Extraction and Characterization of Hemicelluloses from a Softwood Acid Sulfite Pulp
Abstract
:1. Introduction
2. Experiment
2.1. Materials
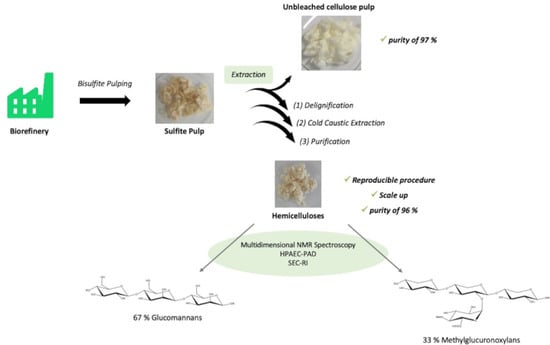
2.2. Hemicellulose Extraction Procedure
2.3. Extraction Yields
2.4. Pulp Characterizations
2.5. Instruments Methods
3. Results and Discussion
3.1. Initial Pulp Characterizations
3.2. Pulp Characterizations at Different Extraction Steps
3.3. Extraction Yields
3.4. Hemicellulose Characterizations
3.4.1. Structural Identification
3.4.2. Hemicellulose Proportions
3.4.3. Hemicellulose Molecular Weights
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Status Report; 2019. Renewables 2019. Available online: https://www.ren21.net (accessed on 1 April 2021).
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Alén, R. Pulp Mills and Wood-Based Biorefineries. In Industrial Biorefineries & White Biotechnology; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 91–126. [Google Scholar]
- Konttinen, Y.; Xu, J.-W.; Patiala, H.; Imai, S.; Waris, V.; Li, T.-F.; Goodman, S.; Nordsletten, L.; Santavirta, S. Cytokines in aseptic loosening of total hip replacement. Curr. Orthop. 1997, 11, 40–47. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the Valorization of Lignocellulosic Materials by Biotechnology: An Overview. BioResources 2013, 8, 3157–3176. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Banerjee, J.; Sasmal, S.; Muir, J.; Arora, A. High xylan recovery using two stage alkali pre-treatment process from high lignin biomass and its valorisation to xylooligosaccharides of low degree of polymerisation. Bioresour. Technol. 2018, 256, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Knill, C.J.; Kennedy, J.F. Degradation of cellulose under alkaline conditions. Carbohydr. Polym. 2003, 51, 281–300. [Google Scholar] [CrossRef]
- Schild, G.; Sixta, H.; Testova, L. Multifunctional Alkaline Pulping, Delignification and Hemicellulose Extraction. Cellul. Chem. Technol. 2010, 44, 35–45. [Google Scholar]
- Alén, R. Analysis of Degradation Products: A New Approach to Characterizing the Combustion Properties of Kraft Black Liquors. J. Pulp Pap. Sci. 1997, 23, J62–J66. [Google Scholar]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Reutilization of Food Waste: One-Step Extration, Purification and Characterization of Ovalbumin from Salted Egg White by Aqueous Two-Phase Flotation. Foods 2019, 8, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wertz, J.-L.; Deleu, M.; Coppée, S.; Richel, A. Valorization of Hemicelluloses. Hemicellul. Lignin Biorefineries 2017, 223–244. [Google Scholar]
- Jin, A.; Ren, J.; Peng, F.; Xu, F.; Zhou, G.; Sun, R.; Kennedy, J. Comparative characterization of degraded and non-degradative hemicelluloses from barley straw and maize stems: Composition, structure, and thermal properties. Carbohydr. Polym. 2009, 78, 609–619. [Google Scholar] [CrossRef]
- Peng, F.; Bian, J.; Ren, J.-L.; Peng, P.; Xu, F.; Sun, R.-C. Fractionation and characterization of alkali-extracted hemicelluloses from peashrub. Biomass Bioenergy 2012, 39, 20–30. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.-L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.-C. Comparative Study of Hemicelluloses Obtained by Graded Ethanol Precipitation from Sugarcane Bagasse. J. Agric. Food Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef]
