A “Wastes-Treat-Wastes” Technology: Role and Potential of Spent Fluid Catalytic Cracking Catalysts Assisted Pyrolysis of Discarded Car Tires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermogravimetric Experiments
2.3. Thermal Analysis Kinetics
3. Results and Discussion
3.1. Thermogravimetric Analysis
3.2. Kinetics Analysis
3.3. Analysis of Catalyst Dosage
3.4. Catalyst Residual Value
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gars, J.; Olovsson, C. Fuel for economic growth? J. Econ. Theory 2019, 184, 104941. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Ming, X.; Jiang, Y.; Hao, J.; Qiao, Y. TG-FTIR and Py-GC / MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energy Convers. Manag. 2018, 175, 288–297. [Google Scholar] [CrossRef]
- Thomas, B.S.; Gupta, R.C. A comprehensive review on the applications of waste tire rubber in cement concrete. Renew. Sustain. Energy Rev. 2016, 54, 1323–1333. [Google Scholar] [CrossRef]
- Koskin, A.P.; Zibareva, I.V.; Vedyagin, A.A. Conversion of Rice Husk and Nutshells into Gaseous, Liquid, and Solid Biofuels. In Biorefinery of Alternative Resources: Targeting Green Fuels and Platform Chemicals; Nanda, S., N. Vo, D.-V., Sarangi, P.K., Eds.; Springer Singapore: Singapore, 2020; pp. 171–194. ISBN 978-981-15-1804-1. [Google Scholar]
- Gao, J.; Zhang, Y.; Meng, D.; Jiao, T.; Qin, X.; Bai, G.; Liang, P. Effect of ash and dolomite on the migration of sulfur from coal pyrolysis volatiles. J. Anal. Appl. Pyrolysis 2019, 140, 349–354. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, M.; Chang, G.; Hu, X.; Guo, Q. A composite obtained from waste automotive plastics and sugarcane skin flour: Mechanical properties and thermo-chemical analysis. Powder Technol. 2019, 347, 27–34. [Google Scholar] [CrossRef]
- Guo, Q.; Yue, X.; Wang, M.; Liu, Y. Pyrolysis of scrap printed circuit board plastic particles in a fluidized bed. Powder Technol. 2010, 198, 422–428. [Google Scholar] [CrossRef]
