A Novel Trace-Level Ammonia Gas Sensing Based on Flexible PAni-CoFe2O4 Nanocomposite Film at Room Temperature
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Flexible PET-PAni-CoFe2O4 Sensor Films
2.3. Characterization and Gas Detection Measurements
3. Results and Discussion
3.1. Magnetic Properties of CoFe2O4 NPs
3.2. FT-IR Analysis
3.3. XRD Analysis
3.4. Morphological Analysis (FE-SEM)
3.5. TEM Analysis
3.6. Roughness Measurements
3.7. Thermal Analyses
3.7.1. Thermogravimetric Analysis (TGA)
3.7.2. Differential Scanning Calorimetry (DSC)
3.8. XPS Spectra
3.9. Gas Sensing Measurements
3.9.1. Selectivity of PET-PAni-CoFe2O4 Film
3.9.2. Response-Dependent Characteristics of PET-PAni-CoFe2O4 (50%) Film
3.9.3. Reproducibility, Response-Recovery Times and Flexibility of the Sensor
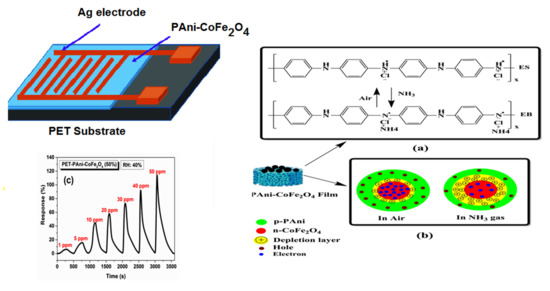
3.9.4. Proposed Mechanism of Gas Sensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Ouyang, J.; Ye, Y.; Li, Z.; Lin, Q.; Chen, T.; Zhang, Z.; Xiang, S. Mixed-valence cobalt (II/III) metal–organic framework for ammonia sensing with naked-eye color switching. ACS Appl. Mater. Interfaces 2018, 10, 27465–27471. [Google Scholar] [CrossRef]
- Wongchoosuk, C.; Jangtawee, P.; Lokavee, P.; Udomrat, S.; Sudkeaw, P.; Kerdcharoen, T. Novel flexible NH3 gas sensor prepared by ink-jet printing technique. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012; Volume 506, pp. 39–42. [Google Scholar]
- Maity, D.; Kumar, R.T.R. Polyaniline anchored MWCNTs on fabric for high performance wearable ammonia sensor. ACS Sens. 2018, 3, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Shen, W.; Lv, D.; Chen, W.; Song, W.; Tan, R. An enhanced flexible room temperature ammonia gas sensor based on GP-PANI/PVDF multi-hierarchical nanocomposite film. Sens. Actuators B Chem. 2021, 334, 129630. [Google Scholar] [CrossRef]
- Moghaddam, H.M.; Malkeshi, H. Self-assembly synthesis and ammonia gas-sensing properties of ZnO/Polythiophene nanofibers. J. Mater. Sci. Mater. Electron. 2016, 27, 8807–8815. [Google Scholar] [CrossRef]
- Jia, X.-S.; Tang, C.-C.; Yan, X.; Yu, G.-F.; Li, J.-T.; Zhang, H.-D.; Li, J.-J.; Gu, C.-Z.; Long, Y.-Z. Flexible polyaniline/poly (methyl methacrylate) composite fibers via electrospinning and in situ polymerization for ammonia gas sensing and strain sensing. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Su, P.-G.; Lee, C.-T.; Chou, C.-Y. Flexible NH3 sensors fabricated by in situ self-assembly of polypyrrole. Talanta 2009, 80, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, J.C.; de Santana Gois, B.H.; Rodrigues de Oliveira, V.J.; da Silva Agostini, D.L.; de Almeida Olivati, C. Gas sensor for ammonia detection based on poly (vinyl alcohol) and polyaniline electrospun. J. Appl. Polym. Sci. 2019, 136, 47288. [Google Scholar] [CrossRef]
- Eising, M.; Cava, C.E.; Salvatierra, R.V.; Zarbin, A.J.G.; Roman, L.S. Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor. Sens. Actuators B Chem. 2017, 245, 25–33. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Q.; Zhou, A.; Huang, Z.; Bai, H.; Li, L. Phase-separated polyaniline/graphene composite electrodes for high-rate electrochemical supercapacitors. Adv. Mater. 2016, 28, 10211–10216. [Google Scholar] [CrossRef]
