Fabrication and Characterization of Cinnamaldehyde-Loaded Mesoporous Bioactive Glass Nanoparticles/PHBV-Based Microspheres for Preventing Bacterial Infection and Promoting Bone Tissue Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CIN-Loaded PHBV/MBGN Microspheres
2.3. Characterization of CIN-Loaded PHBV/MBGN Microspheres
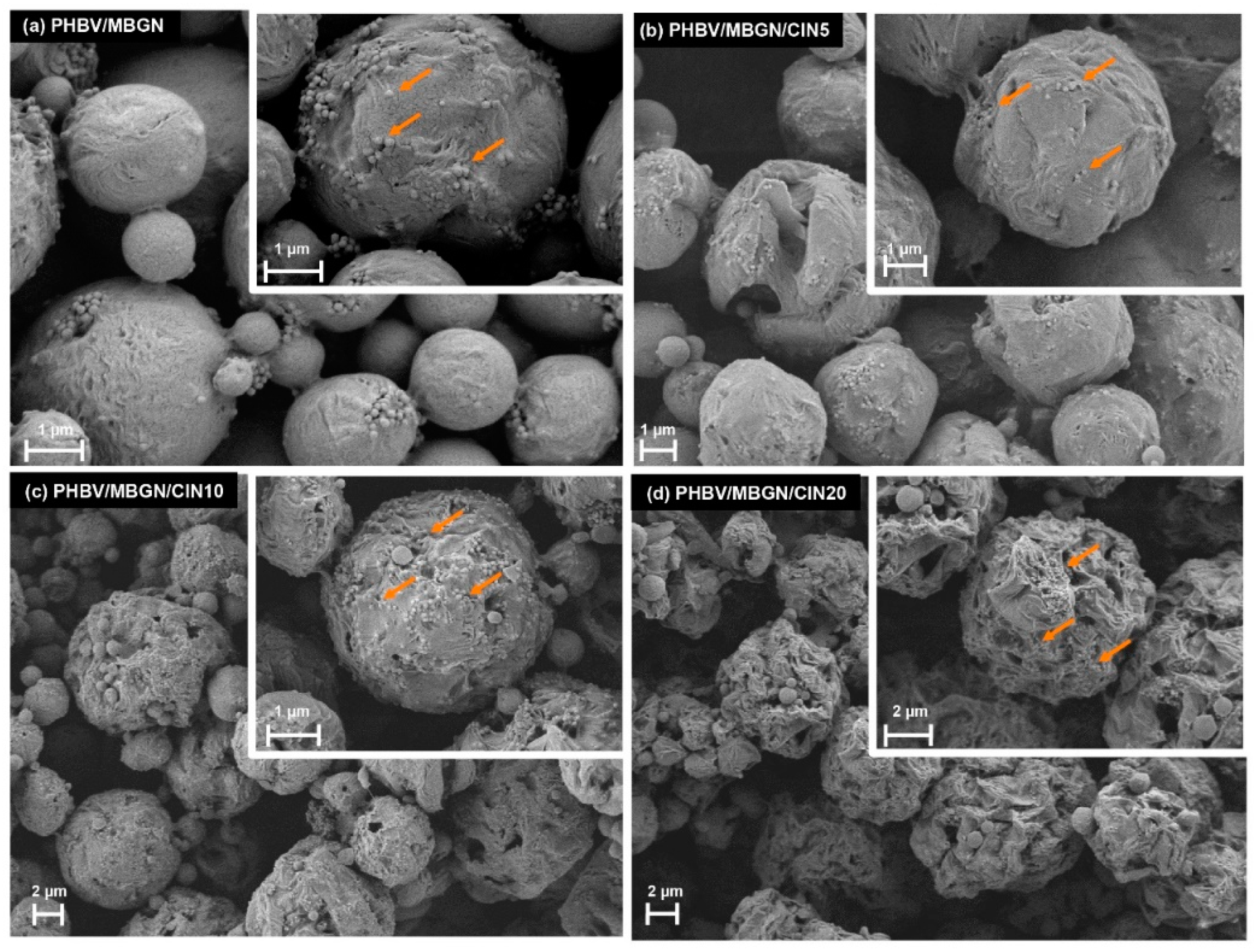
2.3.1. Scanning Electron Microscopy
2.3.2. Mean Particle Size and Zeta Potential Analysis
2.3.3. Fourier Transformed Infrared (FTIR) Spectroscopy
2.3.4. X-ray Diffraction (XRD) Analysis
2.3.5. Energy Dispersive X-ray Spectroscopy (EDS) Analysis
2.4. Encapsulation Efficiency
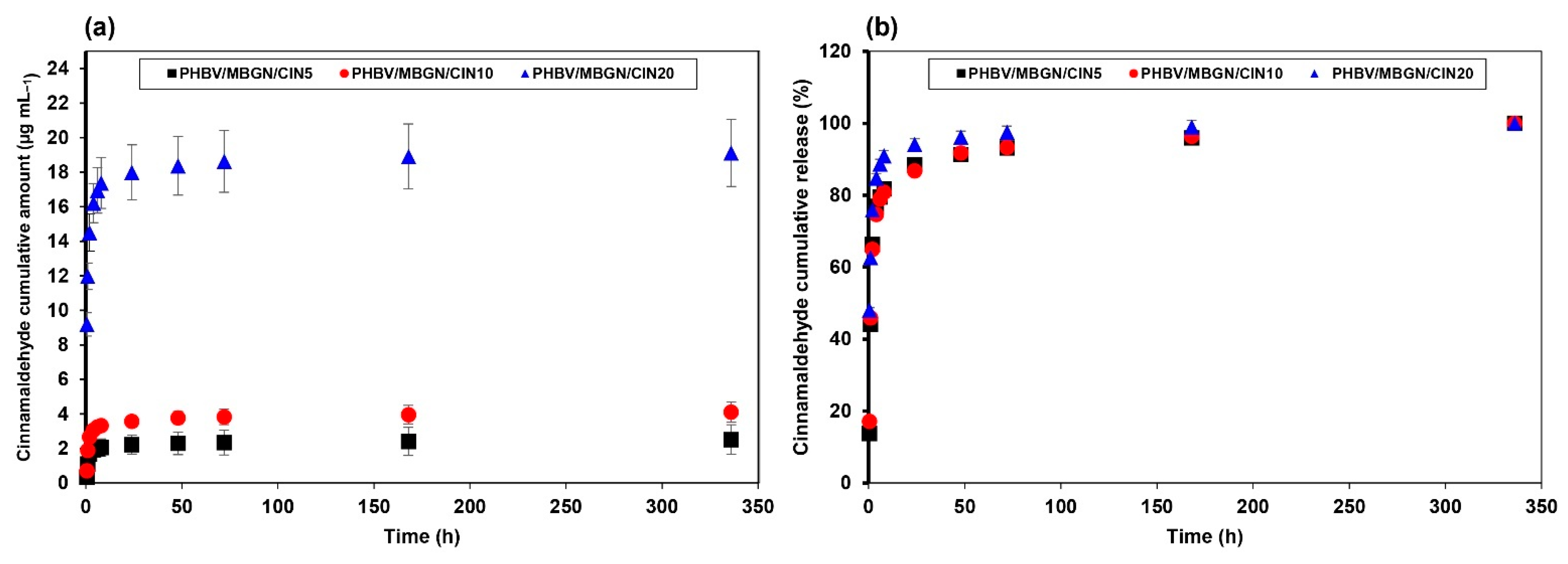
2.5. In Vitro CIN Releasing Behavior
2.6. Antibacterial Assay
2.7. In Vitro Cytotoxicity Test
2.8. In Vitro Bioactivity Test
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Microspheres
3.1.1. Scanning Electron Microscopy
3.1.2. Particle Size, Polydispersity Index, Zeta Potential, and Encapsulation Efficiency
3.1.3. FTIR Analysis
3.2. In Vitro CIN Release Behavior
3.3. Antibacterial Activity
3.4. In Vitro Cytotoxicity and Cell Adhesion Assay
3.5. In Vitro Bioactivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck-Broichsitter, B.E.; Smeets, R.; Heiland, M. Current Concepts in Pathogenesis of Acute and Chronic Osteomyelitis. Curr. Opin. Infect. Dis. 2015, 28, 240–245. [Google Scholar] [CrossRef]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nat. Cell Biol. 2017, 543, 15. [Google Scholar] [CrossRef] [Green Version]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candida activity of Brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef]
- Alshahrani, S.; Ashafaq, M.; Hussain, S.; Mohammed, M.; Sultan, M.; Jali, A.M.; Siddiqui, R.; Islam, F. Renoprotective effects of cinnamon oil against APAP-Induced nephrotoxicity by ameliorating oxidative stress, apoptosis and inflammation in rats. Saudi Pharm. J. 2021, 29, 194–200. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Paudel, S.K.; Bhargava, K.; Kotturi, H. Antimicrobial Activity of Cinnamon Oil Nanoemulsion against Listeria monocytogenes and Salmonella Spp. on Melons. Lebenson. Wiss. Technol. 2019, 111, 682–687. [Google Scholar] [CrossRef]
- Wijesinghe, G.K.; Feiria, S.B.; Maia, F.C.; Oliveira, T.R.; Joia, F.; Barbosa, J.P.; Boni, G.C.; Höfling, J.F. In-vitro Antibacterial and Antibiofilm Activity of Cinnamomum verum Leaf Oil against Pseudomonas aeruginosa, Staphylococcus aureus and Klebsiella pneumoniae. An. Acad. Bras. Ciên. 2021, 93, e20201507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; Moura, L.D.A.G.; de Melo, N.R.; Silva, A.S. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Karumathil, D.P.; Nair, M.S.; Gaffney, J.; Kollanoor-Johny, A.; Venkitanarayanan, K. Trans-Cinnamaldehyde and Eugenol Increase Acinetobacter baumannii Sensitivity to Beta-Lactam Antibiotics. Front. Microbiol. 2018, 9, 1011. [Google Scholar] [CrossRef]