- Sun, R.-C.; Sun, X.-F.; Wen, J.-L. Fractional and Structural Characterization of Lignins Isolated by Alkali and Alkaline Peroxide from Barley Straw. J. Agric. Food Chem. 2001, 49, 5322–5330. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, X.; Sun, R. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 2001, 74, 307–319. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.; Geng, Z.; Liu, C.; Ren, J.; Sun, R.; Fowler, P.; Baird, M. Comparative study of water-soluble and alkali-soluble hemicelluloses from perennial ryegrass leaves (Lolium peree). Carbohydr. Polym. 2007, 67, 56–65. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.-L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.-C. Fractionation of Alkali-Solubilized Hemicelluloses from Delignified Populus gansuensis: Structure and Properties. J. Agric. Food Chem. 2010, 58, 5743–5750. [Google Scholar] [CrossRef]
- He, L.; Chen, D.; Yang, S.; Peng, L.; Zhang, J.; Guan, Q.; Zhang, P. Deep Insights into the Atmospheric Sodium Hydroxide–Hydrogen Peroxide Extraction Process of Hemicellulose in Bagasse Pith: Technical Uncertainty, Dissolution Kinetics Behavior, and Mechanism. Ind. Eng. Chem. Res. 2020, 59, 10150–10159. [Google Scholar] [CrossRef]
- Ebringerová, A.; Heinze, T. Xylan and Xylan Derivatives—Biopolymers with Valuable Properties, 1: Naturally Occurring Xylans Structures, Isolation Procedures and Properties. Macromol. Rapid Commun. 2000, 21, 542–556. [Google Scholar] [CrossRef]
- Rowley, J.; Decker, S.R.; Michener, W.; Black, S.K. Efficient extraction of xylan from delignified corn stover using dimethyl sulfoxide. 3 Biotech 2013, 3, 433–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Wang, Z.; Wang, L.; Chao, Y.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Hemicellulose Composition in Different Cell Wall Fractions Obtained using a DMSO/LiCl Wood Solvent System and Enzyme Hydrolysis. J. Wood Chem. Technol. 2015, 36, 56–62. [Google Scholar] [CrossRef]
- Sun, R. Fractional and structural characterization of hemicelluloses isolated by alkali and alkaline peroxide from barley straw. Carbohydr. Polym. 2002, 49, 415–423. [Google Scholar] [CrossRef]
- Arnoul-Jarriault, B.; Lachenal, D.; Chirat, C.; Heux, L. Upgrading softwood bleached kraft pulp to dissolving pulp by cold caustic treatment and acid-hot caustic treatment. Ind. Crop. Prod. 2015, 65, 565–571. [Google Scholar] [CrossRef]
- Borrega, M.; Concha-Carrasco, S.; Pranovich, A.; Sixta, H. Hot water treatment of hardwood kraft pulp produces high-purity cellulose and polymeric xylan. Cellulose 2017, 24, 5133–5145. [Google Scholar] [CrossRef] [Green Version]
- Gehmayr, V.; Schild, G.; Sixta, H. A precise study on the feasibility of enzyme treatments of a kraft pulp for viscose application. Cellulose 2011, 18, 479–491. [Google Scholar] [CrossRef]