- Wu, T.; Lv, D.; Shen, W.; Song, W.; Tan, R. Trace-level ammonia detection at room temperature based on porous flexible polyaniline/polyvinylidene fluoride sensing film with carbon nanotube additives. Sens. Actuators B Chem. 2020, 316, 128198. [Google Scholar] [CrossRef]
- Bandgar, D.K.; Navale, S.T.; Naushad, M.; Mane, R.S.; Stadler, F.J.; Patil, V.B. Ultra-sensitive polyaniline–iron oxide nanocomposite room temperature flexible ammonia sensor. RSC Adv. 2015, 5, 68964–68971. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Sharma, G.; Rathore, A.S.; Jha, R. Hypersensitive and selective interferometric nose for ultratrace ammonia detection with fast response utilizing PANI@ SnO2 nanocomposite. ACS Photonics 2018, 5, 4402–4412. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Hasani, A.; Nouri, M.; Mahyari, M.; Salehi, A. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens. Actuators B Chem. 2016, 229, 239–248. [Google Scholar] [CrossRef]
- Liu, C.; Tai, H.; Zhang, P.; Yuan, Z.; Du, X.; Xie, G.; Jiang, Y. A high-performance flexible gas sensor based on self-assembled PANI-CeO2 nanocomposite thin film for trace-level NH3 detection at room temperature. Sens. Actuators B Chem. 2018, 261, 587–597. [Google Scholar] [CrossRef]
- Su, P.-G.; Liao, Z.-H. Fabrication of a flexible single-yarn NH3 gas sensor by layer-by-layer self-assembly of graphene oxide. Mater. Chem. Phys. 2019, 224, 349–356. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, H.; Shi, Y.; Yu, X.; Lan, G. Preparation and Gas Sensing Properties of PANI/SnO2 Hybrid Material. Polymers 2021, 13, 1360. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Jiang, Y.; Duan, Z.; Liu, B.; Zhao, Q.; Wang, S.; Yuan, Z.; Tai, H. Ultrasensitive flexible NH3 gas sensor based on polyaniline/SrGe4O9 nanocomposite with ppt-level detection ability at room temperature. Sens. Actuators B Chem. 2020, 319, 128293. [Google Scholar] [CrossRef]
- Ma, J.; Fan, H.; Li, Z.; Jia, Y.; Yadav, A.K.; Dong, G.; Wang, W.; Dong, W.; Wang, S. Multi-walled carbon nanotubes/polyaniline on the ethylenediamine modified polyethylene terephthalate fibers for a flexible room temperature ammonia gas sensor with high responses. Sens. Actuators B Chem. 2021, 334, 129677. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar]
- Kim, W.C.; Lee, S.W.; Kim, S.J.; Yoon, S.H.; Kim, C.S. Magnetic properties of Y-, La-, Nd-, Gd-, and Bi–doped ultrafine CoFe2O4 spinel grown by using a sol–gel method. J. Magn. Magn. Mater. 2000, 215, 217–220. [Google Scholar] [CrossRef]
- Torkian, S.; Ghasemi, A.; Razavi, R.S. Cation distribution and magnetic analysis of wideband microwave absorptive CoxNi1− xFe2O4 ferrites. Ceram. Int. 2017, 43, 6987–6995. [Google Scholar] [CrossRef]
