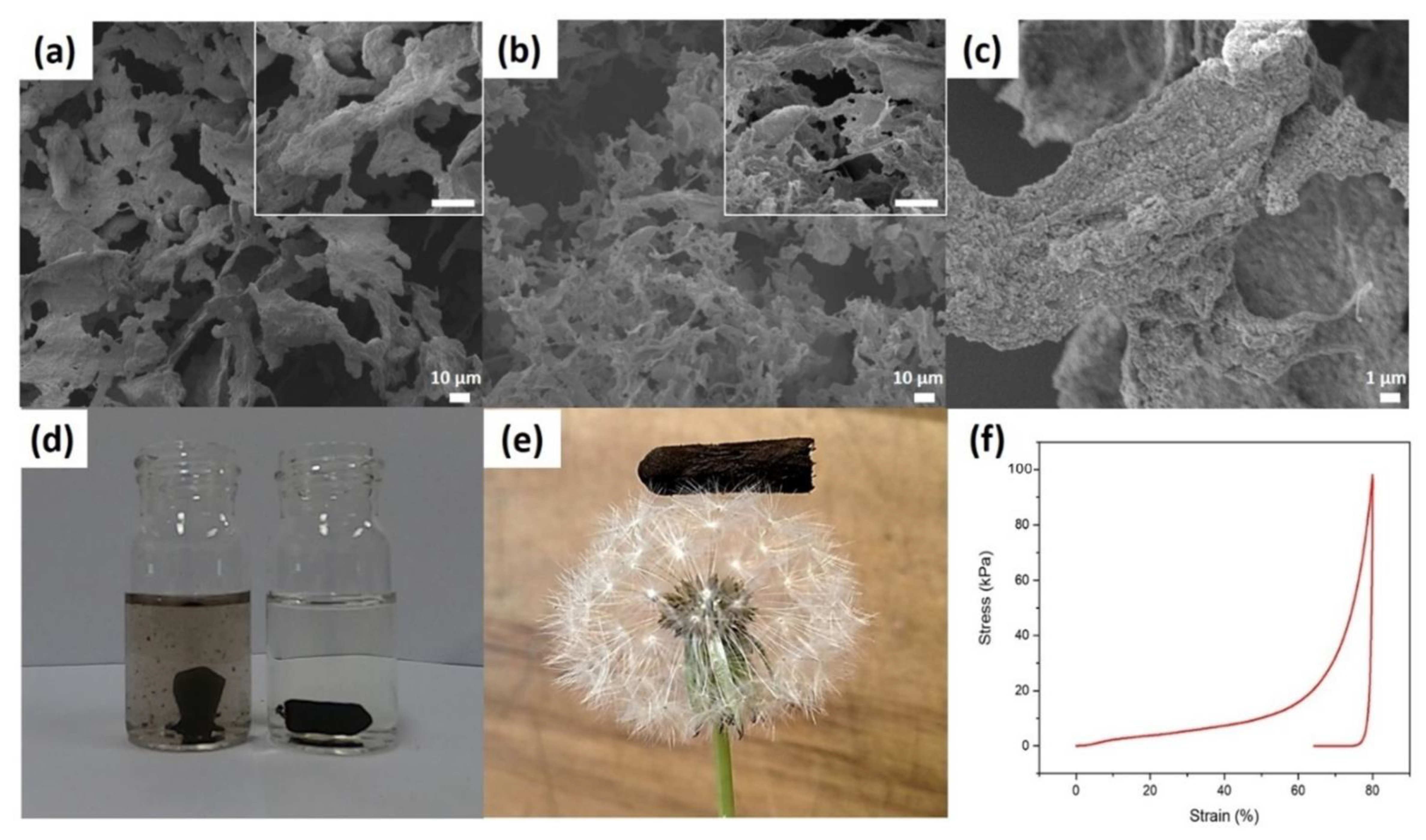
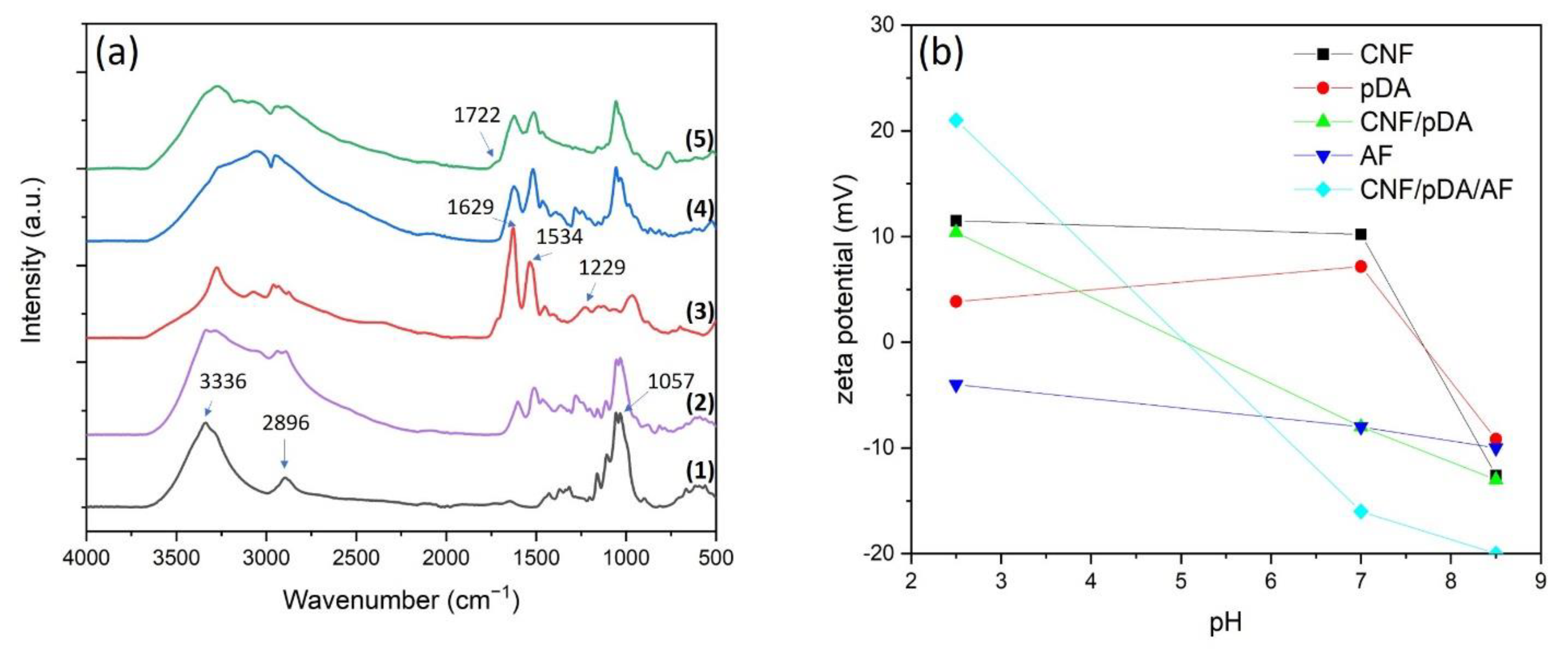
3.1. Synthesis and Characterisation of Aerogel
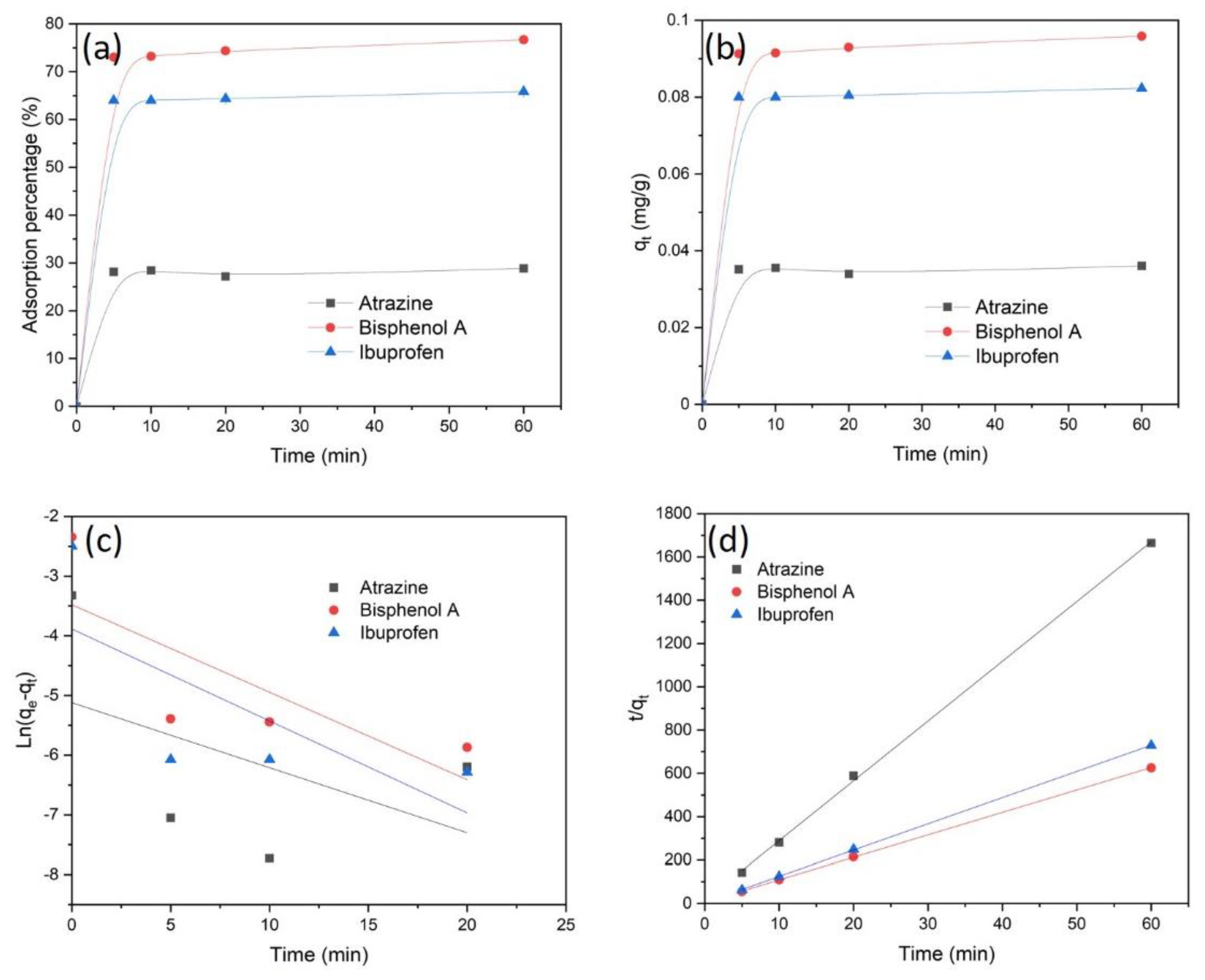
3.2. Adsorption of Trace Organic Compounds by CpA Aerogel
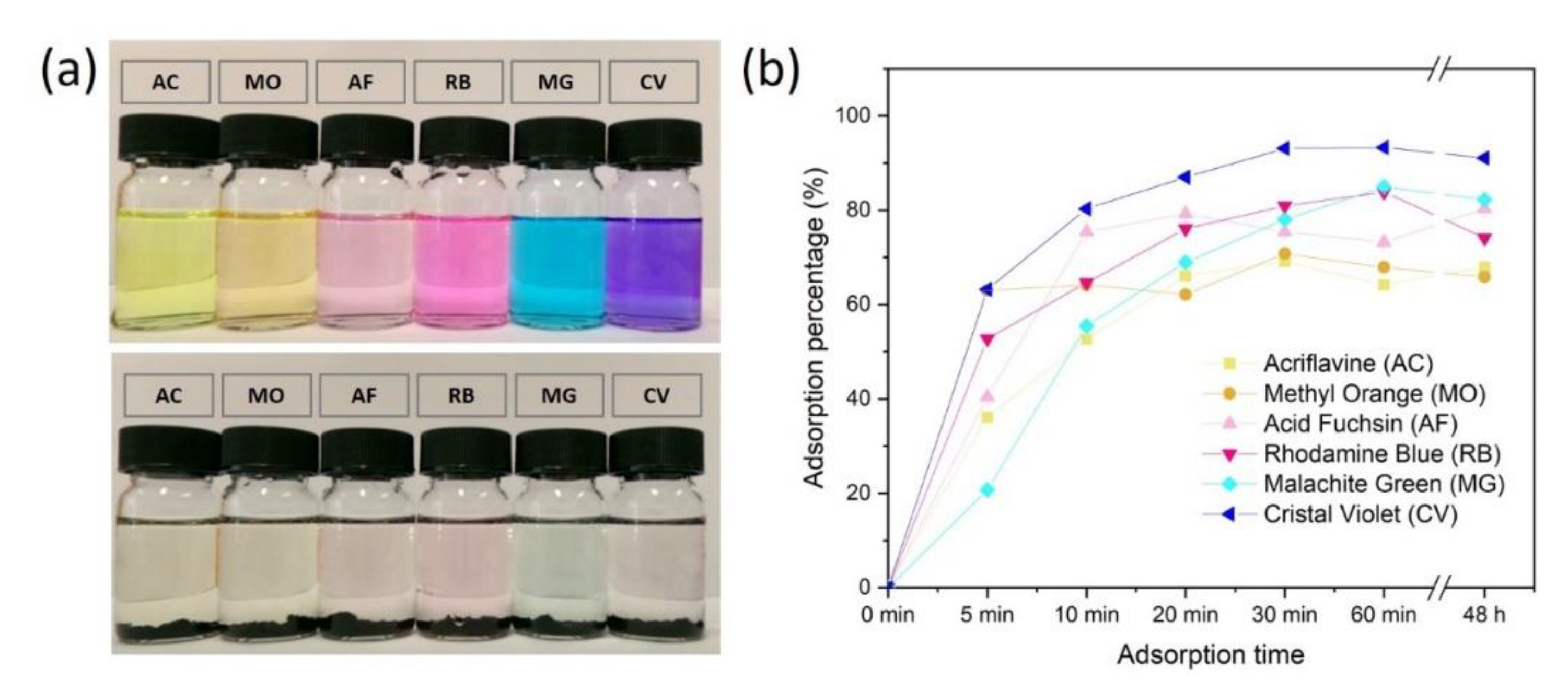
3.3. Adsorption of Dyes by CpA Aerogel
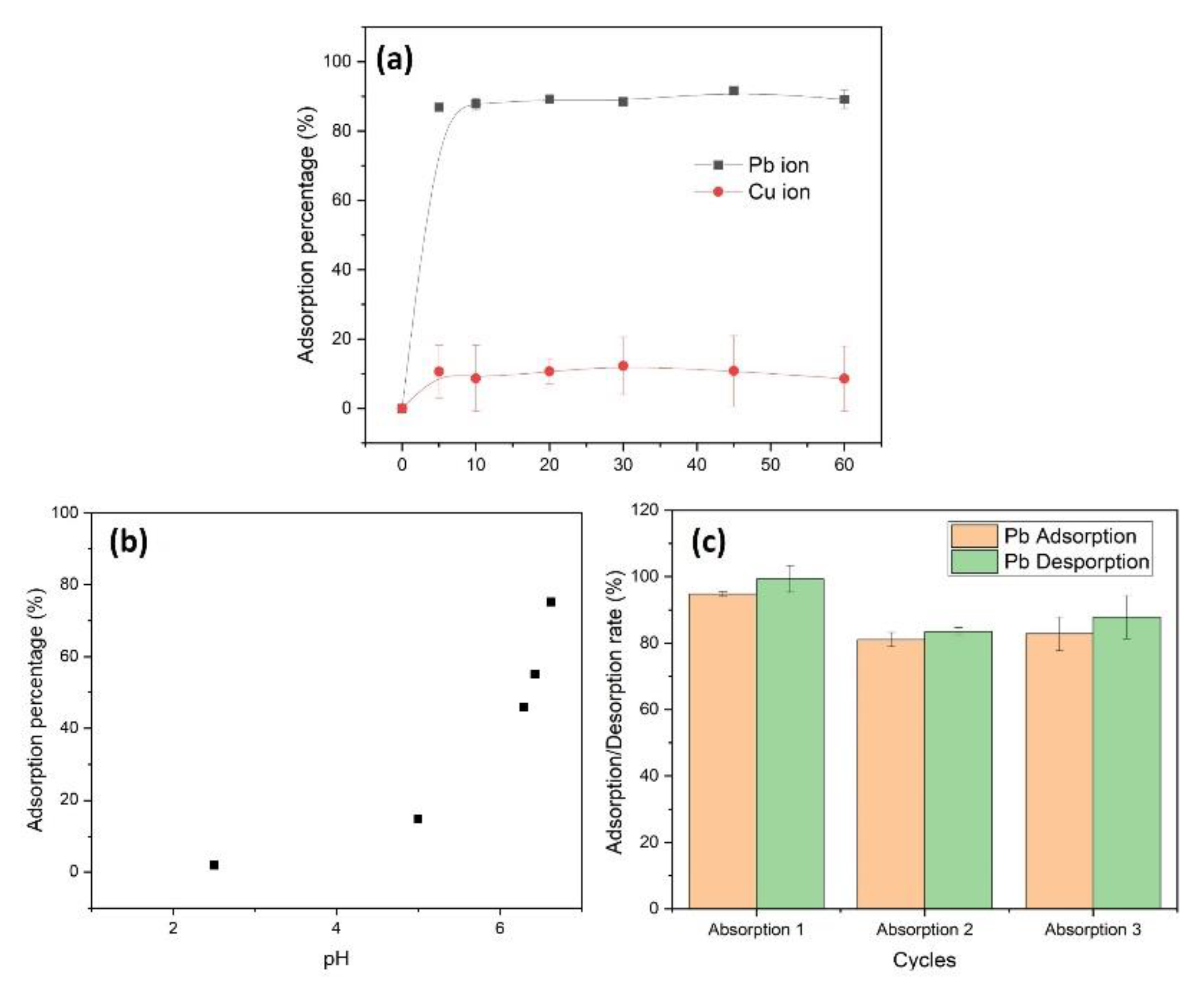
3.4. Adsorption of Heavy Metal Ions by CpA Aerogel
3.5. Performance Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assembly, U.G.; United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development A/RES/70/1; United Nations: Vienna, Austria, 2015. [Google Scholar]
- Ren, Z.J.; Umble, A.K. Recover wastewater resources locally. Nature 2016, 529, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanter, D.R.; Brownlie, W.J. Joint nitrogen and phosphorus management for sustainable development and climate goals. Environ. Sci. Policy 2018, 92, 1–8. [Google Scholar] [CrossRef]
- Aqdam, S.R.; Otzen, D.E.; Mahmoodi, N.M.; Morshedi, D. Adsorption of azo dyes by a novel bio-nanocomposite based on whey protein nanofibrils and nano-clay: Equilibrium isotherm and kinetic modeling. J. Colloid Interface Sci. 2021, 602, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, H.; Zhang, Y.; Zhao, Y.; Quan, X. Effects of Cu(II) and humic acid on atrazine photodegradation. J. Environ. Sci. 2011, 23, 773–777. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Carmalin, S.A.