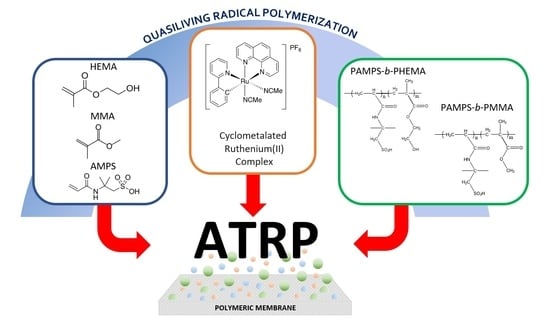
Synthesis of Poly(2-Acrylamido-2-Methylpropane Sulfonic Acid) and its Block Copolymers with Methyl Methacrylate and 2-Hydroxyethyl Methacrylate by Quasiliving Radical Polymerization Catalyzed by a Cyclometalated Ruthenium(II) Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
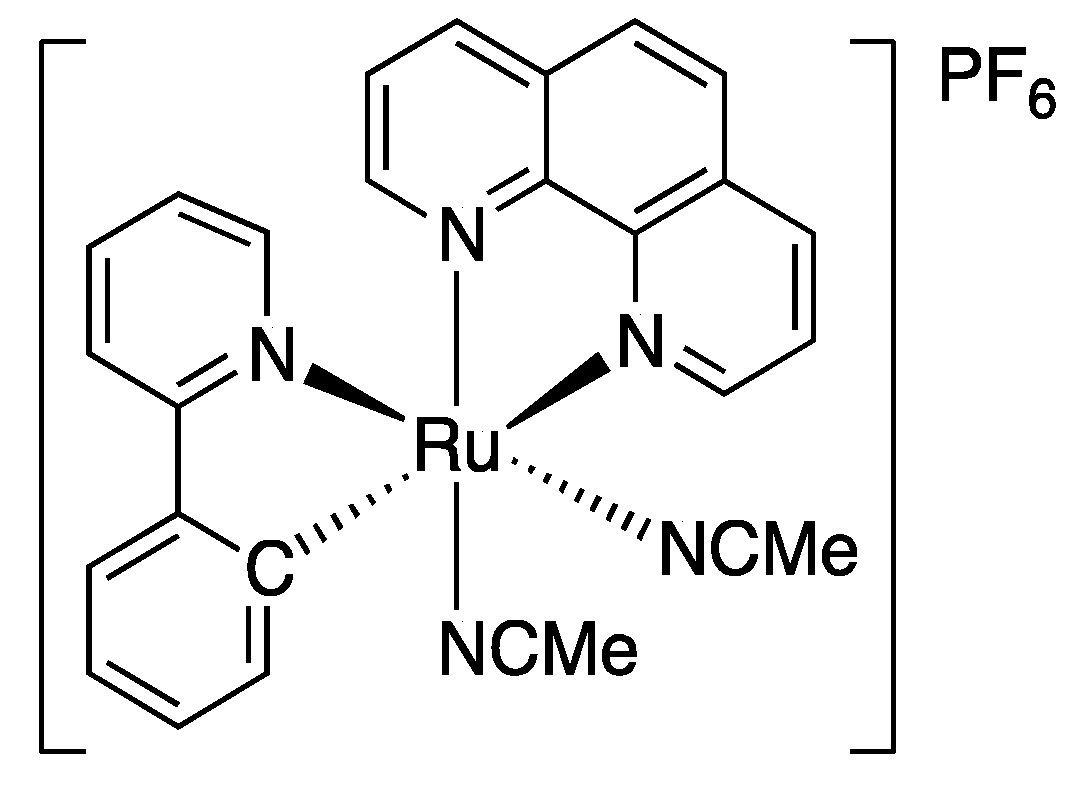
2.2. Synthesis of the Ruthenium(II) Catalyst
2.3. Synthesis of the Homopolymers and Copolymers
Synthesis of PAMPS-b-PMMA Copolymer
2.4. Characterization and Membrane Preparation
2.5. Membrane Preparation
2.6. Determination of Ion Exchange Capacity (IEC)
3. Results and Discussion
3.1. Synthesis and Characterization of AMPS Homopolymer
3.2. Synthesis of Block Copolymers
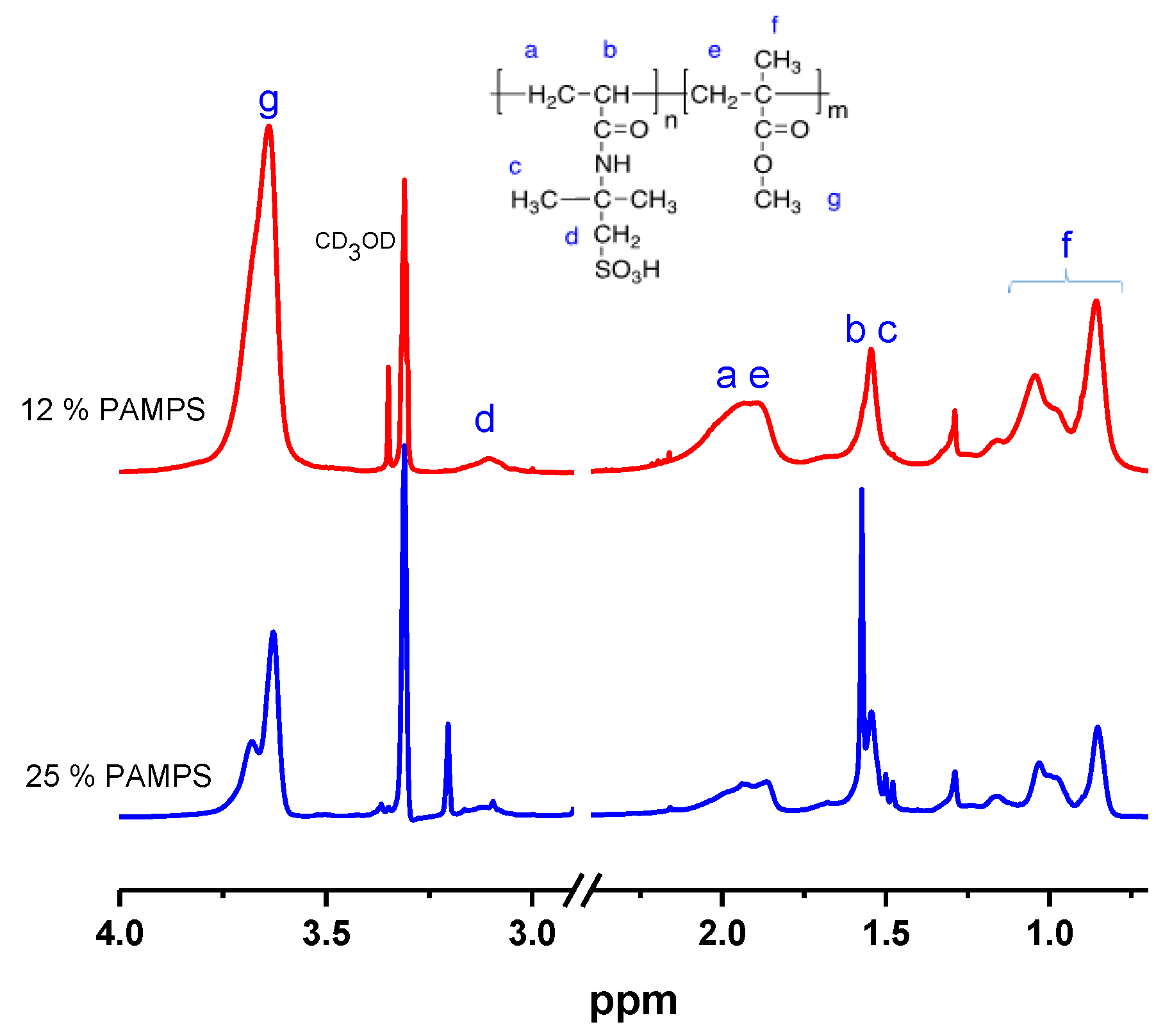
3.2.1. Synthesis of PAMPS-b-PMMA Block Copolymer
3.2.2. Synthesis of PAMPS-b-PHEMA Block Copolymer
3.3. Ion Exchange Capacity (IEC) of Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmadian-Alam, L.; Kheirmand, M.; Mahdavi, H. Preparation, Characterization and Properties of PVDF-g PAMPS/PMMA-Co-PAMPS/Silica Nanoparticle as a New Proton Exchange Nanocomposite Membrane. Chem. Eng. J. 2016, 284, 1035–1048. [Google Scholar] [CrossRef]
- Ahmadian-Alam, L.; Mahdavi, H. Preparation and Characterization of PVDF-Based Blend Membranes as Polymer Electrolyte Membranes in Fuel Cells: Study of Factor Affecting the Proton Conductivity Behavior. Polym. Adv. Technol. 2018, 29, 2287–2299. [Google Scholar] [CrossRef]