- Cao, X.; Li, L.; Shitao, Y.; Liu, S.; Hailong, Y.; Qiong, W.; Ragauskas, A.J. Catalytic conversion of waste cooking oils for the production of liquid hydrocarbon biofuels using in-situ coating metal oxide on SBA-15 as heterogeneous catalyst. J. Anal. Appl. Pyrolysis 2019, 138, 137–144. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Li, H.; Yu, S.; Liu, S.; Yu, H.; Ge, X. SO42−/ZrO2 as catalyst for upgrading of pyrolysis oil by esterification. Fuel 2018, 226, 190–194. [Google Scholar] [CrossRef]
- Li, L.; Ding, Z.; Li, K.; Xu, J.; Liu, F.; Liu, S.; Yu, S.; Xie, C.; Ge, X. Liquid hydrocarbon fuels from catalytic cracking of waste cooking oils using ultrastable zeolite USY as catalyst. J. Anal. Appl. Pyrolysis 2016, 117, 268–272. [Google Scholar] [CrossRef]
- Li, L.; Quan, K.; Xu, J.; Liu, F.; Liu, S.; Yu, S.; Xie, C.; Zhang, B.; Ge, X. Liquid hydrocarbon fuels from catalytic cracking of rubber seed oil using USY as catalyst. Fuel 2014, 123, 189–193. [Google Scholar] [CrossRef]
- Zhong, W.; Guo, Q.; Wang, X.; Zhang, L. Catalytic hydroprocessing of fast pyrolysis bio-oil from Chlorella. J. Fuel Chem. Technol. 2013, 41, 571–578. [Google Scholar] [CrossRef]
- Qi, P.; Chang, G.; Wang, H.; Zhang, X.; Guo, Q. Production of aromatic hydrocarbons by catalytic co-pyrolysis of microalgae and polypropylene using HZSM-5. J. Anal. Appl. Pyrolysis 2018, 136, 178–185. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [Green Version]
- Doğan, O.; Celik, M.B.; Özdalyan, B. The effect of tire derived fuel/diesel fuel blends utilization on diesel engine performance and emissions. Fuel 2012, 95, 340–346. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.; Duan, P.; Wang, F.; Xu, Z. Thermo-chemical conversion of scrap tire waste to produce gasoline fuel. Waste Manag. 2019, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Elbaba, I.F.; Williams, P.T. High yield hydrogen from the pyrolysis–catalytic gasification of waste tyres with a nickel/dolomite catalyst. Fuel 2013, 106, 528–536. [Google Scholar] [CrossRef]
- İlkılıç, C.; Aydın, H. Fuel production from waste vehicle tires by catalytic pyrolysis and its application in a diesel engine. Fuel Process. Technol. 2011, 92, 1129–1135. [Google Scholar] [CrossRef]
- Yuwapornpanit, R.; Jitkarnka, S. Cu-doped catalysts and their impacts on tire-derived oil and sulfur removal. J. Anal. Appl. Pyrolysis 2015, 111, 200–208. [Google Scholar] [CrossRef]