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Quan, L.D.; Dang, N.H.; Tu, T.H.; Linh, V.N.P.; Thy, L.T.M.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Preparation of magnetic iron oxide/graphene aerogel nanocomposites for removal of bisphenol A from water. Synth. Met. 2019, 255, 116106. [Google Scholar] [CrossRef]
- Meena, A.K.; Mishra, G.; Rai, P.; Rajagopal, C.; Nagar, P. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Goel, J.; Rajagopal, C. Sorption of lead, mercury and cadmium ions in multi-component system using carbon aerogel as adsorbent. J. Hazard. Mater. 2008, 153, 502–507. [Google Scholar] [CrossRef]
- Kleemann, C.; Selmer, I.; Smirnova, I.; Kulozik, U. Tailor made protein based aerogel particles from egg white protein, whey protein isolate and sodium caseinate: Influence of the preceding hydrogel characteristics. Food Hydrocoll. 2018, 83, 365–374. [Google Scholar] [CrossRef]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk fibroin aerogels for drug delivery applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Ahmadi, M.; Madadlou, A.; Saboury, A.A. Whey protein aerogel as blended with cellulose crystalline particles or loaded with fish oil. Food Chem. 2016, 196, 1016–1022. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.; Chen, L.; Duan, G.; Mei, C.; Huang, C.; Han, J.; Jiang, S. Anisotropic nanocellulose aerogels with ordered structures fabricated by directional freeze-drying for fast liquid transport. Cellulose 2019, 26, 6653–6667. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Shi, R.; Zhang, H.; Gong, L.; Dai, L. Chitosan/ nanofibrillated cellulose aerogel with highly oriented microchannel structure for rapid removal of Pb (II) ions from aqueous solution. Carbohydr. Polym. 2019, 223, 115048. [Google Scholar] [CrossRef]
- Bagnani, M.; Nyström, G.; De Michele, C.; Mezzenga, R. Amyloid Fibrils Length Controls Shape and Structure of Nematic and Cholesteric Tactoids. ACS Nano 2018, 13, 591–600. [Google Scholar] [CrossRef]
- Adamcik, J.; Jung, J.-M.; Flakowski, J.; Rios, P.D.L.; Dietler, G.; Mezzenga, R. Understanding amyloid aggregation by statistical analysis of atomic force microscopy images. Nat. Nanotechnol. 2010, 5, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Adamcik, J.; Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat. Nanotechnol. 2012, 7, 421–427. [Google Scholar] [CrossRef]
- Kardos, J.; Micsonai, A.; Pál-Gábor, H.; Petrik, E.; Gráf, L.; Kovács, J.; Lee, Y.-H.; Naiki, H.; Goto, Y. Reversible Heat-Induced Dissociation of β2-Microglobulin Amyloid Fibrils. Biochemistry 2011, 50, 3211–3220. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Bolisetty, S.; Tian, T.; Handschin, S.; Lu, C.; Mezzenga, R. Amyloid Fibril-Templated High-Performance Conductive Aerogels with Sensing Properties. Small 2020, 16, e2004932. [Google Scholar] [CrossRef]
- Nystrom, G.; Ronco, M.F.; Bolisetty, S.; Mazzotti, M.; Mezzenga, R. Amyloid Templated Gold Aerogels. Adv. Mater. 2015, 28, 472–478. [Google Scholar] [CrossRef]
