Effect of Relative Humidity on the Electrospinning Performance of Regenerated Silk Solution
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Regenerated Silk
2.2. Electrospinning of Regenerated Silk Solution
2.3. Measurement and Characterization
3. Results and Discussion
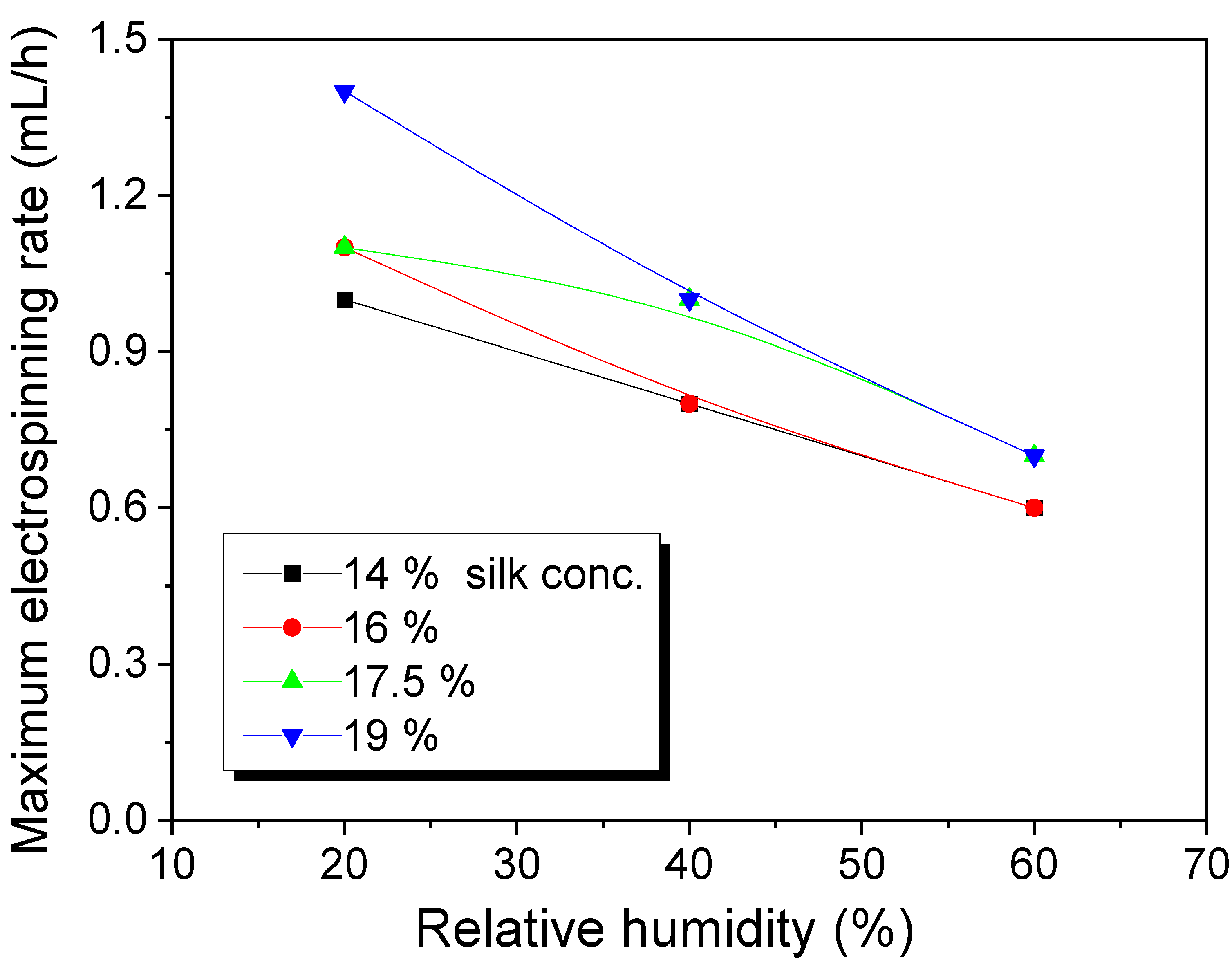
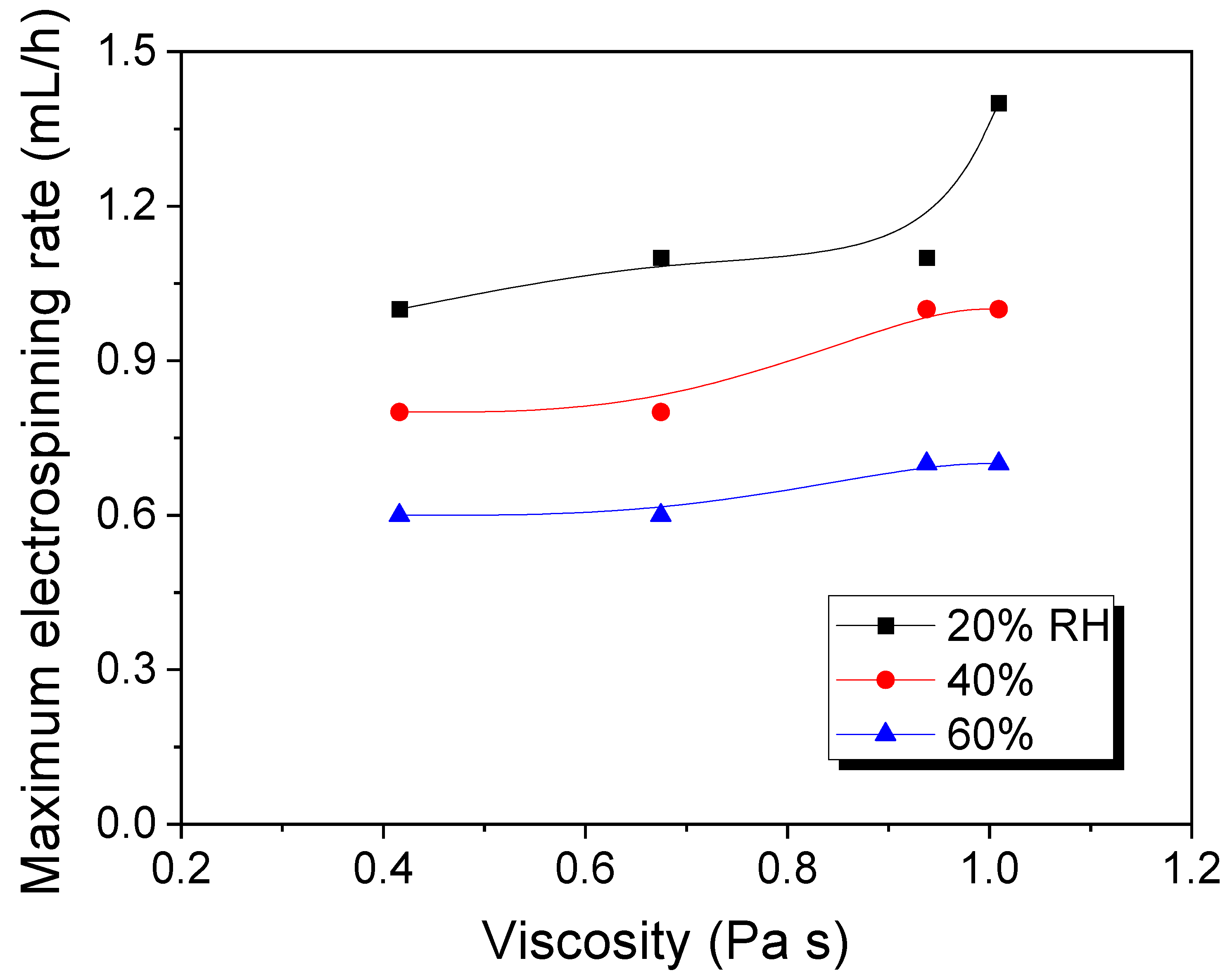
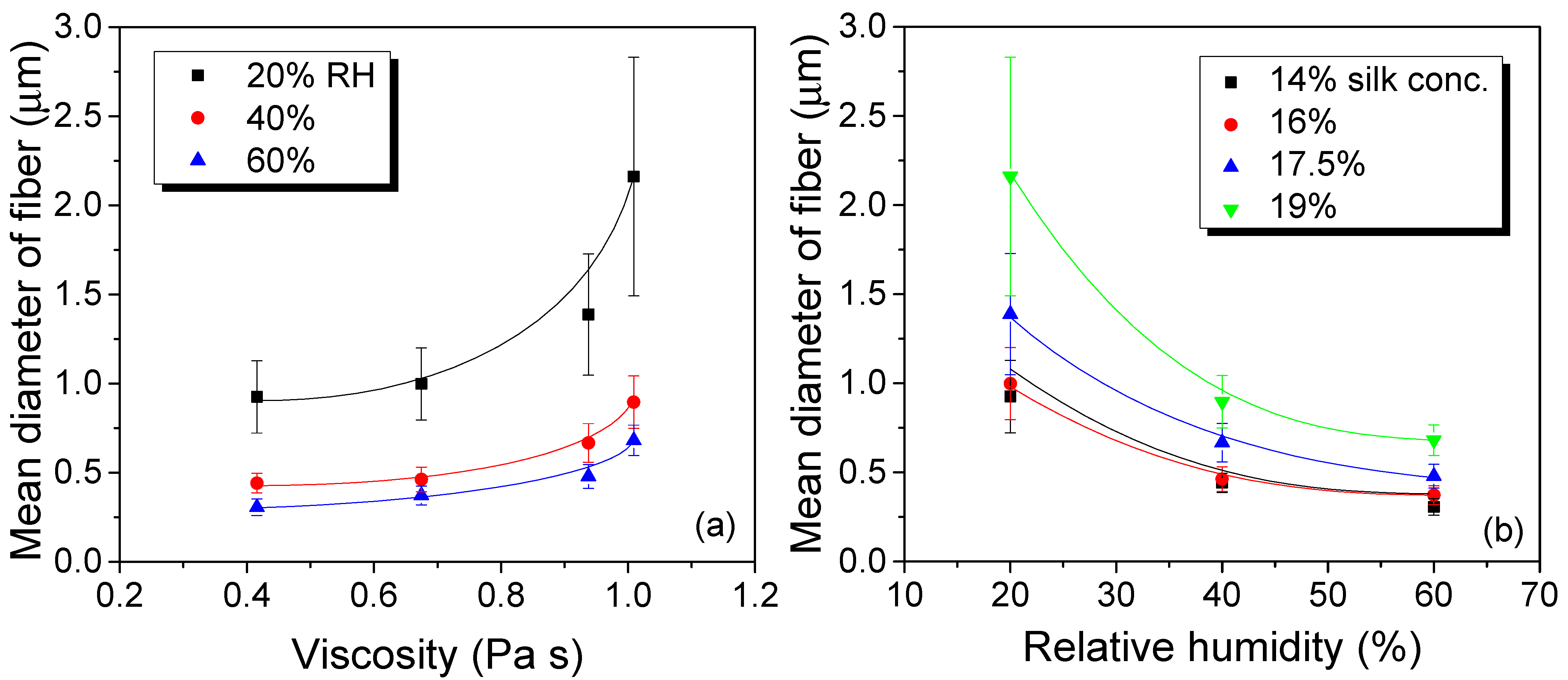
3.1. Effects of Relative Humidity on the Maximum Electrospinning Rate and the Electrospun Morphology of Silk
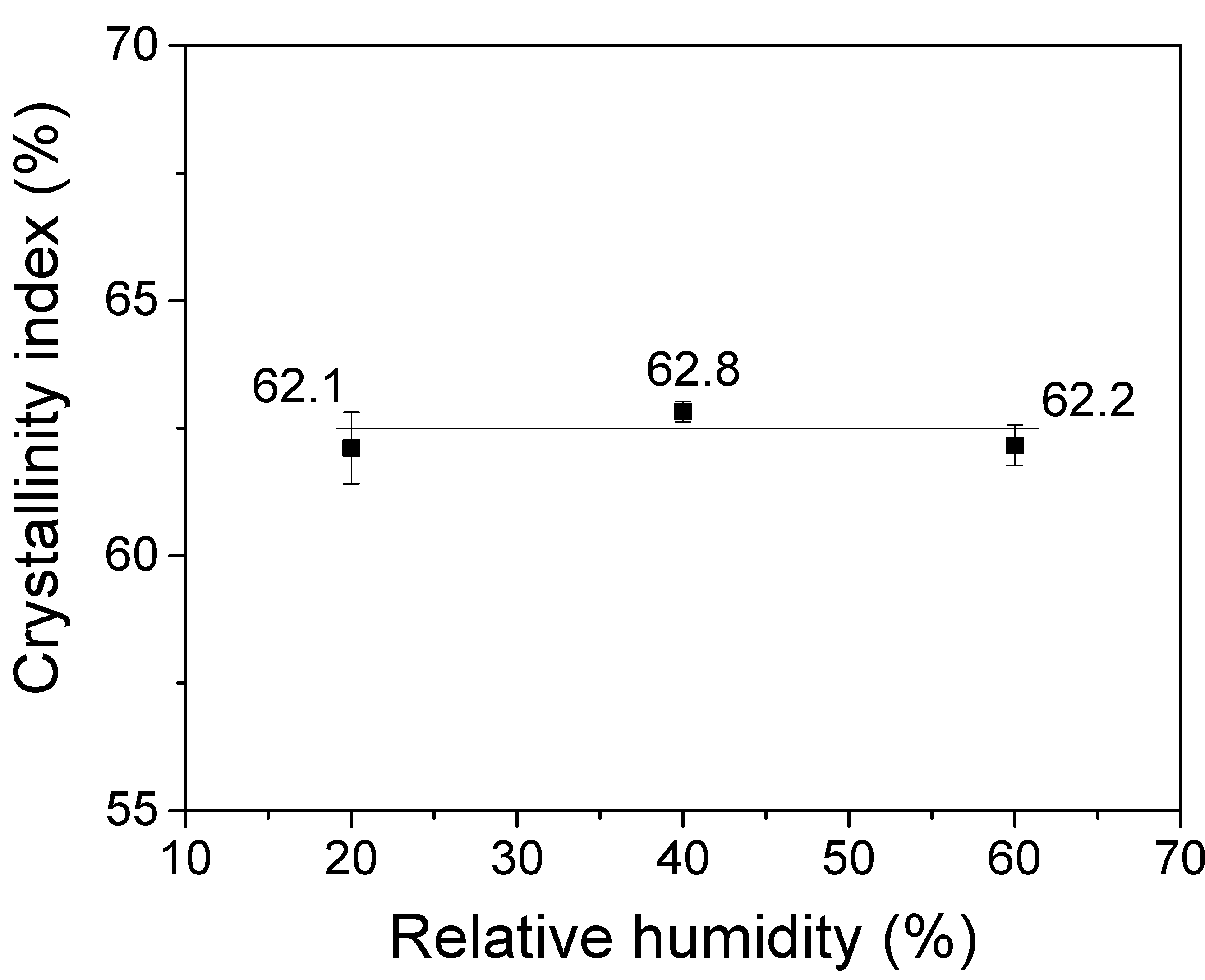
3.2. Effects of Relative Humidity on the Molecular Conformation of Electrospun Silk Web
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Um, I.C.; Kweon, H.Y.; Min, B.G.; Park, Y.H. Structural characteristics and properties of silk fibroin/polyurethane blend films. Int. J. Ind. Entomol. 2002, 5, 163–170. [Google Scholar]
- Sakabe, H.; Ito, H.; Miyamoto, T.; Noishiki, Y.; Ha, W.S. In vivo blood compatibility of regenerated silk fibroin. Seni Gakkaishi 1989, 45, 487–490. [Google Scholar] [CrossRef] [Green Version]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Kirker-Head, C.; McCool, J.; Gronowicz, G.; Zichner, L.; Langer, R.; Vunjak-Novakovic, G.; Kaplan, D.L. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005, 26, 147–155. [Google Scholar] [CrossRef]
- Minoura, N.; Aiba, S.I.; Gotoh, Y.; Tsukada, M.; Imai, Y.J. Attachment and growth of cultured fibroblast cells on silk protein matrices. J. Biomed. Mater. Res. 1995, 29, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.H.; Park, C.H.; Seo, J.N.; Kweon, H.Y.; Kang, S.W.; Lee, K.G. Comparison of methods for the repair of acute tympanic membrane perforations: Silk patch vs. paper patch. Wound Repair Regen. 2010, 18, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.J.; Lee, J.M.; Kim, J.H.; Kim, J.; Kweon, H.Y.; Jo, Y.Y.; Park, C.H. Biodegradation behavior of silk fibroin membranes in repairing tympanic membrane perforations. J. Biomed. Mater. Res. Part A 2012, 100, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef]