- Muenpol, S.; Jitkarnka, S. Effects of Fe supported on zeolites on structures of hydrocarbon compounds and petrochemicals in waste tire-derived pyrolysis oils. J. Anal. Appl. Pyrolysis 2016, 117, 147–156. [Google Scholar] [CrossRef]
- Kordoghli, S.; Khiari, B.; Paraschiv, M.; Zagrouba, F.; Tazerout, M. Production of hydrogen and hydrogen-rich syngas during thermal catalytic supported cracking of waste tyres in a bench-scale fixed bed reactor. Int. J. Hydrogen Energy 2019, 44, 11289–11302. [Google Scholar] [CrossRef]
- Li, Y.; Paranthaman, M.P.; Akato, K.; Naskar, A.K.; Levine, A.M.; Lee, R.J.; Kim, S.-O.; Zhang, J.; Dai, S.; Manthiram, A. Tire-derived carbon composite anodes for sodium-ion batteries. J. Power Sources 2016, 316, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.T.; Brindle, A.J. Catalytic pyrolysis of tyres: Influence of catalyst temperature. Fuel 2002, 81, 2425–2434. [Google Scholar] [CrossRef]
- Li, L.; Quan, K.; Xu, J.; Liu, F.; Liu, S.; Yu, S.; Xie, C.; Ge, X. Preparation of basic mesoporous molecular sieves K2O/Mg-MCM-41 and its catalytic performance on the cracking of soybean oils. J. Anal. Appl. Pyrolysis 2014, 110, 313–317. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Tian, A.; Guo, Q.; Huang, K. Influence of CaO and HZSM-5 on oxygen migration in Chlorella vulgaris polysaccharide pyrolysis. Carbon Resour. Convers. 2019, 2, 111–116. [Google Scholar] [CrossRef]
- Xie, C.; Liu, F.; Yu, S.; Xie, F.; Li, L.; Zhang, S.; Yang, J. Catalytic cracking of polypropylene into liquid hydrocarbons over Zr and Mo modified MCM-41 mesoporous molecular sieve. Catal. Commun. 2008, 10, 79–82. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Li, H.; Yu, S.; Ge, X. Decreasing the acid value of pyrolysis oil via esterification using ZrO2/SBA-15 as a solid acid catalyst. Renew. Energy 2020, 146, 643–650. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Pyrolysis–gasification of post-consumer municipal solid plastic waste for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 949–957. [Google Scholar] [CrossRef]