- Cao, Y.; Bolisetty, S.; Wolfisberg, G.; Adamcik, J.; Mezzenga, R. Amyloid fibril-directed synthesis of silica core–shell nanofilaments, gels, and aerogels. Proc. Natl. Acad. Sci. 2019, 116, 4012–4017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Jia, L.; Zhao, Z.; Han, Y.; Li, Y.; Peng, Q.; Zhang, Q. Fast and robust lead (II) removal from water by bioinspired amyloid lysozyme fibrils conjugated with polyethyleneimine (PEI). Chem. Eng. J. 2020, 390, 124667. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suter, M.K.; Bolisetty, S.; Boulos, S.; Handschin, S.; Nyström, L.; Mezzenga, R. Amyloid Fibrils Aerogel for Sustainable Removal of Organic Contaminants from Water. Adv. Mater. 2020, 32, e1907932. [Google Scholar] [CrossRef] [PubMed]
- Derami, H.G.; Jiang, Q.; Ghim, D.; Cao, S.; Chandar, Y.J.; Morrissey, J.J.; Jun, Y.-S.; Singamaneni, S. A Robust and Scalable Polydopamine/Bacterial Nanocellulose Hybrid Membrane for Efficient Wastewater Treatment. ACS Appl. Nano Mater. 2019, 2, 1092–1101. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, J.; Zhu, J.; Guo, X.; Chen, P.; Duan, G.; Liu, X.; Li, Y. A Mussel-Inspired Polydopamine-Filled Cellulose Aerogel for Solar-Enabled Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 7617–7624. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, F.; Zhang, Z.; Cheng, Y.; Wang, Z.; Li, Y. Stimuli-responsive polydopamine-based smart materials. Chem. Soc. Rev. 2021, 50, 8319–8343. [Google Scholar] [CrossRef]
