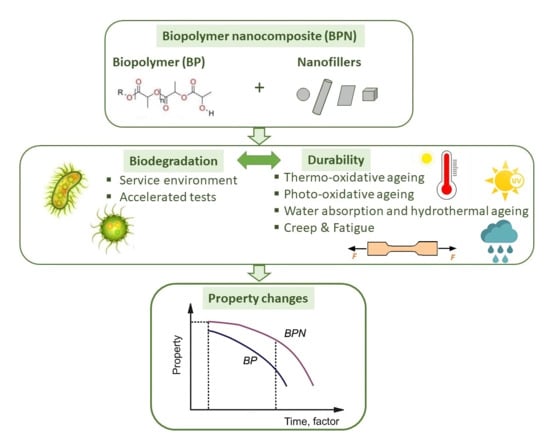
Durability of Biodegradable Polymer Nanocomposites
Abstract
:1. Introduction
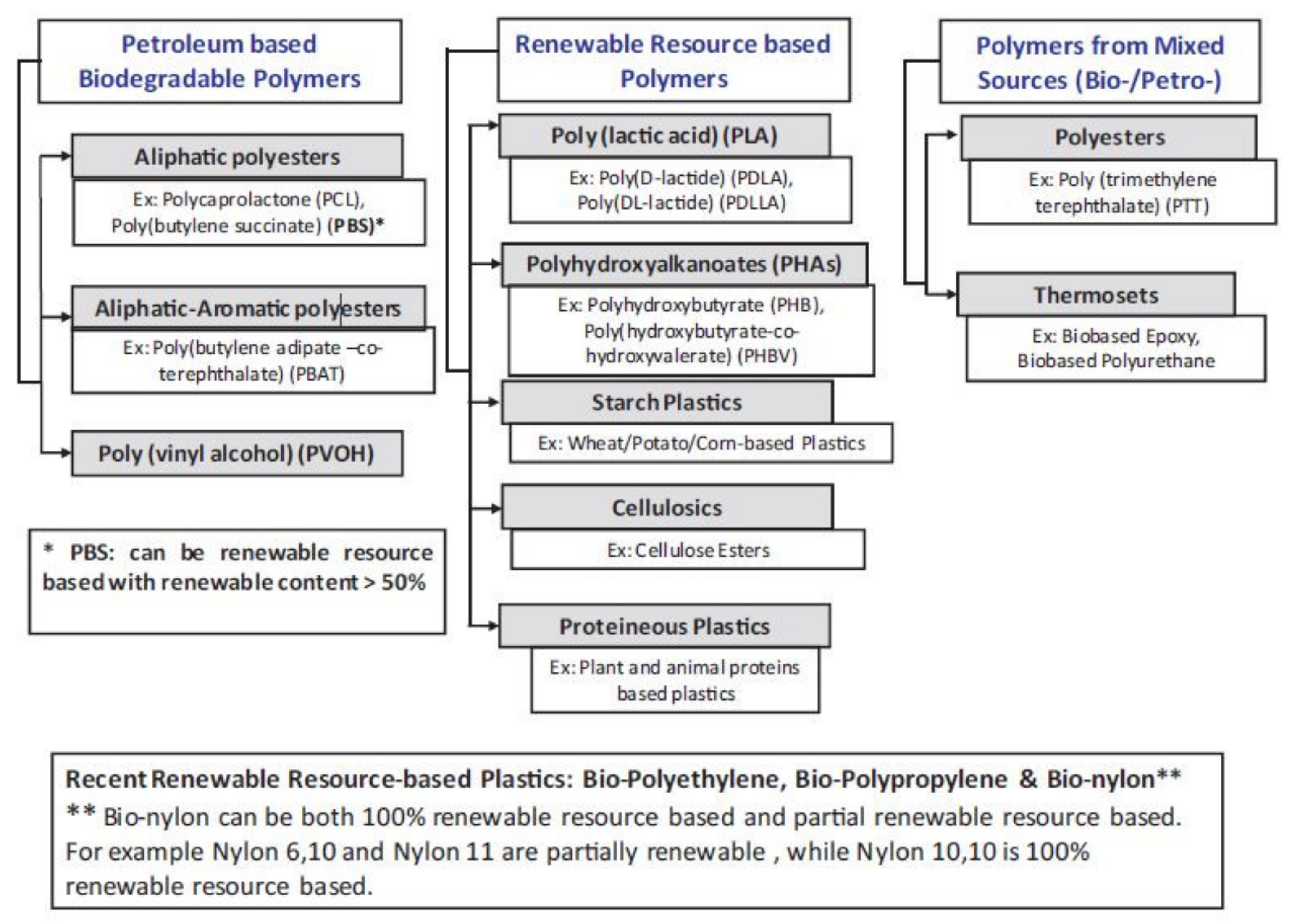
2. Biodegradable Polymers and their Basic Engineering Properties
3. Potential Nanofillers for Biodegradable Polymers
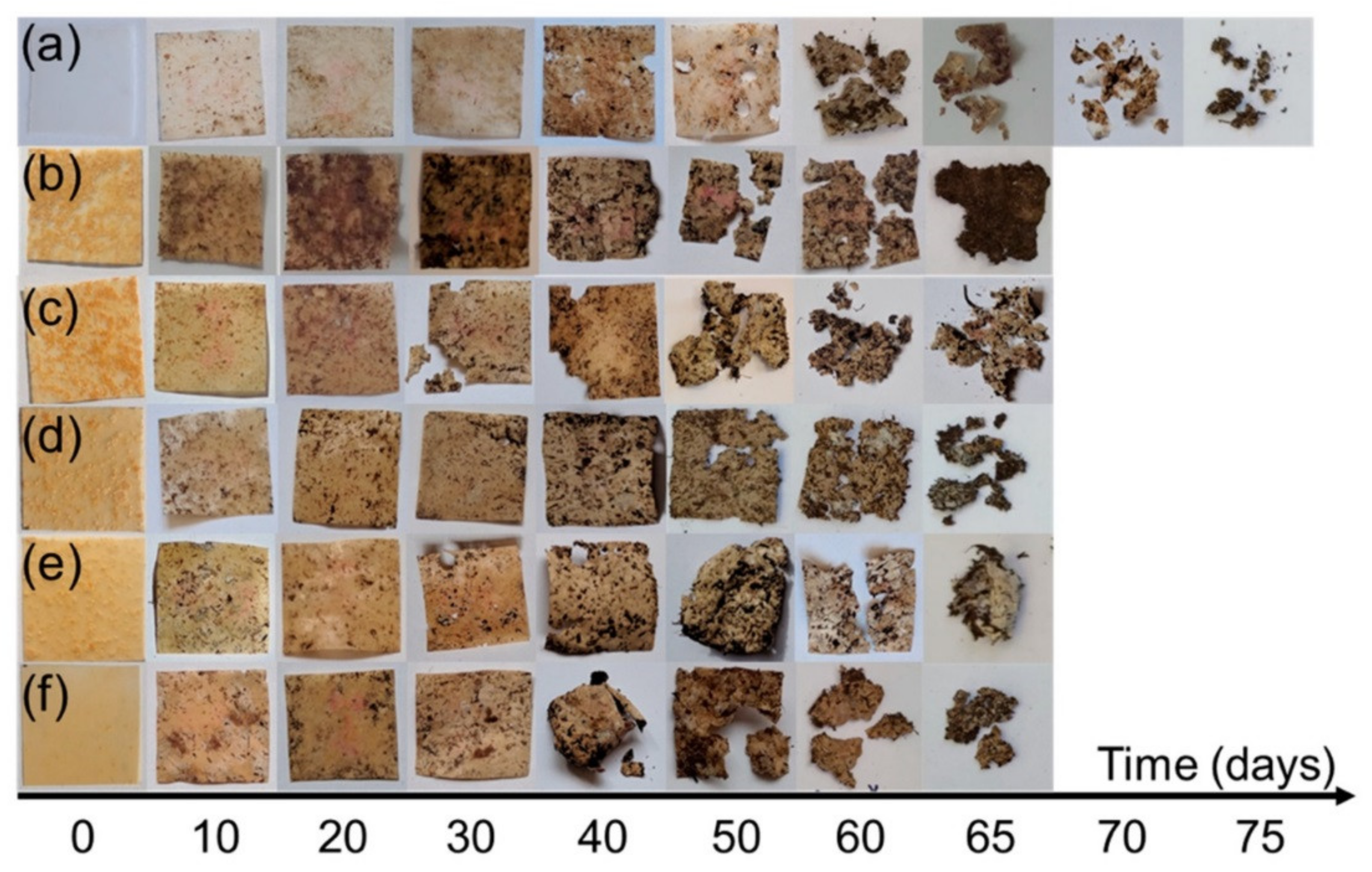
4. Biodegradation of BPN
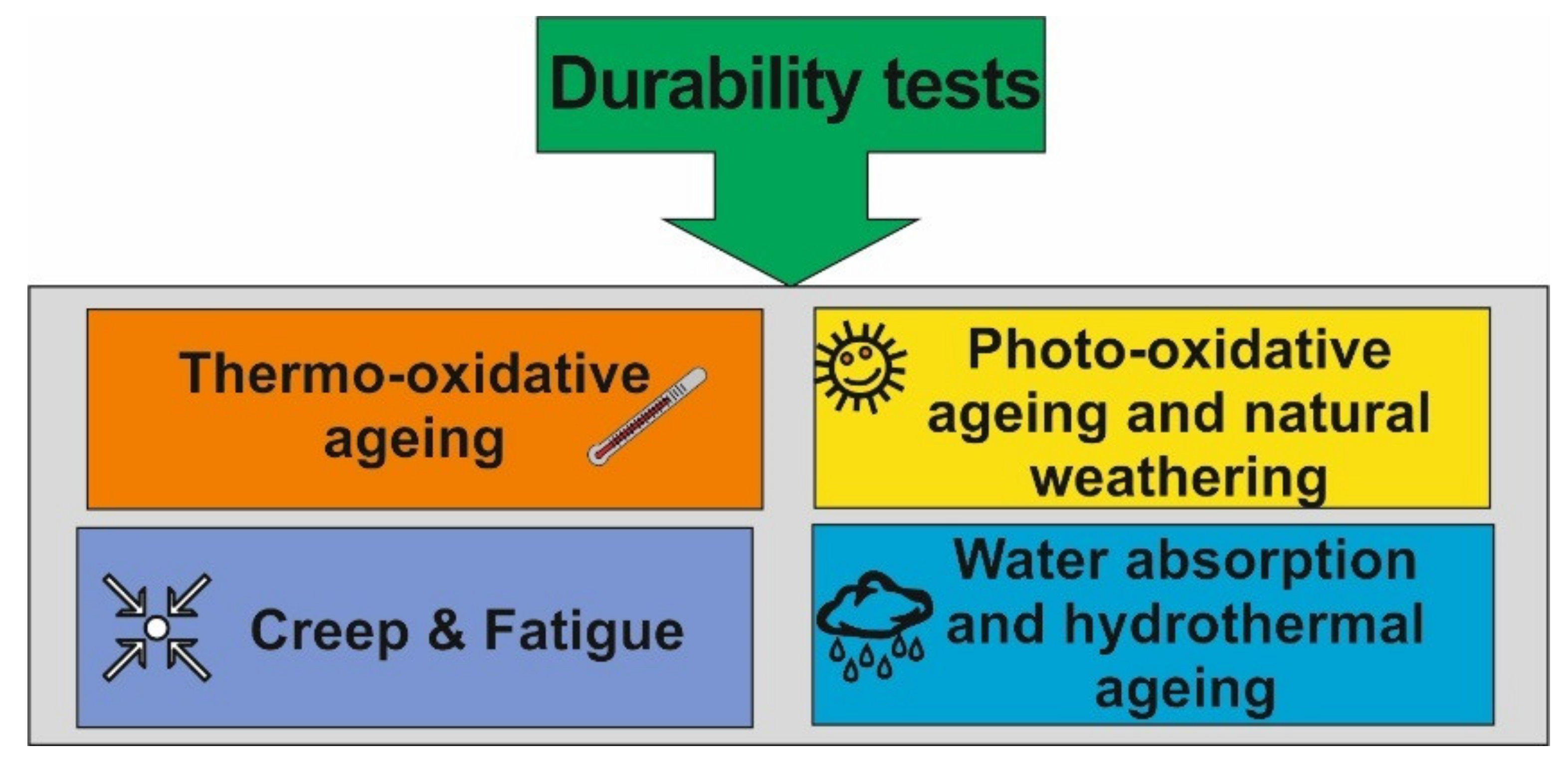
5. Durability Performance of BPN
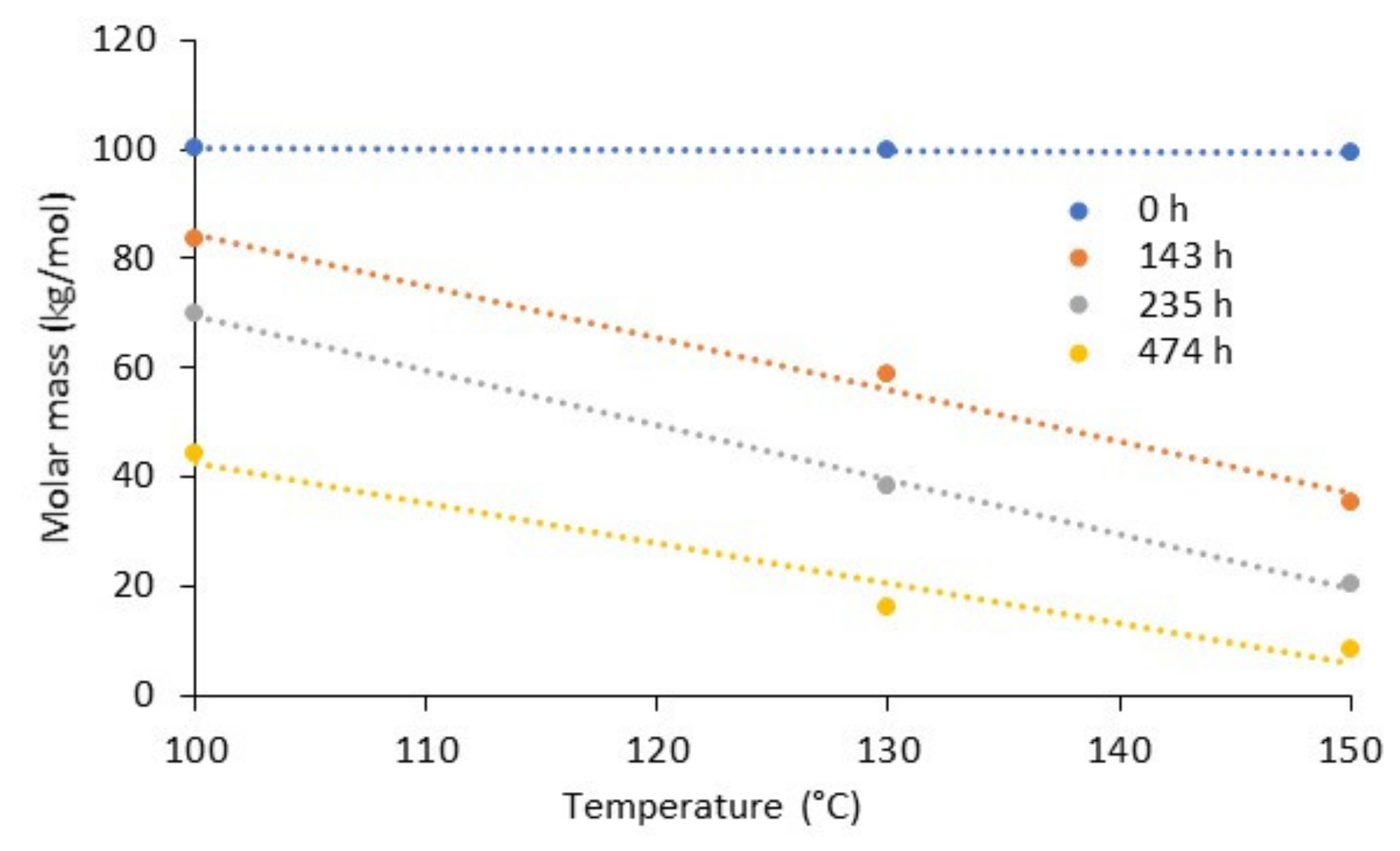
5.1. Thermo-Oxidative Ageing
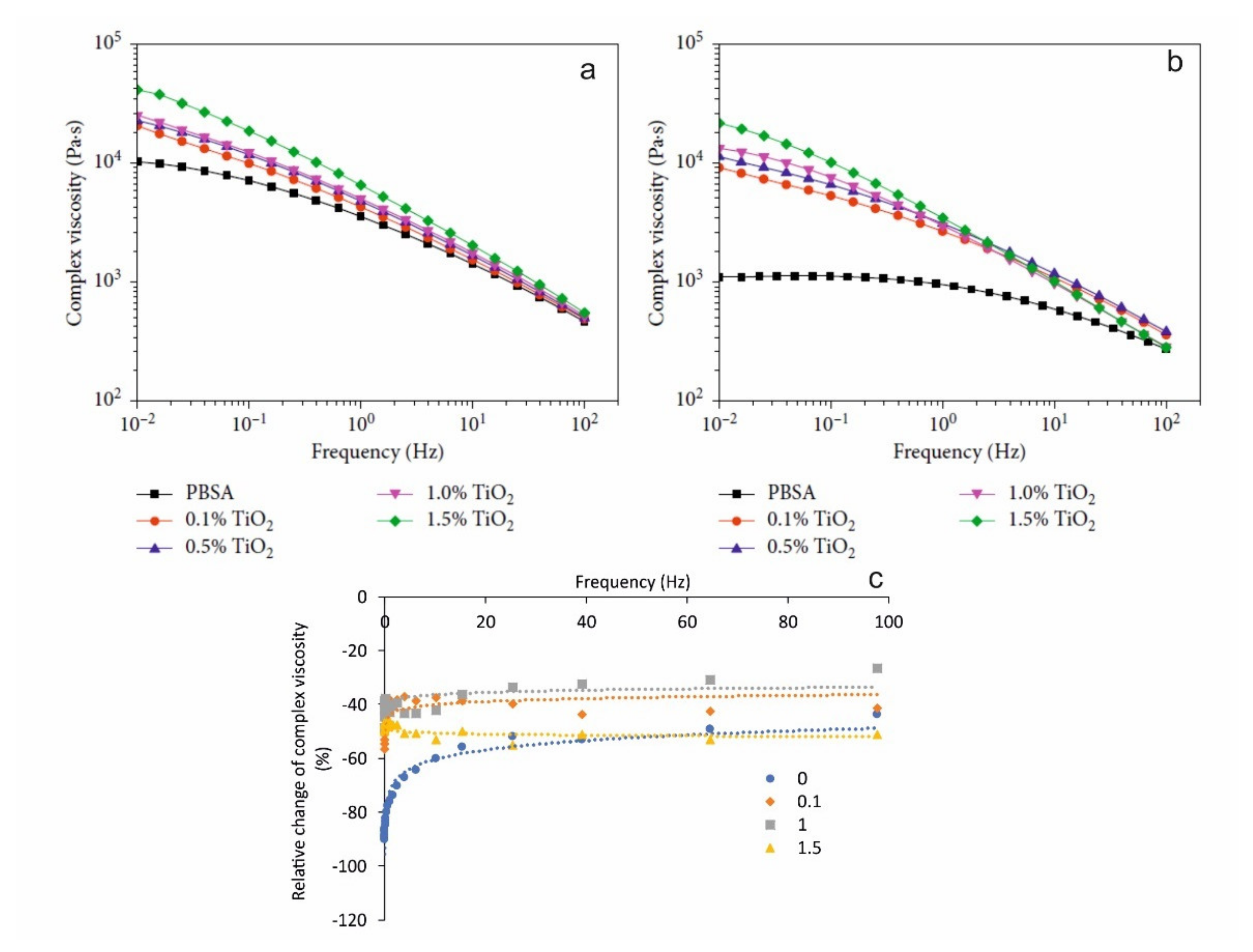
5.2. Photo-Oxidative Ageing
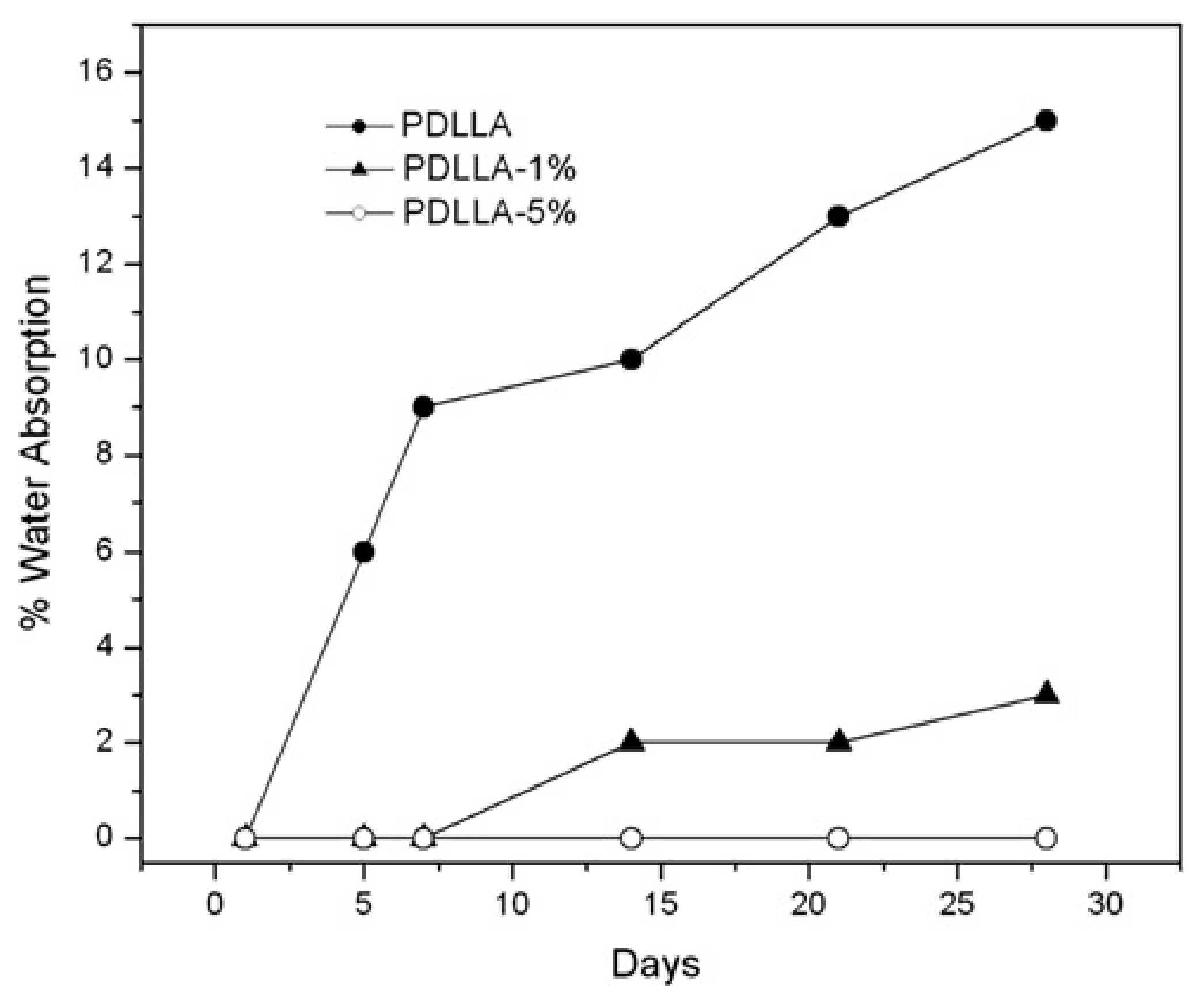
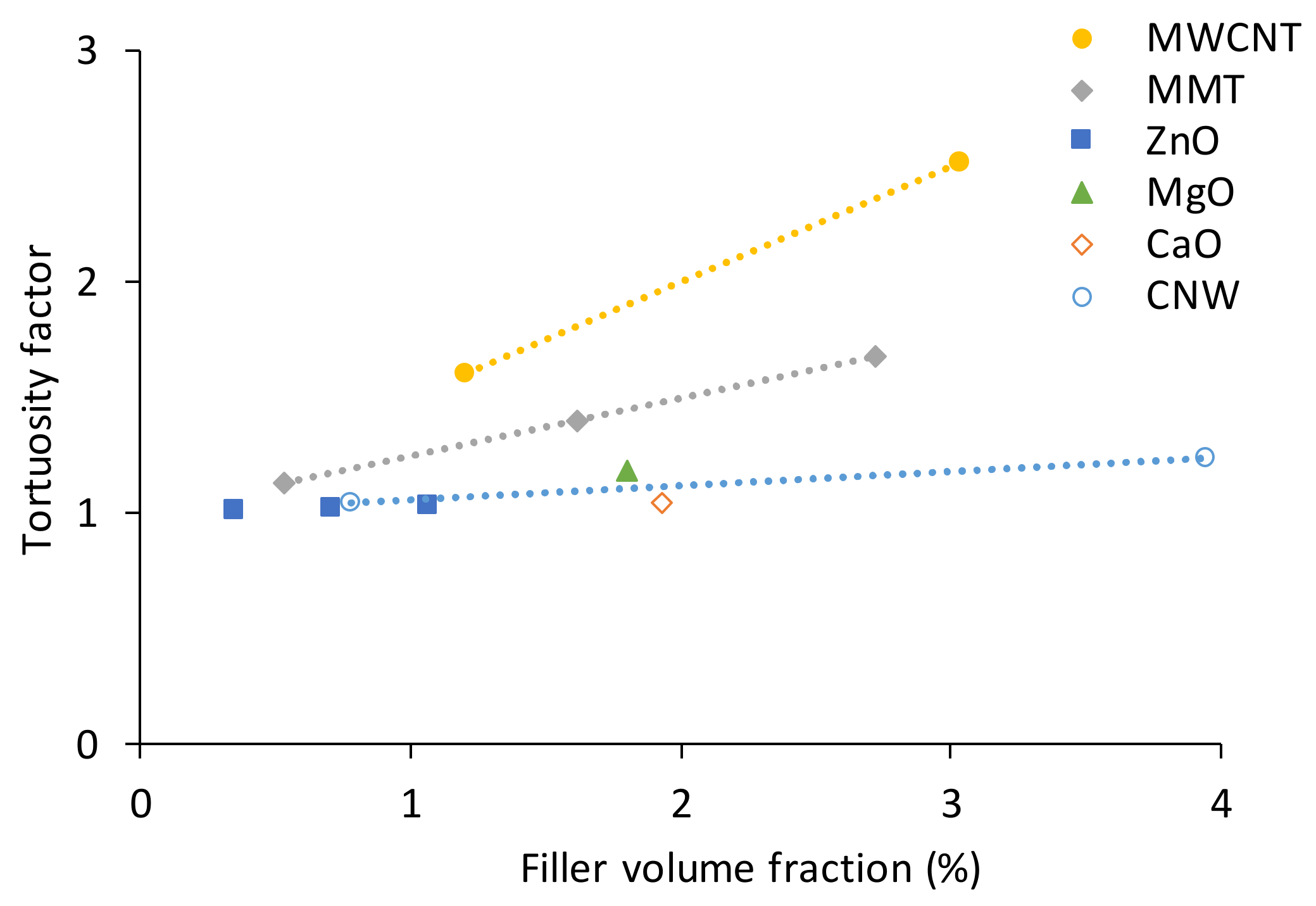
5.3. Water Absorption and Hygrothermal Ageing
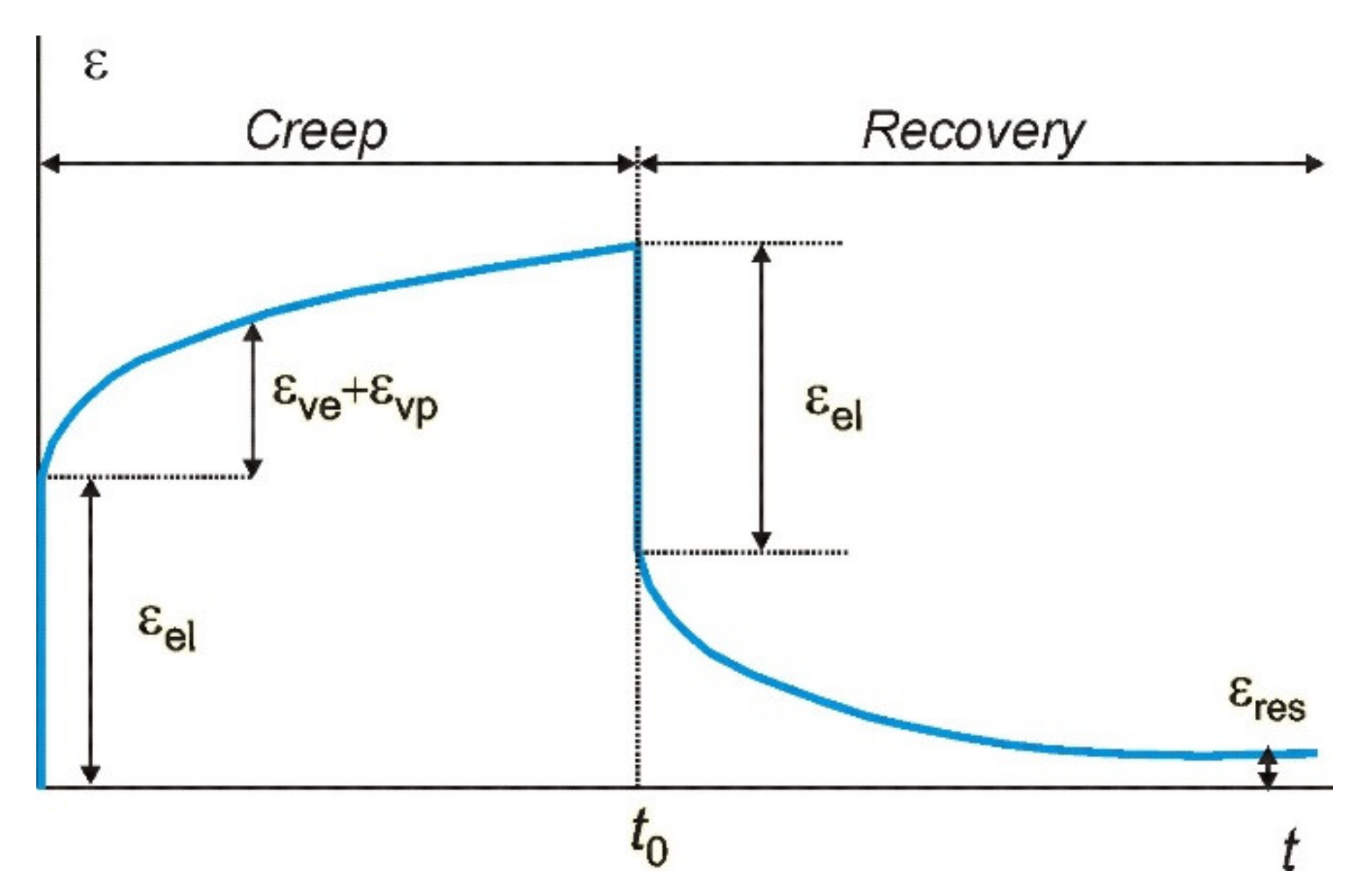
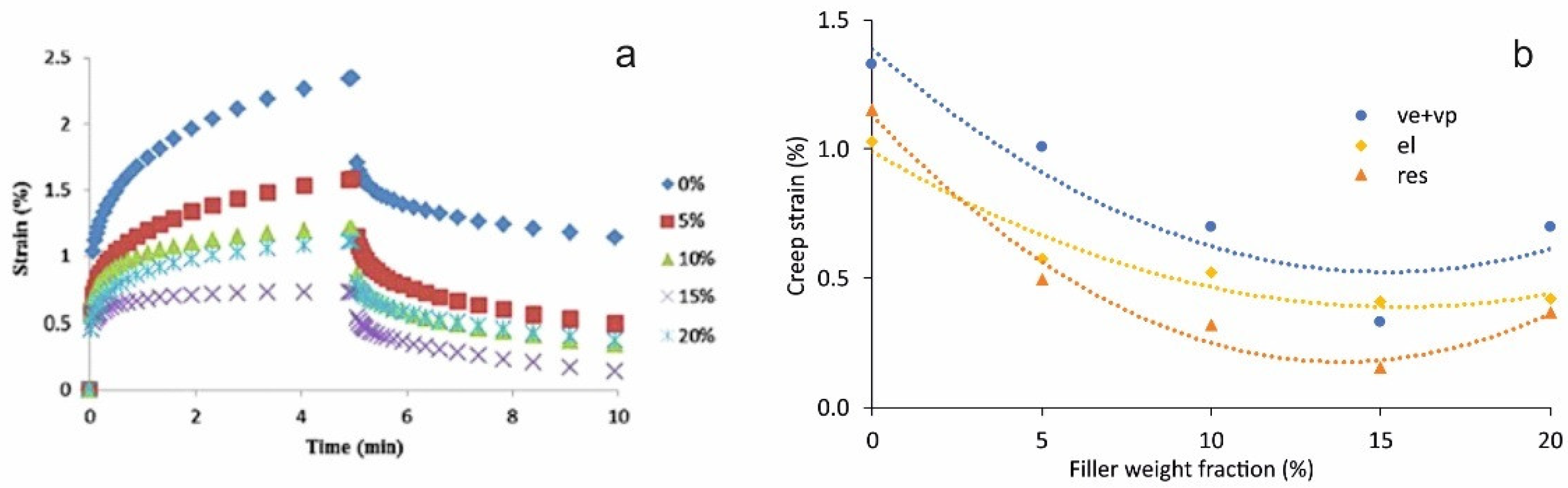
5.4. Creep
5.5. Modelling of Mechanical Properties Accompanied by Biodegradation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | biodegradable polymer |
| BPN | biodegradable polymer nanocomposites |
| CNC | cellulose nanocrystals |
| CNF | cellulose nanofibrils |
| CNT | carbon nanotubes |
| CNW | cellulose nanowhiskers |
| DLS | dynamic light scattering |
| DMTA | dynamic mechanical thermal analysis |
| DSC | differential scanning calorimetry |
| FTIR | Fourier-transform infrared spectroscopy |
| GL | glycerol |
| GnP | graphene nanoplatelets |
| GO | graphene oxide |
| HN | halloysite nanotubes |
| KWW | Kohlrausch–Williams–Watts |
| MCC | microcrystalline cellulose |
| MESS | methacrylated epoxidized sucrose soyate |
| MMT | montmorillonite |
| MWCNT | multiwall carbon nanotubes |
| NFC | nanofibrillated cellulose |
| NMR | nuclear magnetic resonance |
| PBAT | polybutylene adipate terephthalate |
| PBS | polybutylene succinate |
| PBSA | polybutylene succinate adipate |
| PEG | poly(ethylene glycol) |
| PCL | polycaprolactone |
| PDLLA | poly(D,L-lactide) |
| PLA | polylactic acid |
| PHA | polyhydroxyalkanoates |
| PHB | polyhydroxybutyrate |
| PHBV | poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PVOH | polyvinyl alcohol |
| PHV | polyhydroxy valerate |
| SEM | scanning electron microscopy |
| ST | starch |
| TEM | transmission electron microscopy |
| TGA | thermogravimetric analysis |
| TPS | thermoplastic starch |
| TTSP | time–temperature superposition principle |
| UV | ultraviolet |
| WLF | Williams–Landel–Ferry |
References
- Sharma, B.; Malik, P.; Jain, P. Biopolymer reinforced nanocomposites: A comprehensive review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Titone, V.; Correnti, A.; La Mantia, F.P. Effect of moisture content on a biodegradable polyester’s processing and mechanical properties. Polymers 2021, 13, 1616. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 17, 9304–9362. [Google Scholar] [CrossRef]
- Remiš, T.; Belský, P.; Kovárík, T.; Kadlec, J.; Azar, M.G.; Medlín, R.; Vavrunková, V.; Deshmukh, K.; Sadasivuni, K.K. Study on structure, thermal behavior, and viscoelastic properties of nanodiamond-reinforced poly (vinyl alcohol) nanocomposites. Polymers 2021, 13, 1426. [Google Scholar] [CrossRef]
- Rouf, T.B.; Kokini, J.L. Biodegradable biopolymer–graphene nanocomposites. J. Mater. Sci. 2016, 51, 9915–9945. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Pandey, J.K.; Reddy, K.R.; Pratheep Kumar, A.; Singh, R.P. An overview on the degradability of polymer nanocomposites. Polym. Degrad. Stab. 2005, 88, 234–250. [Google Scholar] [CrossRef]
- Chang, B.P.; Mohanty, A.K.; Misra, M. Studies on durability of sustainable biobased composites: A review. RSC Adv. 2020, 10, 17955. [Google Scholar] [CrossRef]
- Li, M.; Li, D.; Wang, L.-J.; Adhikari, B. Creep behavior of starch-based nanocomposite films with cellulose nanofibrils. Carbohydr. Polym. 2015, 117, 957–963. [Google Scholar] [CrossRef]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- De Paula, E.L.; Mano, V.; Pereira, F.V. Influence of cellulose nanowhiskers on the hydrolytic degradation behavior of poly(D,L-lactide). Polym. Degrad. Stab. 2011, 96, 1631–1638. [Google Scholar] [CrossRef]
- De Paula, E.L.; Mano, V.; Duek, E.A.R.; Pereira, F.V. Hydrolytic degradation behavior of PLLA nanocomposites reinforced with modified cellulose nanocrystals. Quím. Nova 2015, 38, 1014–1020. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Zhou, W.; Zhang, M.; Yang, T. Crystallization and biodegradation of polylactide/carbon nanotube composites. Polym. Eng. Sci. 2010, 50, 1721–1733. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S.; Wibooranawong, S.; Naknaen, P. Antimicrobial food packaging prepared from poly(butylene succinate) and zinc oxide. Measurement 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Kumar, B.; Castro, M.; Feller, J.F. Poly(lactic acid)–multi-wall carbon nanotube conductive biopolymernanocomposite vapour sensors. Sens. Actuat. B Chem. 2012, 161, 621–628. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, S.; Xiao, Y.; Yu, X.; Li, X.; Wu, P. Shape memory effect of poly(d,l-lactide)/Fe3O4 nanocomposites by inductive heating of magnetite particles. Colloids Surf. B Biointerfaces 2009, 71, 67–72. [Google Scholar] [CrossRef]