- Namchot, W.; Jitkarnka, S. Catalytic pyrolysis of waste tire using HY/MCM-41 core-shell composite. J. Anal. Appl. Pyrolysis 2016, 121, 297–306. [Google Scholar] [CrossRef]
- Elbaba, I.F.; Wu, C.; Williams, P.T. Hydrogen production from the pyrolysis–gasification of waste tyres with a nickel/cerium catalyst. Int. J. Hydrogen Energy 2011, 36, 6628–6637. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Krzyżyńska, R.; Jouhara, H.; Spencer, N. Use of pyrolytic gas from waste tire as a fuel: A review. Energy 2017, 134, 1121–1131. [Google Scholar] [CrossRef]
- Wang, S.; (Max) Lu, G.Q. Role of CeO2 in Ni/CeO2–Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. B Environ. 1998, 19, 267–277. [Google Scholar] [CrossRef]
- Rodríguez, E.; Félix, G.; Ancheyta, J.; Trejo, F. Modeling of hydrotreating catalyst deactivation for heavy oil hydrocarbons. Fuel 2018, 225, 118–133. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A.; Furimsky, E. Handbook of Spent Hydroprocessing Catalysts: Second Edition. Handb. Spent Hydroprocessing Catal. Second Ed. 2017, 2017, 451–452. [Google Scholar] [CrossRef]
- Pathak, A.; Srichandan, H.; Kim, D.J. Column bioleaching of metals from refinery spent catalyst by Acidithiobacillus thiooxidans: Effect of operational modifications on metal extraction, metal precipitation, and bacterial attachment. J. Environ. Manage. 2019, 242, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhong, H.; Yang, Q.; Yao, E.; Zhang, Y.; Zou, H. Studying the mechanisms of natural rubber pyrolysis gas generation using RMD simulations and TG-FTIR experiments. Energy Convers. Manag. 2019, 189, 143–152. [Google Scholar] [CrossRef]
- Grieco, E.; Bernardi, M.; Baldi, G. Styrene-butadiene rubber pyrolysis: Products, kinetics, modelling. J. Anal. Appl. Pyrolysis 2008, 82, 304–311. [Google Scholar] [CrossRef]
- Cao, L.; Sinha, T.K.; Tao, L.; Li, H.; Zong, C.; Kim, J.K. Synergistic reinforcement of silanized silica-graphene oxide hybrid in natural rubber for tire-tread fabrication: A latex based facile approach. Compos. Part B Eng. 2019, 161, 667–676. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; Wang, C.; He, A. Data on the evolution of curing characteristics and properties during the room-temperature annealing process in SSBR/BR gums and SSBR/BR/SiO2 composites. Data Br. 2019, 27, 104660. [Google Scholar] [CrossRef]