- Liu, R.; Ming, J.; Zhang, H.; Zuo, B. EDC/NHS crosslinked electrospun regenerated tussah silk fibroin nanofiber mats. Fiber. Polym. 2012, 13, 613–617. [Google Scholar] [CrossRef]
- Kim, U.J.; Park, J.H.; Kim, H.J.; Wada, M.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef]
- Ki, C.S.; Park, S.Y.; Kim, H.J.; Jung, H.M.; Woo, K.M.; Lee, J.W.; Park, Y.H. Development of 3-D nanofibrous fibroin scaffold with high porosity by electrospinning: Implications for bone regeneration. Biotechnol. Lett. 2008, 30, 405–410. [Google Scholar] [CrossRef]
- Uttayarat, P.; Jetawattana, S.; Suwanmala, P.; Eamsiri, J.; Tangthong, T.; Pongpat, S. Antimicrobial electrospun silk fibroin mats with silver nanoparticles for wound dressing application. Fiber. Polym. 2012, 13, 999–1006. [Google Scholar] [CrossRef]
- Schneider, A.; Wang, X.Y.; Kaplan, D.L.; Garlick, J.A.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar] [CrossRef] [Green Version]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Um, I.C.; Fang, D.; Hsiao, B.S.; Okamoto, A.; Chu, B. Electro-spinning and electro-blowing of hyaluronic acid. Biomacromolecules 2004, 5, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Um, I.C.; Han, S.M.; Han, S.E. Electrospinning to surpass white natural silk in sunlight rejection for radiative cooling. Adv. Photonics Res. 2021, 2, 2100008. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Wu, S.; Jaffe, M.; Arinzeh, T.L. Structural changes in PVDF fibers due to electrospinning and its effect on biological function. Biomed. Mater. 2013, 8, 045007. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Teo, W.E.; Zhu, X.; Beuerman, R.W.; Ramakrishna, S.; Yung, L.Y.L. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J. Biomed. Mater. Res. Part A 2006, 79, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Sukigara, S.; Gandhi, M.; Ayutsede, J.; Micklus, M.; Ko, F. Regeneration of Bombyx mori silk by electrospinning—Part 1: Processing parameters and geometric properties. Polymer 2003, 44, 5721–5727. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Shao, H.; Hu, X. Electrospinning and rheology of regenerated Bombyx mori silk fibroin aqueous solutions: The effects of pH and concentration. Polymer 2008, 49, 2880–2885. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, C.; Ma, X. A novel three-dimensional tubular scaffold prepared from silk fibroin by electrospinning. Int. J. Biol. Macromol. 2009, 45, 504–510. [Google Scholar] [CrossRef]
- Amiraliyan, N.; Nouri, M.; Kish, M.H. Effects of some electrospinning parameters on morphology of natural silk-based nanofibers. J. Appl. Polym. Sci. 2009, 113, 226–234. [Google Scholar] [CrossRef]
- Yoon, K.; Lee, H.N.; Ki, C.S.; Fang, D.; Hsiao, B.S.; Chu, B.; Um, I.C. Effects of degumming conditions on electro-spinning rate of regenerated silk. Int. J. Biol. Macromol. 2013, 61, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Um, I.C. Effects of electric field on the maximum electro-spinning rate of silk fibroin solutions. Int. J. Biol. Macromol. 2017, 95, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Um, I.C. Effect of molecular weight on electro-spinning performance of regenerated silk. Int. J. Biol. Macromol. 2018, 106, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.S.; Yoon, K.H.; Ki, C.S.; Kim, H.J.; Bae, D.G.; Lee, K.H.; Park, Y.H.; Um, I.C. Effect of degumming condition on the solution properties and electrospinnablity of regenerated silk solution. Int. J. Biol. Macromol. 2013, 55, 161–168. [Google Scholar] [CrossRef]
- Ko, J.S.; Ki, C.S.; Um, I.C. Effect of sericin content on the structural characteristics and properties of electro-spun regenerated silk. Fiber. Polym. 2018, 19, 507–514. [Google Scholar] [CrossRef]
- Cho, H.J.; Yoo, Y.J.; Kim, J.W.; Park, Y.H.; Bae, D.G.; Um, I.C. Effect of molecular weight and storage time on the wet-and electro-spinning of regenerated silk fibroin. Polym. Degrad. Stab. 2012, 97, 1060–1066. [Google Scholar] [CrossRef]