- Li, X.-H.; Xu, C.-L.; Han, X.-H.; Qiao, L.; Wang, T.; Li, F.-S. Synthesis and magnetic properties of nearly monodisperse CoFe2O4 nanoparticles through a simple hydrothermal condition. Nanoscale Res. Lett. 2010, 5, 1039–1044. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.D.; Hemalatha, J. Enhanced magnetic properties of highly crystalline cobalt ferrite fibers and their application as gas sensors. J. Magn. Magn. Mater. 2019, 484, 225–233. [Google Scholar] [CrossRef]
- Bagade, A.A.; Ganbavle, V.V.; Mohite, S.V.; Dongale, T.D.; Sinha, B.B.; Rajpure, K.Y. Assessment of structural, morphological, magnetic and gas sensing properties of CoFe2O4 thin films. J. Colloid Interface Sci. 2017, 497, 181–192. [Google Scholar] [CrossRef]
- Caldeira, L.E.; Guaglianoni, W.C.; Venturini, J.; Arcaro, S.; Bergmann, C.P.; Bragança, S.R. Sintering-dependent mechanical and magnetic properties of spinel cobalt ferrite (CoFe2O4) ceramics prepared via sol-gel synthesis. Ceram. Int. 2020, 46, 2465–2472. [Google Scholar] [CrossRef]
- Zeng, W.; Li, Y.; Miao, B.; Lin, L.; Wang, Z. Recognition of carbon monoxide with SnO2/Ti thick-film sensor and its gas-sensing mechanism. Sens. Actuators B Chem. 2014, 191, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, W.; Wang, Z. Assembly of 2D nanosheets into 3D flower-like NiO: Synthesis and the influence of petal thickness on gas-sensing properties. Ceram. Int. 2016, 42, 4567–4573. [Google Scholar] [CrossRef]
- Guo, W.; Fu, M.; Zhai, C.; Wang, Z. Hydrothermal synthesis and gas-sensing properties of ultrathin hexagonal ZnO nanosheets. Ceram. Int. 2014, 40, 2295–2298. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, H.; Wang, Z. Effects of different petal thickness on gas sensing properties of flower-like WO3·H2O hierarchical architectures. Appl. Surf. Sci. 2015, 347, 73–78. [Google Scholar] [CrossRef]
- Gonzalez-Sandoval, M.P.; Beesley, A.M.; Miki-Yoshida, M.; Fuentes-Cobas, L.; Matutes-Aquino, J.A. Comparative study of the microstructural and magnetic properties of spinel ferrites obtained by co-precipitation. J. Alloys Compd. 2004, 369, 190–194. [Google Scholar] [CrossRef]
- Geng, L.; Zhao, Y.; Huang, X.; Wang, S.; Zhang, S.; Wu, S. Characterization and gas sensitivity study of polyaniline/SnO2 hybrid material prepared by hydrothermal route. Sens. Actuators B Chem. 2007, 120, 568–572. [Google Scholar] [CrossRef]
- Youssef, A.M.; Mohamed, S.A.; Abdel-Aziz, M.S.; Abdel-Aziz, M.E.; Turky, G.; Kamel, S. Biological studies and electrical conductivity of paper sheet based on PANI/PS/Ag-NPs nanocomposite. Carbohydr. Polym. 2016, 147, 333–343. [Google Scholar] [CrossRef]
- Vivekanandan, J.; Ponnusamy, V.; Mahudeswaran, A.; Vijayanand, P.S. Synthesis, characterization and conductivity study of polyaniline prepared by chemical oxidative and electrochemical methods. Arch. Appl. Sci. Res. 2011, 3, 147–153. [Google Scholar]
- Mu, B.; Zhang, W.; Shao, S.; Wang, A. Glycol assisted synthesis of graphene–MnO 2–polyaniline ternary composites for high performance supercapacitor electrodes. Phys. Chem. Chem. Phys. 2014, 16, 7872–7880. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, T.; Philip, J. A facile approach to synthesis of cobalt ferrite nanoparticles with a uniform ultrathin layer of silicon carbide for organic dye removal. J. Mol. Liq. 2020, 317, 114110. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H.; Zoromba, M.S.; Bassyouni, M.; Zwawi, M.; Alshehri, A.A.; Al-Hossainy, A.F. Synthesis and characterization of Co-Al mixed oxide nanoparticles via thermal decomposition route of layered double hydroxide. J. Mol. Struct. 2020, 1206, 127679. [Google Scholar] [CrossRef]