- Kordoghli, S.; Paraschiv, M.; Kuncser, R.; Tazerout, M.; Zagrouba, F. Catalysts’ influence on thermochemical decomposition of waste tires. Environ. Prog. Sustain. Energy 2017, 36, 1560–1567. [Google Scholar] [CrossRef]
- Demirbas, A. Determination of calorific values of bio-chars and pyro-oils from pyrolysis of beech trunkbarks. J. Anal. Appl. Pyrolysis 2004, 72, 215–219. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Hilal DemirbaŞ, A. Yields and heating values of liquids and chars from spruce trunkbark pyrolysis. Energy Sources 2005, 27, 1367–1373. [Google Scholar] [CrossRef]
- Fernandez-Lopez, M.; Pedrosa-Castro, G.J.; Valverde, J.L.; Sanchez-Silva, L. Kinetic analysis of manure pyrolysis and combustion processes. Waste Manag. 2016, 58, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Starink, M.J. A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim. Acta 1996, 288, 97–104. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, B.; Tian, X.; Wang, K.; Tian, Z.; Han, W.; Bian, H. Study on the pyrolysis kinetics and mechanisms of the tread compounds of silica-filled discarded car tires. Polymers 2020, 12, 810. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.J.; Chen, Y.W. Chiuping-Li The interaction of vanadium and nickel in USY zeolite. Zeolites 1995, 15, 77–82. [Google Scholar] [CrossRef]
- Matar, S.; Hatch, L.F. Synthetic Petroleum-Based Polymers. Chem. Petrochem. Processes 2001, 3, 323–373. [Google Scholar]
- Kan, T.; Strezov, V.; Evans, T. Fuel production from pyrolysis of natural and synthetic rubbers. Fuel 2017, 191, 403–410. [Google Scholar] [CrossRef]
- Thakar, N.; Schildhauer, T.J.; Buijs, W.; Kapteijn, F.; Moulijn, J.A. Evaluation of deactivation mechanisms of Pd-catalyzed hydrogenation of 4-isobutylacetophenone. J. Catal. 2007, 248, 249–257. [Google Scholar] [CrossRef]
- Boxiong, S.; Chunfei, W.; Cai, L.; Binbin, G.; Rui, W. Pyrolysis of waste tyres: The influence of USY catalyst/tyre ratio on products. J. Anal. Appl. Pyrolysis 2007, 78, 243–249. [Google Scholar] [CrossRef]