- Kim, H.J.; Um, I.C. Relationship between rheology and electro-spinning performance of regenerated silk fibroin prepared using different degumming methods. Korea-Aust. Rheol. J. 2014, 26, 119–125. [Google Scholar] [CrossRef]
- Park, B.K.; Um, I.C. Effect of Korean Bombyx mori variety on electro-spinning performance of regenerated silk fibroin. Fiber. Polym. 2015, 16, 1935–1940. [Google Scholar] [CrossRef]
- Casper, C.L.; Stephens, J.S.; Tassi, N.G.; Chase, D.B.; Rabolt, J.F. Controlling surface morphology of electrospun polystyrene fibers: Effect of humidity and molecular weight in the electrospinning process. Macromolecules 2004, 37, 573–578. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Mattoso, L.H.C.; Offeman, R.D.; Wood, D.F.; Orts, W.J. Effect of relative humidity on the morphology of electrospun polymer fibers. Can. J. Chem. 2008, 86, 590–599. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; He, A.; Li, J.; Zhang, H.; Han, C.C. Electrospinning of native cellulose from nonvolatile solvent system. Polymer 2008, 49, 2911–2917. [Google Scholar] [CrossRef]
- Hardick, O.; Stevens, B.; Bracewell, D.G. Nanofibre fabrication in a temperature and humidity controlled environment for improved fibre consistency. J. Mater. Sci. 2011, 46, 3890–3898. [Google Scholar] [CrossRef] [Green Version]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Kim, G.T.; Lee, J.S.; Shin, J.H.; Ahn, Y.C.; Hwang, Y.J.; Shin, H.S.; Lee, J.K.; Sung, C.M. Investigation of pore formation for polystyrene electrospun fiber: Effect of relative humidity. Korean J. Chem. Eng. 2005, 22, 783–788. [Google Scholar] [CrossRef]
- De Schoenmaker, B.; Van der Schueren, L.; Zugle, R.; Goethals, A.; Westbroek, P.; Kiekens, P.; Nyokong, T.; De Clerck, K. Effect of the relative humidity on the fibre morphology of polyamide 4.6 and polyamide 6.9 nanofibres. J. Mater. Sci. 2013, 48, 1746–1754. [Google Scholar] [CrossRef]