- Silva, A.P.B.; Montagna, L.S.; Passador, F.R.; Rezende, M.C.; Lemes, A.P. Biodegradable nanocomposites based on PLA/PHBV blend reinforced with carbon nanotubes with potential for electrical and electromagnetic applications. Express Polym. Lett. 2021, 15, 987–1003. [Google Scholar] [CrossRef]
- Armentano, I.; Puglia, D.; Luzi, F.; Arciola, C.R.; Morena, F.; Torre, S.M.; Torre, L. Nanocomposites based on biodegradable polymers. Materials 2018, 11, 795. [Google Scholar] [CrossRef] [Green Version]
- Bari, S.S.; Chatterjee, A.; Mishra, S. Biodegradable polymer nanocomposites: An overview. Polym. Rev. 2016, 56, 287–328. [Google Scholar] [CrossRef]
- Okamoto, M. Biodegradable Polymer/Layered Silicate Nanocomposites: A Review. In Handbook of Biodegradable Polymeric Materials and Their Applications; Mallapragada, S., Narasimhan, B., Eds.; Iowa State University: Ames, IO, USA, 2005; Volume 1, pp. 1–45. ISBN 1-58883-053-5. [Google Scholar]
- Abdoulhdi, A.B.O.; Abdulrahman, A.B.A.M.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian, K.S.S.; Petru, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Anžlovar, A.; Kržan, A.; Žaga, E. Degradation of PLA/ZnO and PHBV/ZnO composites prepared by melt processing. Arab. J. Chem. 2018, 11, 343–352. [Google Scholar] [CrossRef]
- Sawpan, M.A.; Islam, M.R.; Beg, M.D.H.; Pickering, K. Effect of accelerated weathering on physico-mechanical properties of polylactide bio-composites. J. Environ. Polym. Degrad. 2019, 27, 942–955. [Google Scholar] [CrossRef]
- Mtibe, A.; Motloung, M.P.; Bandyopadhyay, J.; Ray, S.S. Synthetic biopolymers and their composites: Advantages and limitations—an overview. Macromol. Rapid Commun. 2021, 42, 2100130. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Esposti, D.M.; Morselli, D.; Fava, F.; Bertin, L.; Cavani, F.; Viaggi, D.; Fabbri, P. The role of biotechnology in the transition from plastics to bioplastics: An opportunity to reconnect global growth with sustainability. FEBS Open Bio 2021, 11, 967–983. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Yiga, V.A.; Lubwama, M.; Pagel, S.; Benz, J.; Olupot, P.W.; Bonten, C. Flame retardancy and thermal stability of agriculturalresidue fiber-reinforc ed polylactic acid: A Review. Polym. Compos. 2021, 42, 15–44. [Google Scholar] [CrossRef]
- Higuchi-Takeuchi, M.; Morisaki, K.; Toyooka, K.; Numata, K. Synthesis of high-molecular-weight polyhydroxyalkanoates by marine photosynthetic purple bacteria. PLoS ONE 2016, 11, e0160981. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.J.; Guo, B.-H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; McLaren, D.C. 16—Thermoplastic Starch in Innovations. In Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 391–412. [Google Scholar] [CrossRef]
- Lackner, M.; Ivanič, F.; Kováčová, M.; Chodák, I. Mechanical properties and structure of mixtures of poly(butylene-adipate-co-terephthalate) (PBAT) with thermoplastic starch (TPS). Int. J. Biobased Plast. 2021, 3, 126–138. [Google Scholar] [CrossRef]
- Wang, X.; Peng, S.; Chen, H.; Yu, X.; Zhao, X. Mechanical properties, rheological behaviors, and phase morphologies of high-toughness PLA/PBAT blends by in-situ reactive compatibilization. Compos. B Eng. 2019, 173, 107028. [Google Scholar] [CrossRef]
- Petersson, L.; Kvien, I.; Oksman, K. Structure and thermal properties of poly(lactic acid)/cellulose whiskers nanocomposite materials. Compos. Sci. Technol. 2007, 67, 2535–2544. [Google Scholar] [CrossRef]
- Vytejčková, S.; Vápenkab, L.; Hradecký, J.; Dobiáš, J.; Hajšlová, J.; Loriot, C.; Vannini, L.; Poustka, J. Testing of polybutylene succinate based films for poultry meat packaging. Polym. Test. 2017, 60, 357–364. [Google Scholar] [CrossRef]
- Wei, D.; Wang, H.; Ziaee, Z.; Chibante, F.; Zheg, A.; Xiao, H. Non-leaching antimicrobial biodegradable PBAT films through a facile and novel approach. Mater. Sci. Eng. C 2016, 58, 986–991. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Râpă, M.; Sivertsvik, M.; Rosnes, J.T.; Popa, E.E.; Dumitriu, R.P.; Marincaș, O.; Matei, E.; Predescu, C.; Vasile, C. PLA-based materials containing bio-plasticizers and chitosan modified with rosehip seed oil for ecological packaging. Polymers 2021, 13, 1610. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.Y.; Park, S.L.; Lee, H.-J.; Kim, S.H.; Suh, M.J.; Ham, S.; Bhatia, S.K.; Gurav, R.; Park, S.-H.; Park, K.; et al. Polyhydroxyalkanoates (PHAs) degradation by the newly isolated marine Bacillus sp. JY14. Chemosphere 2021, 283, 131172. [Google Scholar] [CrossRef]