- Manolakis, I.; Azhar, U. Recent Advances in Mussel-Inspired Synthetic Polymers as Marine Antifouling Coatings. Coatings 2020, 10, 653. [Google Scholar] [CrossRef]
- Kang, H.; Song, X.; Wang, Z.; Zhang, W.; Zhang, S.; Li, J. High-Performance and Fully Renewable Soy Protein Isolate-Based Film from Microcrystalline Cellulose via Bio-Inspired Poly(dopamine) Surface Modification. ACS Sustain. Chem. Eng. 2016, 4, 4354–4360. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.; Messersmith, P. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Song, Y.; Zhao, F.; Spinney, S.; Bernardes, J.D.S.; Tam, K.C. Compressible cellulose nanofibril (CNF) based aerogels produced via a bio-inspired strategy for heavy metal ion and dye removal. Carbohydr. Polym. 2018, 208, 404–412. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.-C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S. Mussel-Inspired Surface Engineering for Water-Remediation Materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Xiong, Y.; Fan, B.; Yao, Q.; Wang, H.; Jin, C.; Sun, Q. Cellulose as an adhesion agent for the synthesis of lignin aerogel with strong mechanical performance, Sound-absorption and thermal Insulation. Sci. Rep. 2016, 6, 32383. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z. Preparation of composite aerogels based on sodium alginate, and its application in removal of Pb2+and Cu2+from water. Int. J. Biol. Macromol. 