- Tripatanasuwan, S.; Zhong, Z.; Reneker, D.H. Effect of evaporation and solidification of the charged jet in electrospinning of poly (ethylene oxide) aqueous solution. Polymer 2007, 48, 5742–5746. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kristl, J.; Janković, B.; Baumgartner, S.; Kocbek, P. The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int. J. Pharm. 2013, 456, 125–134. [Google Scholar] [CrossRef]
- Fashandi, H.; Karimi, M. Pore formation in polystyrene fiber by superimposing temperature and relative humidity of electrospinning atmosphere. Polymer 2012, 53, 5832–5849. [Google Scholar] [CrossRef]
- Lin, S.T.; Sandler, S.I. A priori phase equilibrium prediction from a segment contribution solvation model. Ind. Eng. Chem. Res. 2002, 41, 899–913. [Google Scholar] [CrossRef]
- Cai, Y.; Gevelber, M. The effect of relative humidity and evaporation rate on electrospinning: Fiber diameter and measurement for control implications. J. Mater. Sci. 2013, 48, 7812–7826. [Google Scholar] [CrossRef]
- Mailley, D.; Hébraud, A.; Schlatter, G. A review on the impact of humidity during electrospinning: From the nanofiber structure engineering to the applications. Macromol. Mater. Eng. 2021, 2100115. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Stachewicz, U. The impact of relative humidity on electrospun polymer fibers: From structural changes to fiber morphology. Adv. Colloid Interface Sci. 2020, 102315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zuo, B.; Fan, Z.; Xie, Z.; Lu, Q.; Zhang, X.; Kaplan, D.L. Mechanisms and control of silk-based electrospinning. Biomacromolecules 2012, 13, 798–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.J.; Ki, C.S.; Oh, H.; Lee, K.H.; Um, I.C. Molecular weight distribution and solution properties of silk fibroins with different dissolution conditions. Int. J. Biol. Macromol. 