- Myung, J.; Flanagan, J.C.A.; Waymouth, R.M.; Criddle, C.S. Expanding the range of polyhydroxyalkanoates synthesized by methanotrophic bacteria through the utilization of omega-hydroxyalkanoate co-substrates. AMB Express 2017, 7, 118. [Google Scholar] [CrossRef] [Green Version]
- Signori, F.; Coltelli, M.-B.; Bronco, S. Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Fan, Y.; Nishida, H.; Moria, T.; Shirai, Y.; Endo, T. Thermal degradation of poly(L-lactide): Effect of alkali earth metal oxides for selective L,L-lactide formation. Polymer 2004, 45, 1197–1205. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose re109124inforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Vasile, C.; Râpă, M.; Ștefan, M.; Stan, M.; Macavei, S.; Darie-Niță, R.N.; Barbu-Tudoran, L.; Vodnar, D.C.; Popa, E.E.; Ștefan, R.; et al. New PLA/ZnO:Cu/Ag bionanocomposites for food packaging. Express Polym. Lett. 2017, 11, 531–544. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Chiralt, A.; Torre, L. PLA nanocomposites reinforced with cellulose nanocrystals from posidonia oceanica and ZnO nanoparticles for packaging application. J. Renew. Mater. 2017, 5, 2. [Google Scholar] [CrossRef]
- Sung, S.H.; Chang, Y.; Han, J. Development of polylactic acid nanocomposite films reinforced withcellulose nanocrystals derived from coffee silverskin. Carbohydr. Polym. 2017, 169, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Zinge, C.; Kandasubramanian, B. Nanocellulose based biodegradable polymers. Eur. Polym. J. 2020, 133, 109758. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Guo, B. Photodegradation behavior of poly(butylene succinate-co-butyleneadipate)/ZnO nanocomposites. Colloids Surf. A Physicochem. 2016, 489, 173–181. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Sereda, A.; Gaidukova, G.; Grase, L.; Thakur, V.K.; Filipova, I.; Fridrihsone, V.; Skute, M.; et al. Bio-based poly(butylene succinate)/microcrystalline cellulose/nanofibrillated cellulose-based sustainable polymer composites: Thermo-mechanical and biodegradation studies. Polymers 2020, 12, 1472. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Pakdel, P.M. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf 2019, 22, 100420. [Google Scholar] [CrossRef]
- Gibril, M.E.; Ahmed, K.K.; Lekha, P.; Sithole, B.; Khosla, A.; Furukawa, H. Effect of nanocrystalline cellulose and zinc oxide hybrid organic–inorganic nanofiller on the physical properties of polycaprolactone nanocomposite films. Microsyst. Technol. 2019. [Google Scholar] [CrossRef]
- Chen, C.; Lv, G.; Pan, C.; Song, M.; Wu, C.; Guo, D.; Wang, X.; Chen, B.; Gu, Z. Poly(lactic acid) (PLA) based nanocomposites—A novel way of drug-releasing. Biomed. Mater. 2007, 2, L1–L4. [Google Scholar] [CrossRef] [PubMed]
- Abou-Yousef, H.; Saber, E.; Abdel-Aziz, M.S.; Kamel, S. Efficient alternative of antimicrobial nanocomposites based on cellulose acetate/Cu-NPs. Soft Mater. 2018, 16, 141–150. [Google Scholar] [CrossRef]
- Van der Zee, M. Biodegradability of Polymers Mechanisms and Evaluation Methods. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006. [Google Scholar]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Polish J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Müller, R.J. Biodegradation Behavior of Polymers in Liquid Environments. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006. [Google Scholar]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer biodegradation: Mechanisms and estimation techniques. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.H. Ecological considerations of biodeterioration. Int. Biodeteter. Biodegrad. 2001, 48, 16–25. [Google Scholar] [CrossRef]
- Frits, J. Ecotoxicological Aspects in the Biodegradation Process of Polymers. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006. [Google Scholar]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Muller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly(3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Huang, S.J. Poly(lactic acid) and Copolyesters. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006. [Google Scholar]
- Müller, R.J. Aliphatic-Aromatic Polyesters. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006. [Google Scholar]
- Siracusa, V. Microbial Degradation of Synthetic Biopolymers Waste. Polymers 2019, 11, 1066. [Google Scholar] [CrossRef] [Green Version]
- German Institute for Standardisation. DIN 54900, Part 1–4, Testing of the Compostability of Plastics; German Institute for Standardisation: Berlin, Germany, 1998. [Google Scholar]