- Trad, T.M.; Alvarez, R.M.; McCumiskey, E.J.; Taylor, C.R. Capped CoFe2O4 Nanoparticles: Non-Hydrolytic Synthesis, Characterization, and Potential Applications as Magnetic Extractants and in Ferrofluids. Adv. Nanomater. Nanostructures 2011, 229, 155–162. [Google Scholar]
- He, K.; Li, M.; Guo, L. Preparation and photocatalytic activity of PANI-CdS composites for hydrogen evolution. Int. J. Hydrogen Energy 2012, 37, 755–759. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Cheng, G.; Luo, Q.; An, J.; Wang, Y. Preparation of polyaniline-modified TiO2 nanoparticles and their photocatalytic activity under visible light illumination. Appl. Catal. B Environ. 2008, 81, 267–273. [Google Scholar] [CrossRef]
- Kim, K.N.; Jung, H.-R.; Lee, W.-J. Hollow cobalt ferrite–polyaniline nanofibers as magnetically separable visible-light photocatalyst for photodegradation of methyl orange. J. Photochem. Photobiol. A Chem. 2016, 321, 257–265. [Google Scholar] [CrossRef]
- Šuljagić, M.; Vulić, P.; Jeremić, D.; Pavlović, V.; Filipović, S.; Kilanski, L.; Lewinska, S.; Slawska-Waniewska, A.; Milenković, M.R.; Nikolić, A.S. The influence of the starch coating on the magnetic properties of nanosized cobalt ferrites obtained by different synthetic methods. Mater. Res. Bull. 2021, 134, 111117. [Google Scholar] [CrossRef]
- Abou Hammad, A.B.; Abd El-Aziz, M.E.; Hasanin, M.S.; Kamel, S. A novel electromagnetic biodegradable nanocomposite based on cellulose, polyaniline, and cobalt ferrite nanoparticles. Carbohydr. Polym. 2019, 216, 54–62. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, A.; Kumar, V.; Ahlawat, D.S. Structural, thermal and magnetic investigations of cobalt ferrite doped with Zn2+ and Cd2+ synthesized by auto combustion method. J. Magn. Magn. Mater. 2019, 474, 505–511. [Google Scholar] [CrossRef]
- Bhadra, J.; Al-Thani, N.J.; Madi, N.K.; Al-Maadeed, M.A. Effects of aniline concentrations on the electrical and mechanical properties of polyaniline polyvinyl alcohol blends. Arab. J. Chem. 2017, 10, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Miran, M.S.; Rahman, M.M.; Mollah, M.Y.A.; Susan, M. Polyaniline-silica composite materials: Influence of silica content on the thermal and thermodynamic properties. J. Nanostruct. Polym. Nanocompos. 2013, 9, 83–89. [Google Scholar]
- Abdel-Galil, E.A.; Tourky, A.S.; Kasem, A.E. Sorption of some radionuclides from nuclear waste effluents by polyaniline/SiO2 composite: Characterization, thermal stability, and gamma irradiation studies. Appl. Radiat. Isot. 2020, 156, 109009. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. Polyethylene terephthalate (PET) in the packaging industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Wolinska-Grabczyk, A.; Kubica, P.; Jankowski, A. Effect of the acetate group content on gas permeation through membranes based on poly (ethylene-co-vinyl acetate) and its blends. J. Memb. Sci. 2013, 443, 227–236. [Google Scholar] [CrossRef]