2012, 51, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.K.; Lee, K.H.; Nho, S.K.; Han, M.S.; Um, I.C. Effect of degumming methods on structural characteristics and properties of regenerated silk. Int. J. Biol. Macromol. 2017, 104, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Song, D.W.; Park, Y.H.; Um, I.C. Effect of residual sericin on the structural characteristics and properties of regenerated silk films. Int. J. Biol. Macromol. 2016, 89, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Um, I.C. Effect of degumming ratio on wet spinning and post drawing performance of regenerated silk. Int. J. Biol. Macromol. 2014, 67, 387–393. [Google Scholar] [CrossRef]
- Bae, Y.S.; Um, I.C. Effect of fabrication conditions on structure and properties of mechanically prepared natural silk web and non-woven fabrics. Polymers 2021, 13, 1578. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Um, I.C. Effect of sericin concentration and ethanol content on gelation behavior, rheological properties, and sponge characteristics of silk sericin. Eur. Polym. J. 2017, 93, 761–774. [Google Scholar] [CrossRef]
- Park, C.J.; Ryoo, J.; Ki, C.S.; Kim, J.W.; Kim, I.S.; Bae, D.G.; Um, I.C. Effect of molecular weight on the structure and mechanical properties of silk sericin gel, film, and sponge. Int. J. Biol. Macromol. 2018, 119, 821–832. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, I.C. Effect of silkworm variety on characteristics of raw sericin in silk. Fiber. Polym. 2019, 20, 271–279. [Google Scholar] [CrossRef]
- Tirtaatmadja, V.; McKinley, G.H.; Cooper-White, J.J. Drop formation and breakup of low viscosity elastic fluids: Effects of molecular weight and concentration. Phys. Fluids. 2006, 18, 043101. [Google Scholar] [CrossRef] [Green Version]
- Rošic, R.; Pelipenko, J.; Kocbek, P.; Baumgartner, S.; Bešter-Rogač, M.; Kristl, J. The role of rheology of polymer solutions in predicting nanofiber formation by electrospinning. Eur. Polym. J. 2012, 48, 1374–1384. [Google Scholar] [CrossRef]