- Shen, B.; Wu, C.; Wang, R.; Guo, B.; Liang, C. Pyrolysis of scrap tyres with zeolite USY. J. Hazard. Mater. 2006, 137, 1065–1073. [Google Scholar] [CrossRef]
- Nishikawa, J.; Nakamura, K.; Asadullah, M.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Catalytic performance of Ni/CeO2/Al2O3 modified with noble metals in steam gasification of biomass. Catal. Today 2008, 131, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Mortola, V.B.; Damyanova, S.; Zanchet, D.; Bueno, J.M.C. Surface and structural features of Pt/CeO2-La2O3-Al2O3 catalysts for partial oxidation and steam reforming of methane. Appl. Catal. B Environ. 2011, 107, 221–236. [Google Scholar] [CrossRef]
- Le, T.; Wang, Q.; Ravindra, A.V.; Li, X.; Ju, S. Microwave intensified synthesis of Zeolite-Y from spent FCC catalyst after acid activation. J. Alloys Compd. 2019, 776, 437–446. [Google Scholar] [CrossRef]
- Domingues, C.; Correia, M.J.N.; Carvalho, R.; Henriques, C.; Bordado, J.; Dias, A.P.S. Vanadium phosphate catalysts for biodiesel production from acid industrial by-products. J. Biotechnol. 2013, 164, 433–440. [Google Scholar] [CrossRef] [PubMed]
- El-Akkad, T.M.; Khalil, A.M.; Attia, G.; Nashed, S. Surface and structural properties of 3, 5 and 10A synthetic zeolites. Thermochim. Acta 1982, 52, 19–30. [Google Scholar] [CrossRef]
- Tian, H.; Huang, C.; Fan, Z. Metals on a Novel USY Zeolite after Hydrothermal Aging. In Catalyst Deactivation 2001; Spivey, J.J., Roberts, G.W., Davis, B.H.B.T.-S., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 139, pp. 351–358. ISBN 0167-2991. [Google Scholar]


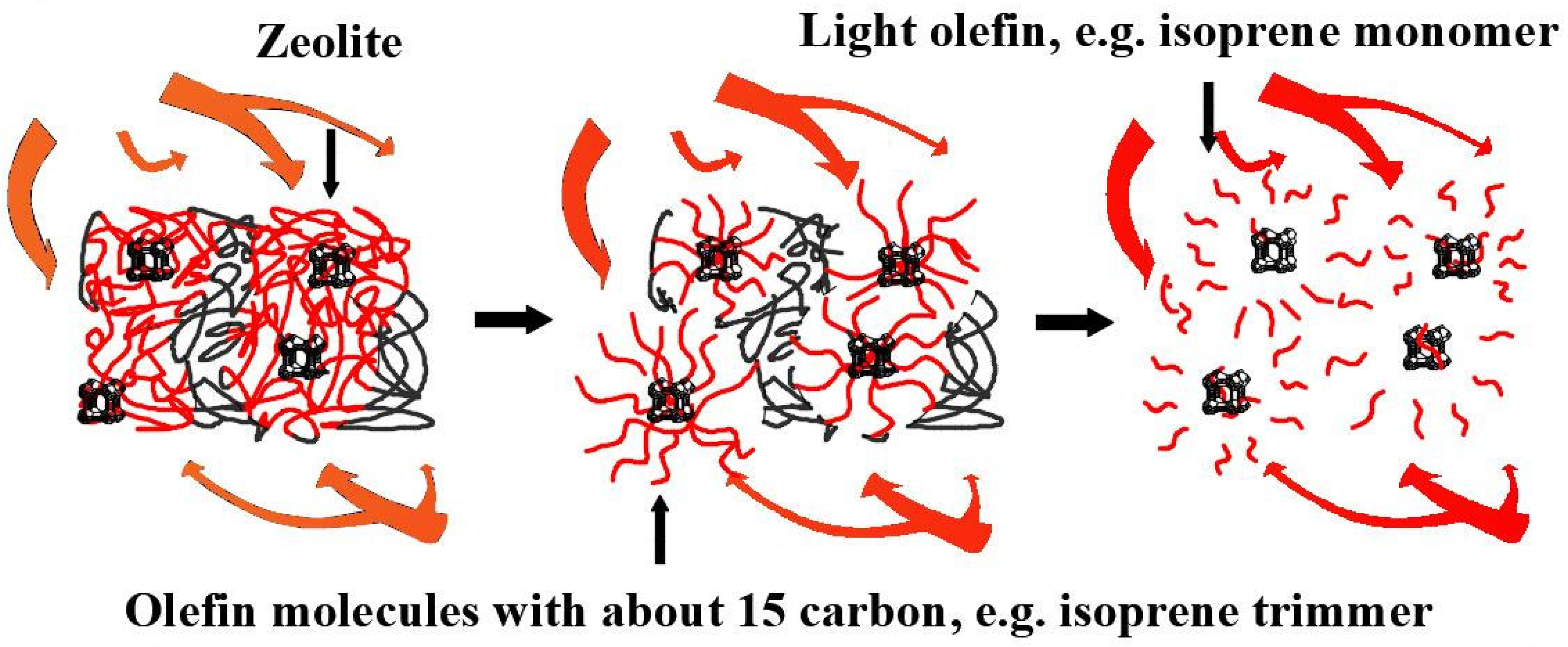

| Tread Rubber | Mixed Ingredients (PHR) | |||||||||||
| SBR | NR | TSR20 | Silica | V700 | N234 | Antilux | ||||||
| 15 | 105 | 20 | 70 | 13 | 18 | 3 | ||||||
| Si69 | SAD | DPG | ZnO | S | CZ | Antioxidant 4020 | ||||||
| 13 | 3 | 1 | 2 | 1.15 | 2 | 2 | ||||||
| Inner liner | Mixed ingredients (PHR) | |||||||||||
| BR9000 | SBR | TSR20 | N234 | TDAE | RD | |||||||
| 15 | 15 | 70 | 45 | 3 | 1.5 | |||||||
| SAD | ZnO | Antilux | 6PPD | CBS | S | |||||||