- Ganguly, S.; Maity, T.; Mondal, S.; Das, P.; Das, N.C. Starch Functionalized Biodegradable Semi-IPN as a PH-Tunable Controlled Release Platform for Memantine. Int. J. Biol. Macromol. 2017, 95, 185–198. [Google Scholar] [CrossRef]
- Corzo-González, Z.; Loria-Bastarrachea, M.I.; Hernández-Nuñez, E.; Aguilar-Vega, M.; González-Díaz, M.O. Preparation and Characterization of Crosslinked PVA/PAMPS Blends Catalytic Membranes for Biodiesel Production. Polym. Bull. 2017, 74, 2741–2754. [Google Scholar] [CrossRef]
- Benkhaled, B.T.; Hadiouch, S.; Olleik, H.; Perrier, J.; Ysacco, C.; Guillaneuf, Y.; Gigmes, D.; Maresca, M.; Lefay, C. Elaboration of Antimicrobial Polymeric Materials by Dispersion of Well-Defined Amphiphilic Methacrylic SG1-Based Copolymers. Polym. Chem. 2018, 9, 3127–3141. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Poly(Ionic Liquid)s as Antimicrobial Materials. Eur. Polym. J. 2018, 105, 135–149. [Google Scholar] [CrossRef]
- Williams, P.A. Handbook of Industrial Water Soluble Polymers, 1st ed.; Wiley-Blackwell: Iowa City, IA, USA, 2007. [Google Scholar]
- Atta, A.M.; El-Mahdy, G.A.; Allohedan, H.A.; Abdullah, M.M.S. Synthesis and Application of Poly Ionic Liquid-Based on 2-Acrylamido-2-Methyl Propane Sulfonic Acid as Corrosion Protective Film of Steel. Int. J. Electrochem. Sci. 2015, 10, 6106–6119. [Google Scholar]
- Son, Y.J.; Kim, S.J.; Kim, Y.J.; Jung, K.H. Selective Vapor Permeation Behavior of Crosslinked PAMPS Membranes. Polymers 2020, 12, 987. [Google Scholar] [CrossRef]