- Yin, L.; Chen, J.; Wang, K.; Geng, Y.; Lai, W.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; Chen, Z.; et al. Study the antibacterial mechanism of cinnamaldehyde against drug-resistant Aeromonas hydrophila in vitro. Microb. Pathog. 2020, 145, 104208. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, N.; Ryan, E.J.; Widaa, A.; Sexton, G.; Fennell, J.; O’Rourke, S.; Cahill, K.C.; Kearney, C.J.; O’Brien, F.J.; Kerrigan, S.W. Staphylococcal Osteomyelitis: Disease Progression, Treatment Challenges, and Future Directions. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Central Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Haponiuk, J.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Tebaldi, M.L.; Maia, A.L.C.; Poletto, F.; de Andrade, F.V.; Soares, D.C.F. Poly(-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Current advances in synthesis methodologies, antitumor applications and biocompatibility. J. Drug Deliv. Sci. Technol. 2019, 51, 115–126. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, D.; Qu, Y.; Peng, J.; Huang, K.; Lei, M.; Wu, T.; Xiao, Y.; Gu, Y.; Qian, Z. Preparation of Adenosine-Loaded Electrospun Nanofibers and Their Application in Bone Regeneration. J. Biomed. Nanotechnol. 2019, 15, 857–877. [Google Scholar] [CrossRef]
- Diermann, S.H.; Lu, M.; Edwards, G.; Dargusch, M.; Huang, H. In Vitro Degradation of a Unique Porous PHBV Scaffold Manufactured Using Selective Laser Sintering. J. Biomed. Mater. Res. A 2019, 107, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Hokmabad, V.R.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Supercritical Assisted Electrospray: An Improved Micronization Process. Polymers 2019, 11, 244. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jansen, J.A.; Yang, F. Electrospraying: Possibilities and Challenges of Engineering Carriers for Biomedical Applications—A Mini Review. Front. Chem. 2019, 7, 258. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ding, Y.; Rai, R.; Roether, J.A.; Schubert, D.W.; Boccaccini, A.R. Preparation and Characterization of PHBV Microsphere/45S5 Bioactive Glass Composite Scaffolds with Vancomycin Releasing Function. Mater. Sci. Eng. C 2014, 41, 320–328. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Liu, Y.; Hu, D.; Gu, L.; Fei, X.; Zhang, J. Effect of Rapamycin Microspheres in Sjögren Syndrome Dry Eye: Preparation and Outcomes. Ocul. Immunol. Inflamm. 2018, 27, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Alagoz, A.S.; Rodriguez-Cabello, J.C.; Hasirci, V. PHBV wet-spun scaffold coated with ELR-REDV improves vascularization for bone tissue engineering. Biomed. Mater. 2018, 13, 055010. [Google Scholar] [CrossRef] [PubMed]
- Dalgıç, A.D.; Atila, D.; Karatas, A.; Tezcaner, A.; Keskin, D. Diatom shell incorporated PHBV/PCL-pullulan co-electrospun scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 735–746. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Moghadam, A.S.; Enderami, S.E.; Islami, M.; Kaabi, M.; Saburi, E.; Farshchi, A.D.; Soleimanifar, F.; Mansouri, V. Aloe Vera–Derived Gel-Blended PHBV Nanofibrous Scaffold for Bone Tissue Engineering. ASAIO J. 2020, 66, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, J. Fabrication and Characterization of Bioactive Wollastonite/PHBV Composite Scaffolds. Biomaterials 2004, 25, 5473–5480. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M. Production and evaluation of biodegradable composites based on PHB–PHV copolymer. Biomaterials 2002, 23, 2631–2639. [Google Scholar] [CrossRef]
- Pan, W.; Xiao, X.; Li, J.; Deng, S.; Shan, Q.; Yue, Y.; Tian, Y.; Nabar, N.R.; Wang, M.; Hao, L. The comparison of biocompatibility and osteoinductivity between multi-walled and single-walled carbon nanotube/PHBV composites. J. Mater. Sci. Mater. Med. 2018, 29, 189. [Google Scholar] [CrossRef]
- Ye, X.; Li, L.; Lin, Z.; Yang, W.; Duan, M.; Chen, L.; Xia, Y.; Chen, Z.; Lu, Y.; Zhang, Y. Integrating 3D-printed PHBV/Calcium sulfate hemihydrate scaffold and chitosan hydrogel for enhanced osteogenic property. Carbohydr. Polym. 2018, 202, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Sui, B.; Ilyas, K.; Boccaccini, A.R. Porous bioactive glass micro- and nanospheres with controlled morphology: Developments, properties and emerging biomedical applications. Mater. Horizons 2021, 8, 300–335. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Mesquita-Guimarães, J.; Magini, R.S.; Souza, J.C.M.; Fredel, M.C.; Boccaccini, A.R. Mesoporous bioactive glass embedding propolis and cranberry antibiofilm compounds. J. Biomed. Mater. Res. Part A 2018, 106, 1614–1625. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, D.; Li, N.; Wen, J.; Jiang, X.; Liu, C.; Li, Y. Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci. Rep. 2016, 6, 19361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Liu, Y.; Tan, Y.; Grover, L.M.; Song, J.; Duan, S.; Zhao, D.; Tan, X. Rubidium-containing mesoporous bioactive glass scaffolds support angiogenesis, osteogenesis and antibacterial activity. Mater. Sci. Eng. C 2019, 105, 110155. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, L.; Zhai, D.; Shi, M.; Luo, Y.; Feng, C.; Fang, B.; Yin, J.; Chang, J.; Wu, C. Mesoporous bioactive glass nanolayer-functionalized 3D-printed scaffolds for accelerating osteogenesis and angiogenesis. Nanoscale 2015, 7, 19207–19221. [Google Scholar] [CrossRef]
- Aguilar-Rabiela, A.; Leal-Egaña, A.; Nawaz, Q.; Boccaccini, A.R. Integration of Mesoporous Bioactive Glass Nanoparticles and Curcumin into PHBV Microspheres as Biocompatible Composite for Drug Delivery Applications. Molecules 2021, 26, 3177. [Google Scholar] [CrossRef]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional Zinc Ion Doped Sol-Gel Derived Mesoporous Bioactive Glass Nanoparticles for Biomedical Applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef]
- Aguilar-Rabiela, A.E.; Hernández-Cooper, E.M.; Otero, J.A.; Vergara-Porras, B. Modeling the release of curcumin from microparticles of poly(hydroxybutyrate) [PHB]. Int. J. Biol. Macromol. 2020, 144, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Murueva, A.V.; Shershneva, A.M.; Abanina, K.V.; Prudnikova, S.; Shishatskaya, E.I. Development and characterization of ceftriaxone-loaded P3HB-based microparticles for drug delivery. Dry. Technol. 2018, 37, 1131–1142. [Google Scholar] [CrossRef] [Green Version]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1. [Google Scholar] [CrossRef]
- Li, X.; Ji, X.; Chen, K.; Ullah, M.W.; Yuan, X.; Lei, Z.; Cao, J.; Xiao, J.; Yang, G. Development of finasteride/PHBV@polyvinylalcohol/chitosan reservoir-type microspheres as a potential embolic agent: From in vitro evaluation to animal study. Biomater. Sci. 2020, 8, 2797–2813. [Google Scholar] [CrossRef] [PubMed]
- Senhorini, G.A.; Zawadzki, S.F.; Farago, P.V.; Zanin, S.M.; Marques, F.A. Microparticles of poly(hydroxybutyrate-co-hydroxyvalerate) loaded with andiroba oil: Preparation and characterization. Mater. Sci. Eng. C 2012, 32, 1121–1126. [Google Scholar] [CrossRef]
- Gonzalez, M.F.; Ruseckaite, R.A.; Cuadrado, T.R. Structural changes of polylactic-acid (PLA) microspheres under hydrolytic degradation. J. Appl. Polym. Sci. 1999, 71, 1223–1230. [Google Scholar] [CrossRef]
- Perveen, K.; Masood, F.; Hameed, A. Preparation, characterization and evaluation of antibacterial properties of epirubicin loaded PHB and PHBV nanoparticles. Int. J. Biol. Macromol. 2020, 144, 259–266. [Google Scholar] [CrossRef]