| 3 | 3.5 | 1.5 | 2 | 1.5 | 1.8 | |||||||
| Component | Content (wt.%) | Component | Content (wt.%) |
|---|---|---|---|
| Na2O | 0.226 | CaO | 0.7 |
| MgO | 0.42 | TiO2 | 0.25 |
| Al2O3 | 51.7 | V2O5 | 0.246 |
| SiO2 | 38.4 | Fe2O3 | 1.12 |
| P2O5 | 0.55 | NiO | 0.854 |
| SO3 | 0.832 | Sb2O3 | 0.245 |
| K2O | 0.252 | La2O3 | 2.73 |
| Material | β/oC·min−1 | W/mg | WSFCC/mg | Te/oC | Tf/oC | (dα/dt)max/% K−1 | Tmax/oC | Wf/% |
|---|---|---|---|---|---|---|---|---|
| Inner liner | 10 | 15.4 | 0 | 361.2 | 441.6 | 6.88 | 421.7 | 45.81 |
| 15.7 | 4.55 | 360.1 | 441.0 | 6.84 | 421.9 | 44.83 | ||
| 15 | 15.42 | 0 | 362.7 | 448.1 | 9.81 | 426.8 | 45.73 | |
| 15.22 | 4.62 | 363.2 | 448.6 | 10.59 | 424.1 | 44.79 | ||
| 20 | 15.35 | 0 | 365.5 | 452.1 | 12.94 | 428.4 | 45.69 | |
| 15.7 | 4.62 | 368.3 | 453.0 | 13.33 | 433.2 | 45.00 | ||
| 25 | 15.5 | 0 | 367.8 | 455.3 | 15.65 | 435.5 | 45.93 | |
| 15.21 | 4.65 | 370.9 | 457.1 | 16.24 | 435.4 | 45.00 | ||
| Tread rubber | 10 | 15.62 | 0 | 362.3 | 469.7 | 5.68 | 432.2 | 35.86 |
| 15.62 | 4.56 | 363.7 | 469.9 | 5.62 | 433.1 | 35.19 | ||
| 15 | 15.01 | 0 | 362.6 | 476.4 | 8.72 | 434.8 | 35.74 | |
| 15.06 | 4.46 | 369.5 | 477.3 | 8.73 | 443.0 | 35.17 | ||
| 20 | 15.07 | 0 | 366.4 | 481.1 | 10.35 | 450.3 | 35.57 | |
| 15.49 | 4.72 | 371.9 | 482.8 | 11.12 | 444.3 | 35.24 | ||
| 25 | 15.02 | 0 | 369.4 | 485.0 | 13.34 | 449.8 | 35.88 | |
| 15.46 | 4.42 | 374.2 | 486.5 | 15.41 | 456.1 | 35.65 |
| Experiment | No. | Matches Name | CAS Number | Molecular Formula | Proportion |
|---|---|---|---|---|---|
| Pyrolysis | 1 | 1-Methylcyclopropene | 003100-04-7 | C4H6 | 48.07% |
| 2 | 1,3-Butadiene, 2-methyl- | 000078-79-5 | C5H8 | 19.34% | |
| 3 | Cyclopropane, 1,1-dimethyl- | 001630-94-0 | C5H10 | 14.08% | |
| 4 | 1-Butene, 3-methyl- | 000563-45-1 | C5H10 | 4.29% | |
| 5 | Propene | 000115-07-1 | C3H6 | 2.04% | |
| 6 | Isobutane | 000075-28-5 | C4H10 | 1.31% | |
| 7 | Cyclopentene | 000142-29-0 | C5H8 | 1.13% | |
| 8 | 2-Pentene, 4-methyl-, (Z)- | 000691-38-3 | C6H12 | 1.07% | |
| ⋯ | ⋯ | ⋯ | ⋯ | ||
| FCC catalytic pyrolysis | 1 | 1-Propene, 2-methyl- | 000115-11-7 | C4H8 | 65.59% |
| 2 | Propylene | 000124-38-9 | C3H6 | 18.11% | |