2018, 107, 741–747. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, L.; Zhang, J.; Hu, J.; Duan, G.; Liu, X.; Li, Y.; Gu, Z. Polydopamine antibacterial materials. Mater. Horizons 2021, 8, 1618–1633. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.; Zhou, Y.; Zhou, F.; Liu, W.; Wang, Z. Mussel-inspired hydrogels: From design principles to promising applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; D’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Burdine, L.; Gillette, T.G.; Lin, A.H.-J.; Kodadek, T. Periodate-Triggered Cross-Linking of DOPA-Containing Peptide−Protein Complexes. J. Am. Chem. Soc. 2004, 126, 11442–11443. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.; Liu, Z.; Ni, Y. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 2015, 22, 1135–1146. [Google Scholar] [CrossRef]
- Dash, R.; Elder, T.; Ragauskas, A.J. Grafting of model primary amine compounds to cellulose nanowhiskers through periodate oxidation. Cellulose 2012, 19, 2069–2079. [Google Scholar] [CrossRef]
- Hollertz, R.; Duran, V.L.; Larsson, P.A.; Wågberg, L. Chemically modified cellulose micro- and nanofibrils as paper-strength additives. Cellulose 2017, 24, 3883–3899. [Google Scholar] [CrossRef]
- Wei, H.; Ren, J.; Han, B.; Xu, L.; Han, L.; Jia, L. Stability of polydopamine and poly(DOPA) melanin-like films on the surface of polymer membranes under strongly acidic and alkaline conditions. Colloids Surf. B Biointerfaces 2013, 110, 22–28. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Freezing as a Path to Build Complex Composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef]