- ASTM. ASTM D6002-96, Standard Guide for Assessing the Compostability of Environmentally Degradable Plastics; ASTM INTERNATIONAL: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Abreu, A.S.; Oliveira, M.; de Sá, A.; Rodrigues, R.M.; Cerqueira, M.A.; Vicente, A.A.; Machado, A. Antimicrobial nanostructured starch based films for packaging. Carbohydr. Polym. 2015, 129, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kim, H.J.; Lee, J.W.; Choi, I.G. Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Neibolts, N.; Platnieks, O.; Gaidukovs, S.; Barkane, A.; Thakur, V.K.; Filipova, I.; Mihai, G.; Zelca, Z.; Yamaguchi, K.; Enachescu, M. Needle-free electrospinning of nanofibrillated cellulose and graphene nanoplatelets based sustainable poly (butylene succinate) nanofibers. Mater. Today Chem. 2020, 17, 100301. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Gaidukova, G.; Grase, L.; Thakur, V.K.; Filipova, I.; Fridrihsone, V.; Skute, M.; Laka, M. Highly loaded cellulose/poly (butylene succinate) sustainable composites for woody-like advanced materials application. Molecules 2020, 25, 121. [Google Scholar] [CrossRef]
- Al Awak, M.M.; Wang, P.; Wang, S.; Tang, Y.; Sun, Y.-P.; Yang, L. Correlation of carbon dots’ light-activated antimicrobial activities and fluorescence quantum yield. RSC Adv. 2017, 7, 30177–30184. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghorbani, M.; Ramazani, S. Preparation and characterization of TiO2NPs and betanin loaded zein/sodium alginate nanofibers. Food Packag. Shelf Life 2020, 24, 100504. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Jacob, H. Preparation and characterization of agar-based nanocomposite films reinforced with bimetallic (Ag-Cu) alloy nanoparticles. Carbohydr. Polym. 2017, 155, 382–390. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Neibolts, N.; Barkane, A.; Gaidukova, G.; Thakur, V.K. Poly(butylene succinate) and graphene nanoplatelet–based sustainable functional nanocomposite materials: Structure-properties relationship. Mater. Today Chem. 2020, 18, 100351. [Google Scholar] [CrossRef]
- Iovino, R.; Zullo, R.; Rao, M.A.; Cassar, L.; Gianfreda, L. Biodegradation of poly(lactic acid)/starch/coir biocomposites under controlled composting conditions. Polym. Degrd. Stab. 2008, 93, 147–157. [Google Scholar] [CrossRef]
- Gaidukova, G.; Platnieks, O.; Aunins, A.; Barkane, A.; Ingrao, C.; Gaidukovs, S. Spent coffee waste as a renewable source for the production of sustainable poly(butylene succinate) biocomposites from a circular economy perspective. RSC Adv. 2021, 11, 18580–18589. [Google Scholar] [CrossRef]
- Platnieks, O.; Barkane, A.; Ijudina, N.; Gaidukova, G.; Thakur, V.K.; Gaidukovs, S. Sustainable tetra pak recycled cellulose / Poly(Butylene succinate) based woody-like composites for a circular economy. J. Clean. Prod. 2020, 270, 122321. [Google Scholar] [CrossRef]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Zaidi, N.S.R.; Mah, S.-K. Poly (vinyl) alcohol crosslinked composite packaging film containing gold nanoparticles on shelf life extension of banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- Venkatesh, C.; Laurenti, M.; Bandeira, M.; Lanzagorta, E.; Lucherini, L.; Cauda, V.; Devine, D.M. Biodegradation and Antimicrobial Properties of Zinc Oxide–Polymer Composite Materials for Urinary Stent Applications. Coatings 2020, 10, 1002. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite filmswith combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef] [Green Version]
- Achilias, D.S.; Panayotidou, E.; Zuburtikudis, I. Thermal degradation kinetics and isoconversional analysis of biodegradable poly(3-hydroxybutyrate)/organomodified montmorillonite nanocomposites. Thermochim. Acta 2011, 514, 58–66. [Google Scholar] [CrossRef]
- Bagde, P.; Nadanathangam, V. Mechanical, antibacterial and biodegradable properties of starch film containing bacteriocin immobilized crystalline nanocellulose. Carbohydr. Polym. 2019, 222, 115021. [Google Scholar] [CrossRef]
- Aniskevich, K.; Glaskova, T.; Janson, Y. Elastic and sorption characteristics of an epoxy binder in a composite during its moistening. Mech. Compos. Mater. 2005, 41, 341–350. [Google Scholar] [CrossRef]
- Aniskevich, A.; Glaskova-Kuzmina, T.C. 3. Effect of moisture on elastic and viscoelastic properties of fibre reinforced plastics: Retrospective and current trends. In Creep and Fatigue in Polymer Matrix Composites, 2nd ed.; Guedes, R.M., Ed.; Woodhead Publishing: Oxford, UK, 2019; pp. 83–120. [Google Scholar] [CrossRef]
- Glaskova-Kuzmina, T.; Zotti, A.; Borriello, A.; Zarrelli, M.; Aniskevich, A. Basalt fibre composite with carbon nanomodified epoxy matrix under hydrothermal ageing. Polymers 2021, 13, 532. [Google Scholar] [CrossRef] [PubMed]