- Özen, İ.; Bozoklu, G.; Dalgıçdir, C.; Yücel, O.; Ünsal, E.; Çakmak, M.; Menceloğlu, Y.Z. Improvement in gas permeability of biaxially stretched PET films blended with high barrier polymers: The role of chemistry and processing conditions. Eur. Polym. J. 2010, 46, 226–237. [Google Scholar] [CrossRef]
- Han, M.G.; Im, S.S. X-ray photoelectron spectroscopy study of electrically conducting polyaniline/polyimide blends. Polymer 2000, 41, 3253–3262. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Attia, S.Y.; Barakat, Y.F.; Hassan, H.H.; Zoubi, W. Al Hydrothermal synthesis of α-MnS nanoflakes@ nitrogen and sulfur Co-doped rGO for high-performance hybrid supercapacitor. ChemistrySelect 2018, 3, 6061–6072. [Google Scholar] [CrossRef]
- Zha, D.; Xiong, P.; Wang, X. Strongly coupled manganese ferrite/carbon black/polyaniline hybrid for low-cost supercapacitors with high rate capability. Electrochim. Acta 2015, 185, 218–228. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.; Jang, J. Fabrication of highly flexible, scalable, and high-performance supercapacitors using polyaniline/reduced graphene oxide film with enhanced electrical conductivity and crystallinity. Adv. Funct. Mater. 2014, 24, 2489–2499. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Attia, S.Y.; Hassan, H.H. Spinel-structured FeCo2O4 mesoporous nanosheets as efficient electrode for supercapacitor applications. Microporous Mesoporous Mater. 2017, 251, 26–33. [Google Scholar] [CrossRef]
- WP, W.; Yang, H.; Xian, T.; JL, J. XPS and magnetic properties of CoFe2O4 nanoparticles synthesized by a polyacrylamide gel route. Mater. Trans. 2012, 53, 1586–1589. [Google Scholar]
- Fantauzzi, M.; Secci, F.; Angotzi, M.S.; Passiu, C.; Cannas, C.; Rossi, A. Nanostructured spinel cobalt ferrites: Fe and Co chemical state, cation distribution and size effects by X-ray photoelectron spectroscopy. RSC Adv. 2019, 9, 19171–19179. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.B.; Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Ramgir, N.S.; Patil, V.B. Hybrid polyaniline-WO3 flexible sensor: A room temperature competence towards NH3 gas. Sens. Actuators B Chem. 2019, 288, 279–288. [Google Scholar] [CrossRef]
- Cho, J.-H.; Yu, J.-B.; Kim, J.-S.; Sohn, S.-O.; Lee, D.-D.; Huh, J.-S. Sensing behaviors of polypyrrole sensor under humidity condition. Sens. Actuators B Chem. 2005, 108, 389–392. [Google Scholar] [CrossRef]
- Bissell, R.A.; Persaud, K.C.; Travers, P. The influence of non-specific molecular partitioning of analytes on the electrical responses of conducting organic polymer gas sensors. Phys. Chem. Chem. Phys. 2002, 4, 3482–3490. [Google Scholar] [CrossRef]
- Bandgar, D.K.; Navale, S.T.; Nalage, S.R.; Mane, R.S.; Stadler, F.J.; Aswal, D.K.; Gupta, S.K.; Patil, V.B. Simple and low-temperature polyaniline-based flexible ammonia sensor: A step towards laboratory synthesis to economical device design. J. Mater. Chem. C 2015, 3, 9461–9468. [Google Scholar] [CrossRef]