- Scotti, K.L.; Dunand, D.C. Freeze casting—A review of processing, microstructure and properties via the open data repository, FreezeCasting.net. Prog. Mater. Sci. 2018, 94, 243–305. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Supramaniam, J.; Wong, T.W.; Soottitantawat, A.; Ruktanonchai, U.R.; Tey, B.T.; Tang, S.Y. Synthesis of bio-inspired cellulose nanocrystals-soy protein isolate nanoconjugate for stabilization of oil-in-water Pickering emulsions. Carbohydr. Res. 2021, 504, 108336. [Google Scholar] [CrossRef]
- Li, D.; Li, Q.; Mao, D.; Bai, N.; Dong, H. A versatile bio-based material for efficiently removing toxic dyes, heavy metal ions and emulsified oil droplets from water simultaneously. Bioresour. Technol. 2017, 245, 649–655. [Google Scholar] [CrossRef]
- Amarasinghe, B.; Williams, R. Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem. Eng. J. 2007, 132, 299–309. [Google Scholar] [CrossRef]
- Nematidil, N.; Sadeghi, M.; Nezami, S.; Sadeghi, H. Synthesis and characterization of Schiff-base based chitosan-g-glutaraldehyde/NaMMTNPs-APTES for removal Pb2+ and Hg2+ ions. Carbohydr. Polym. 2019, 222, 114971. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Xiong, Q.; Li, C.; Shen, Y.; Uyama, H. Hierarchical porous cellulose/activated carbon composite monolith for efficient adsorption of dyes. Cellulose 2017, 24, 4275–4289. [Google Scholar] [CrossRef]