- Cardoso, J.; Ricci-Júnior, E.; Gentili, D.; Spinelli, L.; Lucas, E. Influence of Cardanol Encapsulated on the Properties of Poly(Lactic Acid) Microparticles. Quim. Nova 2017, 41, 273–283. [Google Scholar] [CrossRef]
- Ding, A.; Teng, L.; Zhou, Y.; Chen, P.; Nie, W. Synthesis and characterization of bovine serum albumin-loaded microspheres based on star-shaped PLLA with a xylitol core and their drug release behaviors. Polym. Bull. 2017, 75, 2917–2931. [Google Scholar] [CrossRef]
- Dos Santos, P.P.; Andrade, L.D.A.; Flôres, S.H.; Rios, A.D.O. Nanoencapsulation of carotenoids: A focus on different delivery systems and evaluation parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Barua, S.; Barua, D. A multiscale modeling study of particle size effects on the tissue penetration efficacy of drug-delivery nanoparticles. BMC Syst. Biol. 2017, 11, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Z.-M.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Study on Drug Release Behaviors of Poly-Alpha,Beta-[n-(2-Hydroxyethyl)-L-Aspartamide]-g-Poly(Epsilon-Caprolactone) Nano- and Microparticles. Biomacromolecules 2006, 7, 2020–2026. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Chen, P.; Yasin, T.; Fatima, N.; Hasan, F.; Hameed, A. Encapsulation of Ellipticine in poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) based nanoparticles and its in vitro application. Mater. Sci. Eng. C 2013, 33, 1054–1060. [Google Scholar] [CrossRef]
- Musumeci, T.; Ventura, C.A.; Giannone, I.; Ruozi, B.; Montenegro, L.; Pignatello, R.; Puglisi, G. PLA/PLGA nanoparticles for sustained release of docetaxel. Int. J. Pharm. 2006, 325, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Yang, C.; Plackett, D.; Needham, D.; Burt, H.M. PLGA and PHBV Microsphere Formulations and Solid-State Characterization: Possible Implications for Local Delivery of Fusidic Acid for the Treatment and Prevention of Orthopaedic Infections. Pharm. Res. 2009, 26, 1644–1656. [Google Scholar] [CrossRef]
- Otroj, M.; Taymouri, S.; Varshosaz, J.; Mirian, M. Preparation and characterization of dry powder containing sunitinib loaded PHBV nanoparticles for enhanced pulmonary delivery. J. Drug Deliv. Sci. Technol. 2020, 56, 101570. [Google Scholar] [CrossRef]
- Vardhan, H.; Mittal, P.; Adena, S.K.R.; Mishra, B. Long-circulating polyhydroxybutyrate-co-hydroxyvalerate nanoparticles for tumor targeted docetaxel delivery: Formulation, optimization and in vitro characterization. Eur. J. Pharm. Sci. 2017, 99, 85–94. [Google Scholar] [CrossRef]