- Kopp, A.; Smeets, R.; Gosau, M.; Kröger, N.; Fuest, S.; Köpf, M.; Kruse, M.; Krieger, J.; Rutkowski, R.; Henningsen, A.; et al. Effect of process parameters on additive-free electrospinning of regenerated silk fibroin nonwovens. Bioact. Mater. 2020, 5, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Raksa, A.; Numpaisal, P.O.; Ruksakulpiwat, Y. The effect of humidity during electrospinning on morphology and mechanical properties of SF/PVA nanofibers. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Collins, G.; Federici, J.; Imura, Y.; Catalani, L.H. Charge generation, charge transport, and residual charge in the electrospinning of polymers: A review of issues and complications. J. Appl. Phys. 2012, 111, 44701. [Google Scholar] [CrossRef]
- Guerrero, J.; Rivero, J.; Gundabala, V.R.; Perez-Saborid, M.; Fernandez-Nieves, A. Whipping of electrified liquid jets. Proc. Natl. Acad. Sci. USA 2014, 111, 13763–13767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Bae, Y.S.; Kim, S.J.; Song, D.W.; Park, Y.H.; Bae, D.G.; Choi, J.H.; Um, I.C. Preparation of new natural silk non-woven fabrics by using adhesion characteristics of sericin and their characterization. Int. J. Biol. Macromol. 2018, 106, 39–47. [Google Scholar] [CrossRef]
- Chung, D.E.; Kim, H.H.; Kim, M.K.; Lee, K.H.; Park, Y.H.; Um, I.C. Effects of different bombyx mori silkworm varieties on the structural characteristics and properties of silk. Int. J. Biol. Macromol. 2015, 79, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Um, I.C.; Park, Y.H. The effect of casting solvent on the structural characteristics and miscibility of regenerated silk fibroin/poly(vinyl alcohol) blends. Fibers Polym. 2007, 8, 579–585. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.Y.; Park, Y.H.; Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef]
- Chung, D.E.; Um, I.C. Effect of molecular weight and concentration on crystallinity and post drawing of wet spun silk fibroin fiber. Fibers Polym. 2014, 15, 153–160. [Google Scholar] [CrossRef]