| 3 | Cyclopropane, 1,1-dimethyl- | 001630-94-0 | C5H10 | 3.70% | |
| 4 | 2-Pentene, (E)- | 000646-04-8 | C5H10 | 2.45% | |
| 5 | 1,4-Pentadiene | 000591-93-5 | C5H8 | 2.16% | |
| 6 | Isobutane | 000075-28-5 | C4H10 | 1.68% | |
| ⋯ | ⋯ | ⋯ | ⋯ |
| Testing Content | BET Surface Area (m2/g) | Matrix Surface Area (m2/g) | Micropore Surface Area (m2/g) | Total Pore Volume (cc/g) | Micropore Volume (cc/g) |
|---|---|---|---|---|---|
| Before the experiment | 129 | 60 | 69 | 0.154 | 0.031 |
| After the experiment | 104 | 52 | 52 | 0.128 | 0.025 |
| Inner Liner | Tread Rubber | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
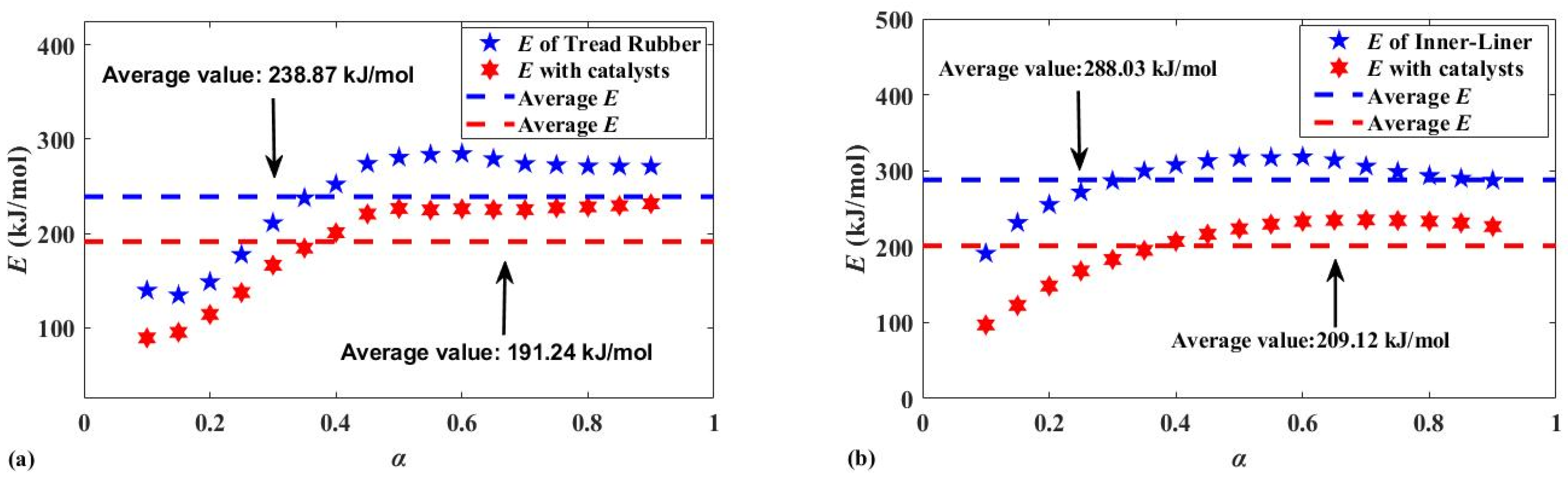
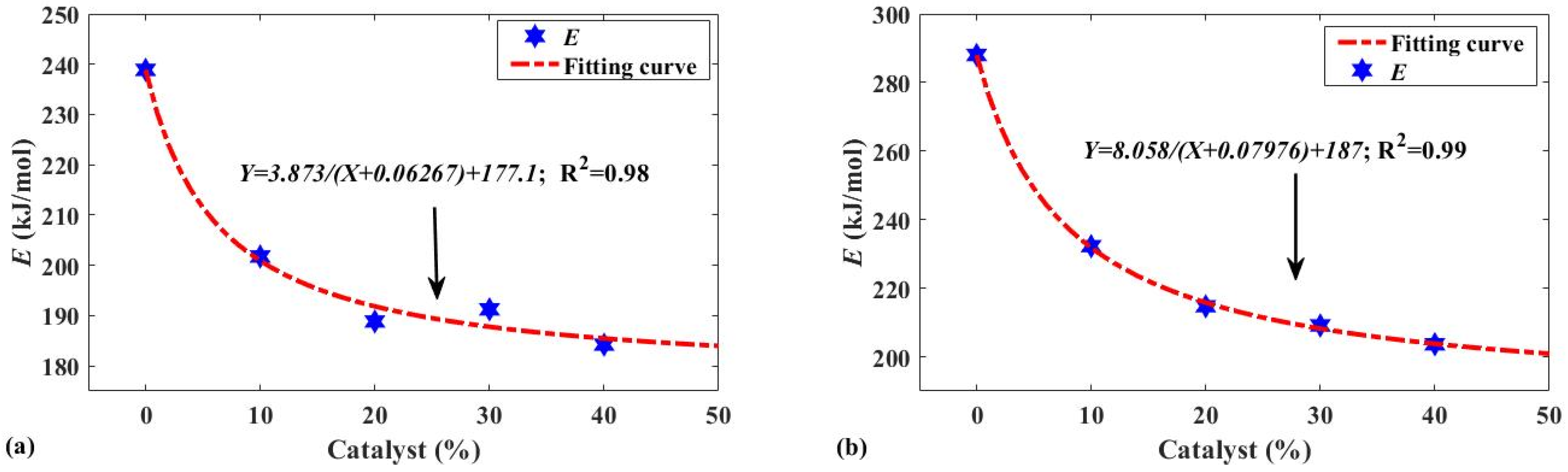
| Wt/% | 0 | 10 | 20 | 30 | 40 | 0 | 10 | 20 | 30 | 40 |
| E/kJ·mol−1 | 288 | 232.3 | 214.7 | 209.1 | 203.7 | 238.9 | 201.8 | 188.8 | 191.3 | 184.2 |
| Fitting function | Y = a/(X + b) + c | |||||||||
| Fitting result | Y = 8.058/(X + 0.0776) + 187 | Y = 3.873/(X + 0.06267) + 177.1 | ||||||||
| R2 | 0.99 | 0.98 | ||||||||
| No. of Experiment | Rubber | WR/mg | Catalyst | WC/mg | TH/°C | E/kJ·mol−1 |
|---|---|---|---|---|---|---|
| 1 | Inner liner | 15.44 | 288.03 | |||
| 2 | 15.34 | I | 4.60 | 209.19 | ||
| 3 | 15.67 | I | 4.64 | 1000 | 287.03 | |
| 4 | 15.54 | II | 4.59 | 257.39 | ||
| 5 | 15.11 | II | 4.58 | 1000 | 279.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Wang, C.; Bian, H. A “Wastes-Treat-Wastes” Technology: Role and Potential of Spent Fluid Catalytic Cracking Catalysts Assisted Pyrolysis of Discarded Car Tires. Polymers 2021, 13, 2732. https://doi.org/10.3390/polym13162732
Zhao B, Wang C, Bian H. A “Wastes-Treat-Wastes” Technology: Role and Potential of Spent Fluid Catalytic Cracking Catalysts Assisted Pyrolysis of Discarded Car Tires. Polymers. 2021; 13(16):2732. https://doi.org/10.3390/polym13162732
Chicago/Turabian StyleZhao, Baishun, Chuansheng Wang, and Huiguang Bian. 2021. "A “Wastes-Treat-Wastes” Technology: Role and Potential of Spent Fluid Catalytic Cracking Catalysts Assisted Pyrolysis of Discarded Car Tires" Polymers 13, no. 16: 2732. https://doi.org/10.3390/polym13162732