- Chen, J.; Davis, S.S. The release of diazepam from poly(hydroxybutyrate-hydroxyvalerate) microspheres. J. Microencapsul. 2002, 19, 191–201. [Google Scholar] [CrossRef]
- Chotchindakun, K.; Pathom-Aree, W.; Dumri, K.; Ruangsuriya, J.; Pumas, C.; Pekkoh, J. Low Crystallinity of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Bioproduction by Hot Spring Cyanobacterium Cyanosarcina Sp. AARL T020. Plants 2021, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Kang, J.; Rutkowski, B.; Gawȩda, M.; Zhang, J.; Wang, Y.; Founier, N.; Sitarz, M.; Taccardi, N.; Boccaccini, A.R. Toward Highly Dispersed Mesoporous Bioactive Glass Nanoparticles With High Cu Concentration Using Cu/Ascorbic Acid Complex as Precursor. Front. Chem. 2019, 7, 497. [Google Scholar] [CrossRef] [Green Version]
- Al-Bayati, F.A.; Mohammed, M.J. Isolation, Identification, and Purification of Cinnamaldehyde FromCinnamomum Zeylanicumbark Oil. An Antibacterial Study. Pharm. Biol. 2009, 47, 61–66. [Google Scholar] [CrossRef]
- Monnier, A.; Rombouts, C.; Kouider, D.; About, I.; Fessi, H.; Sheibat-Othman, N. Preparation and Characterization of Biodegradable Polyhydroxybutyrate-Co-Hydroxyvalerate/Polyethylene Glycol-Based Microspheres. Int. J. Pharm. 2016, 513, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Pereira, A.; De Melo, N.F.S.; Porto, R.M.; Feitosa, L.O.; Tonello, P.S.; Filho, N.L.D.; Rosa, A.H.; Lima, R.; Fraceto, L. Controlled release system for ametryn using polymer microspheres: Preparation, characterization and release kinetics in water. J. Hazard. Mater. 2011, 186, 1645–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Palazzo, A.; Hennink, W.E.; Kok, R.J. Effect of Particle Size on Drug Loading and Release Kinetics of Gefitinib-Loaded PLGA Microspheres. Mol. Pharm. 2017, 14, 459–467. [Google Scholar] [CrossRef]
- Wang, J.; Helder, L.; Shao, J.; Jansen, J.A.; Yang, M.; Yang, F. Encapsulation and Release of Doxycycline from Electrospray-Generated PLGA Microspheres: Effect of Polymer End Groups. Int. J. Pharm. 2019, 564, 1–9. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Livshits, V.A.; Makhina, T.A.; Myshkina, V.L.; Bonartseva, G.A.; Iordanskii, A.L. Controlled release profiles of dipyridamole from biodegradable microspheres on the base of poly(3-hydroxybutyrate). Express Polym. Lett. 2007, 1, 797–803. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and Non-Fickian Drug Release from Polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar] [PubMed]