- Ogunsona, E.O.; Misra, M.; Mohanty, A.K. Accelerated hydrothermal aging of biocarbon reinforced nylon biocomposites. Polym. Degrad. Stab. 2017, 139, 76–88. [Google Scholar] [CrossRef]
- Goliszek, M.; Podkościelna, B.; Sevastyanova, O.; Fila, K.; Chabros, A.; Pączkowski, P. Investigation of accelerated aging of lignin-containing polymermaterials. Int. J. Biol. Macromol. 2019, 123, 910–922. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Capote, G.; Patiño, J.P. Effect of surface treatment of fibers on the accelerated aging of biocomposites. Constr. Build. Mater. 2021, 271, 121875. [Google Scholar] [CrossRef]
- Phua, Y.J.; Chow, W.S.; Mohd Ishak, Z.A. The hydrolytic effect of moisture and hygrothermal aging on poly(butylene succinate)/organo-montmorillonite nanocomposites. Polym. Degrad. Stab. 2011, 96, 1194–1203. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.M.; Chiralt, A.; Torre, L. Production and characterization of PLA PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crop. Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Impact of Nanoclays on the Biodegradation of Poly(Lactic Acid) Nanocomposites. Polymers 2018, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Dorigato, A.; Sebastiani, M.; Pegoretti, A.; Fambri, L. Effect of Silica Nanoparticles on the Mechanical Performances of Poly(Lactic Acid). J. Polym. Environ. 2012, 20, 713–725. [Google Scholar] [CrossRef]
- Cosquer, R.; Pruvost, S.; Gouanvé, F. Improvement of Barrier Properties of Biodegradable Polybutylene Succinate/Graphene Nanoplatelets Nanocomposites Prepared by Melt Process. Membranes 2021, 11, 151. [Google Scholar] [CrossRef]
- Cai, L.; Qi, Z.; Xu, J.; Guo, B.; Huang, Z. Study on the photodegradation stability of poly(butylene succinate-co-butylene adipate)/TiO2 nanocomposites. J. Chem. 2019, 5036019. [Google Scholar] [CrossRef]
- Ahuja, D.; Kumar, L.; Kaushik, A. Thermal stability of starch bionanocomposites films: Exploring the role of esterified cellulose nanofibers isolated from crop residue. Carbohydr. Polym. 2021, 255, 117466. [Google Scholar] [CrossRef]
- Famá, L.; Rojoe, P.G.; Bernal, C.; Goyanes, S. Biodegradable starch based nanocomposites with low water vapor permeability and high storage modulus. Carbohydr. Polym. 2012, 87, 1989–1993. [Google Scholar] [CrossRef]
- Ollier, R.P.; D’Amico, D.A.; Schroeder, W.F.; Cyras, V.P.; Alvarez, V.A. Effect of clay treatment on the thermal degradation of PHB based nanocomposites. Appl. Clay Sci. 2018, 163, 146–152. [Google Scholar] [CrossRef]
- Jayakumar, A.; Prabhu, K.; Shah, L.; Radha, P. Biologically and environmentally benign approach for PHB-silver nanocomposite synthesis and its characterization. Polym. Test. 2020, 81, 106197. [Google Scholar] [CrossRef]
- Nivedita, S.; Shiny, J. Effect of unmodified and modified montmorillonite on the properties of PCL based ultrafiltration membrane for water treatment applications. J. Water Process. Eng. 2018, 21, 61–68. [Google Scholar] [CrossRef]
- Georgiopoulos, P.; Kontou, E.; Christopoulos, A. Short-term creep behavior of a biodegradable polymer reinforced with wood-fibers. Composites 2015, 80, 134–144. [Google Scholar] [CrossRef]
- Ramazani, S.; Karimin, M. Study the molecular structure of poly(ε-caprolactone)/graphene oxide and graphene nanocompositenanofibers. J. Mech. Behav. Biomed. Mater. 2016, 61, 484–492. [Google Scholar] [CrossRef]
- Bai, H.; Liang, Z.; Wang, D.; Guo, J.; Zhang, S.; Ma, P.; Dong, W. Biopolymer nanocomposites with customized mechanical property and exceptionally antibacterial performance. Compos. Sci. Technol. 2020, 199, 108338. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Morphology and thermal degradation studies of melt-mixed PLA/PHBV biodegradable polymer blend nanocomposites with TiO2 as filler. J. Appl. Polym. Sci. 2015, 50, 10. [Google Scholar] [CrossRef]
- Ding, K.; Wei, N.; Zhou, Y. Viscoelastic behavior and model simulations of poly(butylene adipateco-terephthalate) biocomposites with carbon nanotubes: Hierarchical structures and relaxation. J. Compos. Mater. 2016, 50, 1805–1816. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and Water Resistant Poly(vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front Mater. 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch 2013, 65, 48–60. [Google Scholar] [CrossRef]
- Amaro, L.P.; Cicogna, F.; Passaglia, E.; Morici, E.; Oberhauser, W.; Al-Malaika, S.; Tzankova Dintcheva, N.; Coiai, S. Thermo-oxidative stabilization of poly(lactic acid) with antioxidant intercalated layered double hydroxides. Polym. Degrad. Stab. 2016, 133, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Cooney, R.P. Ch. 9. Thermal degradation of polymer and polymer composites. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; William Andrew Applied Science Publishers: Oxford, UK, 2018; pp. 185–206. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Remmler, M.; Mackenzie, K.; Moder, M.; Wachsen, O. Thermal decomposition of biodegradable polyesters—II. Poly(lactic acid). Polym. Degrad. Stab. 1996, 53, 329–342. [Google Scholar] [CrossRef]