- Bae, Y.S.; Um, I.C. Preparation, structural characteristics, and properties of airlaid nonwoven silk fabric. Polym. Korea 2020, 44, 809–816. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.Y.; Lee, K.G.; Park, Y.H. The role of formic acid in solution stability and crystallization of silk protein polymer. Int. J. Biol. Macromol. 2003, 33, 203–213. [Google Scholar] [CrossRef]
- Magoshi, J.; Mizuide, M.; Magoshi, Y.; Takahashi, K.; Kubo, M.; Nakamura, S. Physical properties and structure of silk. VI. Conformational changes in silk fibroin induced by immersion in water at 2 to 130 C. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 515–520. [Google Scholar] [CrossRef]
- Jeong, L.; Lee, K.Y.; Liu, J.W.; Park, W.H. Time-resolved structural investigation of regenerated silk fibroin nanofibers treated with solvent vapor. Int. J. Biol. Macromol. 2006, 38, 140–144. [Google Scholar] [CrossRef]
- Yazawa, K.; Ishida, K.; Masunaga, H.; Hikima, T.; Numata, K. Influence of water content on the β-sheet formation, thermal stability, water removal, and mechanical properties of silk materials. Biomacromolecules 2016, 17, 1057–1066. [Google Scholar] [CrossRef]


| Relative Humidity (%) | Silk Concentration (%(w/w)) | |||
|---|---|---|---|---|
| 14 | 16 | 17.5 | 19 | |
| 20 |  |  |  |  |
| 40 |  |  |  |  |
| 60 |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.K.; Um, I.C. Effect of Relative Humidity on the Electrospinning Performance of Regenerated Silk Solution. Polymers 2021, 13, 2479. https://doi.org/10.3390/polym13152479
Park BK, Um IC. Effect of Relative Humidity on the Electrospinning Performance of Regenerated Silk Solution. Polymers. 2021; 13(15):2479. https://doi.org/10.3390/polym13152479
Chicago/Turabian StylePark, Bo Kyung, and In Chul Um. 2021. "Effect of Relative Humidity on the Electrospinning Performance of Regenerated Silk Solution" Polymers 13, no. 15: 2479. https://doi.org/10.3390/polym13152479