- Shuai, C.; Wang, C.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. Enhanced Crystallinity and Antibacterial of PHBV Scaffolds Incorporated with Zinc Oxide. J. Nanomater. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.-Y.; Wang, C.; Yao, J. Effect of silver contents in cellulose nanocrystal/silver nanohybrids on PHBV crystallization and property improvements. Carbohydr. Polym. 2017, 173, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Balasubramanian, P.; Paterson, T.; Stein, R.; MacNeil, S.; Fiorilli, S.; Vitale-Brovarone, C.; Shepherd, J.; Boccaccini, A.R. Ag modified mesoporous bioactive glass nanoparticles for enhanced antibacterial activity in 3D infected skin model. Mater. Sci. Eng. C 2019, 103, 109764. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Xu, Y.; Feng, P.; Wang, G.; Xiong, S.; Peng, S. Antibacterial polymer scaffold based on mesoporous bioactive glass loaded with in situ grown silver. Chem. Eng. J. 2019, 374, 304–315. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Burt, S.; Reinders, R. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.; Zhu, D.-H.; Wu, H.; Zong, M.-H.; Jing, Y.-R.; Han, S.-Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control. 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and Antibacterial Properties of Peppermint Essential Oil Loaded Poly (ε-caprolactone) (PCL) Electrospun Fiber Mats for Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 346. [Google Scholar] [CrossRef] [Green Version]
- Clapp, P.W.; Lavrich, K.S.; Van Heusden, C.A.; Lazarowski, E.R.; Carson, J.L.; Jaspers, I. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L470–L486. [Google Scholar] [CrossRef] [PubMed]
- Absalan, A.; Mesbah-Namin, S.A.; Tiraihi, T.; Taheri, T. The Effects of Cinnamaldehyde and Eugenol on Human Adipose-Derived Mesenchymal Stem Cells Viability, Growth and Differentiation: A Cheminformatics and in Vitro Study. Avicenna J. Phytomed. 2016, 6, 643–657. [Google Scholar] [PubMed]
- Zheng, K.; Solodovnyk, A.; Li, W.; Goudouri, O.-M.; Stähli, C.; Nazhat, S.N.; Boccaccini, A.R. Aging Time and Temperature Effects on the Structure and Bioactivity of Gel-Derived 45S5 Glass-Ceramics. J. Am. Ceram. Soc. 2015, 98, 30–38. [Google Scholar] [CrossRef]
- Joshi, K.J.; Shah, N.M. Study of Hydroxyapatite Nano-particles synthesized using sono-chemical supported hydrothermal method. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Zheng, K.; Fan, Y.; Torre, E.; Balasubramanian, P.; Taccardi, N.; Cassinelli, C.; Morra, M.; Iviglia, G.; Boccaccini, A.R. Incorporation of Boron in Mesoporous Bioactive Glass Nanoparticles Reduces Inflammatory Response and Delays Osteogenic Differentiation. Part. Part. Syst. Charact. 2020, 37, 2000054. [Google Scholar] [CrossRef]