- Rasselet, D.; Ruellan, A.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C.; Fayolle, B. Oxidative degradation of polylactide (PLA) and its effects on physical and mechanical properties. Eur. Polym. J. 2014, 50, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J. Additives for Plastics Handbook, 3rd ed.; Elsevier Advanced Technology: Oxford, UK, 2001; 484p. [Google Scholar]
- Plota, A.; Masek, A. Lifetime prediction methods for degradable polymeric materials—A short review. Materials 2020, 13, 4507. [Google Scholar] [CrossRef]
- Barkane, A.; Platnieks, O.; Jurinovs, M.; Gaidukovs, S. Thermal stability of UV-cured vegetable oil epoxidized acrylate-based polymer system for 3D printing application. Polym. Degrad. Stab. 2020, 181, 109347. [Google Scholar] [CrossRef]
- Chrissafis, K.; Antoniadis, G.; Paraskevopoulos, K.M.; Vassiliou, A.; Bikiaris, D.N. Comparative study of the effect of different nanoparticles onthe mechanical properties and thermal degradation mechanism ofin situ prepared poly(e-caprolactone) nanocomposites. Compos. Sci. Technol. 2007, 67, 2165–2174. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, J.; Wang, H.; Xue, L. Investigation of microstructures and ultraviolet aging properties of organomontmorillonite/SBS modified bitumen. Mater. Chem. Phys. 2011, 129, 769–776. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, M.E.; del Campo, A.S.M.; Robledo-Ortíz, J.R.; Arellano, M.; Perez-Fonseca, A.A. Accelerated weathering of poly(lactic acid) and its biocomposites: A review. Polym. Degrad. Stabil. 2020, 179, 109290. [Google Scholar] [CrossRef]
- Beg, M.D.H.; Pickering, K.L. Accelerated weathering of unbleached and bleached Kraft wood fibre reinforced polypropylene composites. Polym. Degrad. Stabil. 2008, 93, 1939–1946. [Google Scholar] [CrossRef]
- Glaskova, T.I.; Guedes, R.M.; Morais, J.J.; Aniskevich, A.N. A comparative analysis of moisture transport models applied to epoxy binder. Mech. Compos. Mater. 2007, 43, 377–388. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A.D. Free volume and mass transport in polymer nanocomposites. Curr. Opin. Colloid Interface Sci. 2012, 17, 132–140. [Google Scholar] [CrossRef]
- Nielsen, L.E. Models for the Permeability of Filled Polymer Systems. J. Macromol. Sci. Part A Chem. 1967, 1, 929–942. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. Tortuosity model to predict the combined effects of crystallinity and nano-sized clay mineral on the water vapour barrier properties of polylactic acid. Appl. Clay Sci. 2017, 141, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Murariu, M.; Benali, S.; Paint, Y.; Dechief, A.-L.; Murariu, O.; Raquez, J.-M.; Dubois, P. Adding value in production of multifunctional polylactide (PLA)–ZnO nanocomposite films through alternative manufacturing methods. Molecules 2021, 26, 2043. [Google Scholar] [CrossRef] [PubMed]
- Rostamzad, H.; Paighambari, S.Y.; Shabanpour, B.; Ojagh, S.M.; Mousavi, S.M. Improvement of fish protein film with nanoclay and transglutaminase for food packaging. Food Packag. Shelf Life 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Nanthakumar, K.; Yeng, C.M.; Chun, K.S. Tensile and water absorption properties of solvent cast biofilms of sugarcane leaves fibre-filled poly(lactic) acid. J. Thermoplast. Compos. Mater. 2020, 33, 289–304. [Google Scholar] [CrossRef]
- Chan, M.Y.; Koay, S.C.; Husseinsyah, S.; San, S.T. Cross-linked chitosan/corn cob biocomposite films with salicylaldehyde on tensile, thermal, and biodegradable properties: A comparative study. Adv. Polym. Technol. 2016, 34, 1229–1239. [Google Scholar] [CrossRef]
- Bharadwaj, R.K. Modeling the Barrier Properties of Polymer-Layered Silicate Nanocomposites. Macromolecules 2001, 34, 9189–9192. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Pukanszky, B. Influence of interface interaction on the ultimate tensile properties of polymer composites. Composites 1990, 21, 255–262. [Google Scholar] [CrossRef]