| Absorbent | Compound | Adsorption Capacity (mg/g) | Adsorption Efficiency (%) | Contact Time (min) | Ref. |
|---|---|---|---|---|---|
| CpA | Crystal Violet | 18.66 (10 ppm) | 93.1 | 30 min | This work |
| CpA | Bisphenol A | 0.0958 (1 ppm) | 72.9 | 5 min | This work |
| CpA | Pb (II) ion | 17.8254 (20 ppm) | 91.7 | 5 min | This work |
| Amyloid | Bisphenol A | 0.0968 (1 ppm) | 78 | >60 min | [24] |
| Porous chitosan | Pb (II) ion | 29.1 (50 ppm) | 80.83 | 40 min | [53] |
| cellulose/active carbon | Methylene blue | 26.7 (1000 ppm) | 89 | 50 h | [54] |
| Chitosan/nanocellulose | Pb (II) ion | 252.6 (150 ppm) | 82 | 5 min | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorriaux, M.; Sorieul, M.; Chen, Y. Bio-Based and Robust Polydopamine Coated Nanocellulose/Amyloid Composite Aerogel for Fast and Wide-Spectrum Water Purification. Polymers 2021, 13, 3442. https://doi.org/10.3390/polym13193442
Sorriaux M, Sorieul M, Chen Y. Bio-Based and Robust Polydopamine Coated Nanocellulose/Amyloid Composite Aerogel for Fast and Wide-Spectrum Water Purification. Polymers. 2021; 13(19):3442. https://doi.org/10.3390/polym13193442
Chicago/Turabian StyleSorriaux, Maxime, Mathias Sorieul, and Yi Chen. 2021. "Bio-Based and Robust Polydopamine Coated Nanocellulose/Amyloid Composite Aerogel for Fast and Wide-Spectrum Water Purification" Polymers 13, no. 19: 3442. https://doi.org/10.3390/polym13193442