- Nawaz, Q.; Rehman, M.A.U.; Burkovski, A.; Schmidt, J.; Beltrán, A.M.; Shahid, A.; Alber, N.K.; Peukert, W.; Boccaccini, A.R. Synthesis and Characterization of Manganese Containing Mesoporous Bioactive Glass Nanoparticles for Biomedical Applications. J. Mater. Sci. Mater. Med. 2018, 29, 64. [Google Scholar] [CrossRef] [PubMed]
- Karbowniczek, J.E.; Kaniuk, Ł.; Berniak, K.; Gruszczyński, A.; Stachewicz, U. Enhanced Cells Anchoring to Electrospun Hybrid Scaffolds with PHBV and HA Particles for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 632029. [Google Scholar] [CrossRef] [PubMed]




| Samples | Diameter (µm) | PDI | Zeta Potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| PHBV/MBGN | 6.1 ± 0.7 a | 0.9 ± 0.1 a | −20.7 ± 0.4 a | – |
| PHBV/MBGN/CIN5 | 7.2 ± 1.5 a | 0.4 ± 0.1 b | −21.3 ± 0.5 a | 99.96 ± 0.01 a |
| PHBV/MBGN/CIN10 | 11.4 ± 1.6 b | 0.6 ± 0.2 ab | −20.4 ± 0.5 a | 99.83 ± 0.02 a |
| PHBV/MBGN/CIN20 | 12.5 ± 2.3 b | 0.5 ± 0.2 ab | −12.2 ± 2.7 b | 99.26 ± 0.04 a |
| Systems | Kinetic Models | ||||||
|---|---|---|---|---|---|---|---|
| Zero-Order | First Order | Higuchi | Hixson-Crowell | Korsmeyer–Peppas | |||
| R2 | R2 | K1 h−1 | R2 | R2 | R2 | n | |
| PHBV/MBGN/CIN5 | −5.22 | 0.87 | 0.42 | −2.24 | −3.01 | 0.68 | 0.12 |
| PHBV/MBGN/CIN10 | −5.63 | 0.84 | 0.41 | −2.42 | −3.24 | 0.72 | 0.12 |
| PHBV/MBGN/CIN20 | −18.29 | 0.76 | 0.93 | −9.95 | −12.41 | 0.74 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotchindakun, K.; Pekkoh, J.; Ruangsuriya, J.; Zheng, K.; Unalan, I.; Boccaccini, A.R. Fabrication and Characterization of Cinnamaldehyde-Loaded Mesoporous Bioactive Glass Nanoparticles/PHBV-Based Microspheres for Preventing Bacterial Infection and Promoting Bone Tissue Regeneration. Polymers 2021, 13, 1794. https://doi.org/10.3390/polym13111794
Chotchindakun K, Pekkoh J, Ruangsuriya J, Zheng K, Unalan I, Boccaccini AR. Fabrication and Characterization of Cinnamaldehyde-Loaded Mesoporous Bioactive Glass Nanoparticles/PHBV-Based Microspheres for Preventing Bacterial Infection and Promoting Bone Tissue Regeneration. Polymers. 2021; 13(11):1794. https://doi.org/10.3390/polym13111794
Chicago/Turabian StyleChotchindakun, Kittipat, Jeeraporn Pekkoh, Jetsada Ruangsuriya, Kai Zheng, Irem Unalan, and Aldo R. Boccaccini. 2021. "Fabrication and Characterization of Cinnamaldehyde-Loaded Mesoporous Bioactive Glass Nanoparticles/PHBV-Based Microspheres for Preventing Bacterial Infection and Promoting Bone Tissue Regeneration" Polymers 13, no. 11: 1794. https://doi.org/10.3390/polym13111794