- Danyadi, L.; Janecska, T.; Szabo, Z.; Nagy, G.; Moczo, J.; Pukanszky, B. Wood flour filled PP composites: Compatibilization and adhesion. Compos. Sci. Technol. 2007, 67, 2838–2846. [Google Scholar] [CrossRef] [Green Version]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Gaidukovs, S.; Platnieks, O.; Gaidukova, G.; Starkova, O.; Barkane, A.; Beluns, S.; Thakur. Understanding the Impact of Microcrystalline Cellulose Modification on Durability and Biodegradation of Highly Loaded Biocomposites for Woody like Materials Applications. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. Effect of the wine lees wastes as cost-advantage and natural fillers on the thermal and mechanical properties of poly(3-hydroxybutyrate-cohydroxyhexanoate) (PHBH) and poly(3-hydroxybutyrate-cohydroxyvalerate) (PHBV). J. Appl. Polym. Sci. 2019, 137, 48869. [Google Scholar] [CrossRef]
- Sugiman, S.; Salman, S.; Maryudi, M. Effects of volume fraction on water uptake and tensile properties of epoxy filled with inorganic fillers having different reactivity to water. Mater. Today Commun. 2020, 24, 101360. [Google Scholar] [CrossRef]
- Starkova, O.; Gaidukovs, S.; Platnieks, O.; Barkane, A.; Garkusina, K.; Palitis, E.; Grase, L. Water absorption and hydrothermal ageing of epoxy adhesives reinforced with amino-functionalized graphene oxide nanoparticles. Polym. Degrad. Stabil. 2021, 191, 109670. [Google Scholar] [CrossRef]
- Platnieks, O.; Sereda, A.; Gaidukovs, S.; Thakur, V.K.; Barkane, A.; Gaidukova, G.; Filipova, I.; Ogurcovs, A.; Fridrihsone, V. Adding value to poly (butylene succinate) and nanofibrillated cellulose-based sustainable nanocomposites by applying masterbatch process. Ind. Crop. Prod. 2021, 169, 113669. [Google Scholar] [CrossRef]
- Jyoti, J.; Singh, P.B.; Arya, A.K.; Dhakate, S.R. Dynamic mechanical properties of multiwall carbon nanotube reinforced ABS composites and their correlation with entanglement density, adhesion, reinforcement and C factor. RSC Adv. 2016, 6, 3997. [Google Scholar] [CrossRef]
- Glaskova, T.; Aniskevich, K.; Borisova, A. Modeling of creep for MWCNT/epoxy nanocomposite. J. Appl. Polym. Sci. 2013, 129, 3314–3324. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Saha, P.; Adhikari, J.; Deshmukh, K.; Ahamed, M.B.; Cabibihan, J.-J. Recent advances in mechanical properties of biopolymer composites: A review. Polym. Compos. 2019, 41, 32–59. [Google Scholar] [CrossRef]
- Aniskevich, K.; Starkova, O.; Jansons, J.; Aniskevich, A. Long-Term Deformability and Aging of Polymer Matrix Composites; Nova Science Publishers: Hauppauge, NY, USA, 2011; ISBN 978-1-61470-406-5. [Google Scholar]
- Brinson, H.F.; Brinson, L.C. Polymer Engineering Science and Viscoelasticity. An Introduction; Springer Science Business Media: Berlin, Germany, 2008; ISBN 978-0-387-73860-4. [Google Scholar]
- Amiri, A.; Yu, A.; Webster, D.; Ulven, C. Bio-Based Resin Reinforced with Flax Fiber as Thermorheologically Complex Materials. Polymers 2016, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Ollier, R.P.; Casado, U.; Nicolini, A.T.; Alvarez, V.A.; Pérez, C.J.; Ludueña, L.N. Improved creep performance of melt-extruded polycaprolactone/organo-bentonite nanocomposites. J. Appl. Polym. Sci. 2021, 138, e50961. [Google Scholar] [CrossRef]
- Guedes, R.M.; Singh, A.; Pinto, V. Viscoelastic modelling of creep and stress relaxation behaviour in PLA-PCL fibres. Fiber Polym. 2017, 18, 2443–2453. [Google Scholar] [CrossRef]
- Kontou, E.; Spathis, G.; Georgiopoulos, P. Modeling of nonlinear viscoelasticity-viscoplasticity of bio-based polymer composites. Polym. Degrad. Stabil. 2014, 110, 203–207. [Google Scholar] [CrossRef]
- Vieira, A.C.; Vieira, J.C.; Ferra, J.M.; Magalhães, F.D.; Guedes, R.M.; Marques, A.T. Mechanical study of PLA–PCL fibers during in vitro degradation. J. Mech. Behav. Biomed. 2011, 4, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.C.; Guedes, R.M.; Tita, V. Constitutive modeling of biodegradable polymers: Hydrolytic degradation and time-dependent behavior. Int. J. Solids Struct. 2014, 51, 1164–1174. [Google Scholar] [CrossRef] [Green Version]
- Breche, Q.; Chagnon, G.; Machado, G.; Girarda, E.; Nottelet, B.; Garricc, X.; Faviera, D. Mechanical behaviour’s evolution of a PLA-b-PEG-b-PLA triblock copolymer during hydrolytic degradation. J. Mech. Behav. Biomed. 2016, 60, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Qiu, T.Y.; Song, M.; Zhao, L.G. Testing, characterization and modelling of mechanical behaviour of poly (lactic-acid) and poly (butylene succinate) blends. Mech. Adv. Mater. Mod. Process. 2016, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Guedes, R.M.; Paiva, D.; Magalhães, F.D. Experiment and modelling of the strain-rate-dependent response during in vitro degradation of PLA fbres. SN Appl. Sci. 2020, 2, 177. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.S.; Lopez-Pamies, O.; Kazmi, R. Thermo-mechanical large deformation response and constitutive modeling of viscoelastic polymers over a wide range of strain rates and temperatures. Int. J. Plast. 2006, 22, 581–601. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, G.; Han, X.; Gao, Y.; Zeng, Q.; Hou, B.; Zhang, D. Multiscale modelling for the heterogeneous strength of biodegradable polyesters. J. Mech. Behav. Biomed. 2019, 90, 337–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| PLA | PCL | PBS | PBAT | PHA | TPS | |