- Bandgar, D.K.; Navale, S.T.; Navale, Y.H.; Ingole, S.M.; Stadler, F.J.; Ramgir, N.; Aswal, D.K.; Gupta, S.K.; Mane, R.S.; Patil, V.B. Flexible camphor sulfonic acid-doped PAni/α-Fe2O3 nanocomposite films and their room temperature ammonia sensing activity. Mater. Chem. Phys. 2017, 189, 191–197. [Google Scholar] [CrossRef] [Green Version]
- She, C.; Li, G.; Zhang, W.; Xie, G.; Zhang, Y.; Li, L.; Yue, F.; Liu, S.; Jing, C.; Cheng, Y. A flexible polypyrrole/silk-fiber ammonia sensor assisted by silica nanosphere template. Sens. Actuators A Phys. 2021, 317, 112436. [Google Scholar] [CrossRef]
- Li, Z.-F.; Blum, F.D.; Bertino, M.F.; Kim, C.-S.; Pillalamarri, S.K. One-step fabrication of a polyaniline nanofiber vapor sensor. Sens. Actuators B Chem. 2008, 134, 31–35. [Google Scholar] [CrossRef]
- Arora, R.; Srivastav, A.; Mandal, U.K. Polyaniline Based Polymeric Nanocomposite Containing TiO2 and SnO2 for Environmental and Energy Applications. Int. J. Mod. Eng. Res. 2012, 2, 2384–2395. [Google Scholar]
- Patil, U.V.; Ramgir, N.S.; Karmakar, N.; Bhogale, A.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K.; Kothari, D.C. Room temperature ammonia sensor based on copper nanoparticle intercalated polyaniline nanocomposite thin films. Appl. Surf. Sci. 2015, 339, 69–74. [Google Scholar] [CrossRef]















| Material | Substrate | Detection Limit | Response % | Response Time (s) | Flexibility | Ref. |
|---|---|---|---|---|---|---|
| PAni | PET | <5 ppm | 26 (100 ppm) | 33 | - | [61] |
| S, N: GQDs/PAni | PET | 1 ppm | 42.3 (100 ppm) | 115 | The response at bending angle 80° was more than at 0° | [14] |
| MWCNT-PAni | PVDF | 0.1 ppm | 32 (1 ppm) | 76 | Less than 10% deviation after 500 bending cycles @ 60° | [11] |
| PAni-α-Fe2O3 | PET | <2.5 ppm | 72 (100 ppm) | 50 | - | [62] |
| GP-PAni | PVDF | 100 ppb | 60 (1 ppm) | 46 | The reponse decreased from 60 to 49% at 1500 bending cycles | [4] |
| PAni-WO3 | PET | 1 ppm | 121 (100 ppm) | 32 | the response decreased by 9%@60° bending, 900 s | [58] |
| MWCNTs-PAni | Modified PET | 33 ppm | 117 (50 ppm) | 47 | - | [19] |
| PPy | silk | 1 ppm | 73.25 (100 ppm) | 24 | The response decreased by 10.61%@30° after 200 bending cycles | [63] |
| PAni-CoFe2O4 | PET | 25 ppb | 118.3 (50 ppm) | 24.3 | Stable response after 500 bending cycle with 3.4% decrease@60° | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthy, R.D.; Saleh, A. A Novel Trace-Level Ammonia Gas Sensing Based on Flexible PAni-CoFe2O4 Nanocomposite Film at Room Temperature. Polymers 2021, 13, 3077. https://doi.org/10.3390/polym13183077
Alharthy RD, Saleh A. A Novel Trace-Level Ammonia Gas Sensing Based on Flexible PAni-CoFe2O4 Nanocomposite Film at Room Temperature. Polymers. 2021; 13(18):3077. https://doi.org/10.3390/polym13183077
Chicago/Turabian StyleAlharthy, Rima D., and Ahmed Saleh. 2021. "A Novel Trace-Level Ammonia Gas Sensing Based on Flexible PAni-CoFe2O4 Nanocomposite Film at Room Temperature" Polymers 13, no. 18: 3077. https://doi.org/10.3390/polym13183077