- Yang, S.; Yang, B.; Duan, C.; Fuller, D.A.; Wang, X.; Chowdhury, S.P.; Stavik, J.; Zhang, H.; Ni, Y. Applications of enzymatic technologies to the production of high-quality dissolving pulp: A review. Bioresour. Technol. 2019, 281, 440–448. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR Spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Sassaki, G.L.; Guerrini, M.; Serrato, R.V.; Filho, A.P.S.; Carlotto, J.; Simas-Tosin, F.; Cipriani, T.R.; Iacomini, M.; Torri, G.; Gorin, P.A.J. Monosaccharide composition of glycans based on Q-HSQC NMR. Carbohydr. Polym. 2014, 104, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kiemle, D.J.; Stipanovic, A.J.; Mayo, K.E. Proton NMR Methods in the Compositional Characterization of Polysaccharides. In Proceedings of the ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 2003; pp. 122–139. [Google Scholar]
- Sjöström, E. Wood Chemistry, Fundamentals and Applications; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Vinogradov, E.; Petersen, B.; Bock, K. Discussion. Carbohydr. Res. 1998, 307, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Capek, P.; Alfo, J. An Acetylated Galactoglucomannan From. Carbohydr. Res. 2002, 337, 1033–1037. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Qi, R.-T.; Pang, M.-R.; Liu, C.; Li, G.-Y.; Zhang, Y. Isolation, chemical characterization, and immunomodulatory activity of naturally acetylated hemicelluloses from bamboo shavings. J. Zhejiang Univ. Sci. B 2017, 18, 138–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemin, M.; Wirotius, A.-L.; Ham-Pichavant, F.; Chollet, G.; Perez, D.D.S.; Petit-Conil, M.; Cramail, H.; Grelier, S. Well-defined oligosaccharides by mild acidic hydrolysis of hemicelluloses. Eur. Polym. J. 2015, 66, 190–197. [Google Scholar] [CrossRef]




| Time (Min) | Eluent A (%) | Eluent B (%) | Eluent C (%) |
|---|---|---|---|
| 0 | 90 | 10 | 0 |
| 8 | 90 | 10 | 0 |
| 8.1 | 0 | 0 | 100 |
| 15 | 0 | 0 | 100 |
| 15.1 | 90 | 10 | 0 |
| 30 | 90 | 10 | 0 |
| Maritime Pine | Sulfite Pulps | Sulfite Pulp, RYAM * | |
|---|---|---|---|
| Dry matter | - | - | 29.6 ± 0.2 (%) |
| Cellulose | 42–50% | 83–87% | 79.7 ± 0.6 (%) |
| Hemicelluloses (S18) | 24–27% | 10–15% | 15.4 ± 1.0 (%) |
| Lignin (Kappa *, 0.147) | 20% | 2–5% | 5.2 ± 0.1 (%) |
| Proportions (wt.%) | |
|---|---|
| Monosaccharides | 100 |
| Arabinose | 0.1 |
| Galactose | 0.4 |
| Glucose | 16.5 |
| Mannose | 51.0 |
| Xylose | 32.0 |
| Hemicelluloses | 96 |
| Glucomannans | 64 |
| Methylglucuronoxylans | 32 |
| Cellulose | 4 |
| MW (g/mol) | Mn (g/mol) | Ɖ | |
|---|---|---|---|
| HC-A | 26,400 | 15,700 | 1.7 |
| HC-B | 18,900 | 13,000 | 1.5 |
| HC-C | 30,000 | 17,600 | 1.7 |
| HC-D | 19,800 | 14,000 | 1.4 |
| HC-E | 26,600 | 13,800 | 1.9 |
| HC-F | 25,000 | 12,400 | 2.0 |
| HC-RYAM | 24,000 | 13,500 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincent, P.; Ham-Pichavant, F.; Michaud, C.; Mignani, G.; Mastroianni, S.; Cramail, H.; Grelier, S. Extraction and Characterization of Hemicelluloses from a Softwood Acid Sulfite Pulp. Polymers 2021, 13, 2044. https://doi.org/10.3390/polym13132044
Vincent P, Ham-Pichavant F, Michaud C, Mignani G, Mastroianni S, Cramail H, Grelier S. Extraction and Characterization of Hemicelluloses from a Softwood Acid Sulfite Pulp. Polymers. 2021; 13(13):2044. https://doi.org/10.3390/polym13132044
Chicago/Turabian StyleVincent, Pauline, Frédérique Ham-Pichavant, Christelle Michaud, Gérard Mignani, Sergio Mastroianni, Henri Cramail, and Stéphane Grelier. 2021. "Extraction and Characterization of Hemicelluloses from a Softwood Acid Sulfite Pulp" Polymers 13, no. 13: 2044. https://doi.org/10.3390/polym13132044