|---|---|---|---|---|---|---|
| Density, g/cm3 | 1.21–1.30 | 1.11–1.15 | 1.22–1.26 | 1.26 | 1.18–1.26 | 0.85–1.00 |
| Melting point, °C | 165–170 | 58–65 | 110–115 | 89 | 160–190 | 100–160 * |
| Glass transition, °C | 55–65 | −65–60 | −35–20 | −30–20 | 10–40 | −60–10 |
| Tensile strength, MPa | 30–60 | 20–45 | 20–35 | 15–25 | 30–50 | 0.5–50 |
| Young’s modulus, GPa | 2–4 | 0.2–0.4 | 0.2–0.4 | 0.05–0.10 | 3–4 | 0.05–0.50 |
| Elongation at break, % | 2–10 | 300–1000 | 30–500 | 500–1100 | 4–12 | 10–300 |
| Type of Application | Biopolymer | Nanofiller | References |
|---|---|---|---|
| Packaging | PLA | ZnO | [11,48,49,50,51] |
| PLA | MMT | [17,49] | |
| PLA | Nanocellulose | [48,51,52,53] | |
| PBS | ZnO | [54] | |
| PBS | Nanocellulose | [48,55] | |
| Starch | Ag, ZnO, CuO | [56] | |
| Starch | Nanocellulose | [48] | |
| PCL | ZnO/nanocellulose | [57] | |
| Biomedical applications | PLA | ZnO | [26] |
| PLA | TiO2 | [58] | |
| PLA | Fe3O4 | [47] | |
| Antimicrobial applications | PLA | Ag | [49] |
| PLA | MMT | [1,17] | |
| PBS | ZnO | [16,17] | |
| Cellulose acetate | Cu | [59] | |
| Smart applications | PLA | MWCNT | [18] |
| Poly(d,l-lactide) | Fe3O4 | [19] | |
| PLA/PHBV | MWCNT | [20] |
| BP Matrix | Filler (Content) | Type of Durability Testing | Indicator | Reference |
|---|---|---|---|---|
| PLA | ZnO (0.1, 1 wt.%) | Thermal | Glass trans. temperature | [26] |
| ZnO (1, 2, 3 wt%) | Water absorption | Diffusivity | [11] | |
| CaO, MgO (5 wt%) | Thermal | Pyrolysis | [47] | |
| MMT (5 wt.%) | Microbial | Molecular weight | [17] | |
| CNT (2, 5 wt%) | Thermal | Crystal. temperature | [15] | |
| CNF (1, 5 wt.%) | Hydrothermal | Glass trans. temperature | [13] | |
| CNC (1, 5 wt.%) | Water absorption | Hydrolytic degradation rate | [96] | |
| ZnO: Cu/Ag (0.5–1.5 wt%) | Microbial | SEM images | [50] | |
| Nanoclays (OMMT, HNT, Laponite®, 1, 5 wt.%) | Microbial | CO2 evolution | [97] | |
| SiO2 | Creep tests | Creep resistance | [98] | |
| PBS | ZnO (0.5, 1, 3 wt.%) | Photo-oxidative | Crystal. temperature | [54] |
| ZnO (2–10 wt.%) | Microbial | Inhibition zone diameter | [16] | |
| MMT (0–10 wt.%) | Hydrothermal | Tensile strength and modulus | [95] | |
| GnP | Water absorption | Permeability | [99] | |
| CNF (12–40 wt.%) | Thermal | Crystal. temperature | [55] | |
| PBSA | TiO2 (0.5–1.5 wt.%) | Photo-oxidative | Crystal. temperature | [100] |
| Starch | CNF (5–20 wt.%) | Thermal | Creep resistance | [10] |
| Ag, ZnO, CuO (0.66–3 wt%) | Microbial | SEM images | [56] | |
| Cellulose nanofibres (10 wt.%) | Thermal | Activation energy | [101] | |
| MWCNT (0.005–0.055 wt%)) | Thermal | Glass trans. temperature | [102] | |
| PHB | Bentonite (2–6 wt.%) | Thermal | Crystal. temperature | [103] |
| nAg (0.25–1.25 mM) | Microbial, hydrolytic | SEM, glass trans. temperature | [104] | |
| MMT (1–10 wt.%) | Thermal | Glass trans. temperature | [87] | |
| PCL | Nanoclay (6–26 wt.%) | Thermal | Glass trans. temperature | [105] |
| Nanocellulose/ZnO (2–8 wt.%) | Thermal | Phase trans. temperature | [57] | |
| Bentonite (1.5, 3 wt.%) | Creep | Creep resistance | [22] | |
| MMT, MWCNT, SiO2 (0.5–2.5 wt.%) | Thermal | Activation energy | [106] | |
| GO (0.1 wt%) | Creep | Creep resistance | [107] | |
| Cellulose acetate | Cu (2, 6 mol.%) | Microbial | SEM images | [59] |
| Ag/MMT (3, 5 wt.%) | Microbial, thermal | Inhibition reduction rate, glass trans. temperature | [86] | |
| PVA | CNC/GO/Ag (0.5 wt.%) | Bacterial | Antibacterial efficiency | [108] |
| PLA/PHBV | TiO2 | Thermal | Activation energy | [109] |
| PLA/PBS | CNC (1–3wt.%) | Barrier | Permeability, oxygen transmission rate | [96] |
| PBAT | CNT (1–5wt.%) | Creep and stress relaxation | Creep resistance | [110] |
| PVA/ST/GL | HN (0.25–5 wt.%) | Water absorption | Water solubility, water contact angle | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glaskova-Kuzmina, T.; Starkova, O.; Gaidukovs, S.; Platnieks, O.; Gaidukova, G. Durability of Biodegradable Polymer Nanocomposites. Polymers 2021, 13, 3375. https://doi.org/10.3390/polym13193375
Glaskova-Kuzmina T, Starkova O, Gaidukovs S, Platnieks O, Gaidukova G. Durability of Biodegradable Polymer Nanocomposites. Polymers. 2021; 13(19):3375. https://doi.org/10.3390/polym13193375
Chicago/Turabian StyleGlaskova-Kuzmina, Tatjana, Olesja Starkova, Sergejs Gaidukovs, Oskars Platnieks, and Gerda Gaidukova. 2021. "Durability of Biodegradable Polymer Nanocomposites" Polymers 13, no. 19: 3375. https://doi.org/10.3390/polym13193375