- Shen, Y.; Xi, J.; Qiu, X.; Zhu, W. A New Proton Conducting Membrane Based on Copolymer of Methyl Methacrylate and 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid for Direct Methanol Fuel Cells. Electrochim. Acta 2007, 52, 6956–6961. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Iván, B. Macromolecular Nomenclature Note No. 19: Terminology and Classification of Quasiliving Polymerizations and Ideal Living Polymerizations on the Basis of the Logic of Elementary Polymerization Reactions, and Comments on Using the Term “Controlled”. Macromol. Chem. Phys. 2000, 201, 2621–2628. [Google Scholar]
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef]
- Masci, G.; Giacomelli, L.; Crescenzi, V. Atom Transfer Radical Polymerization of Sodium 2-Acrylamido-2- Methylpropanesulfonate. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4446–4454. [Google Scholar] [CrossRef]
- Masci, G.; Diociaiuti, M.; Crescenzi, V. ATRP Synthesis and Association Properties of Thermoresponsive Anionic Block Copolymers. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4830–4842. [Google Scholar] [CrossRef]
- Tolstov, A.; Gromadzki, D.; Netopilík, M.; Makuška, R. Aqueous AGET ATRP of Sodium 2-Acrylamido-2-Methyl-N-Propane Sulfonate Yielding Strong Anionic Comb Polyelectrolytes. E-Polymers 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Lin, J. PAMPS-Graft-Ni3Si2O5(OH)4 Multiwalled Nanotubes as a Novel Nano-Sorbent for the Effective Removal of Pb(Ii) Ions. Rsc Adv. 2020, 10, 7619–7627. [Google Scholar] [CrossRef] [Green Version]
- Motlaq, V.F.; Momtazi, L.; Zhu, K.; Knudsen, K.D.; Nyström, B. Differences in Self-Assembly Features of Thermoresponsive Anionic Triblock Copolymers Synthesized via One-Pot or Two-Pot by Atom Transfer Radical Polymerization. J. Polym. Sci. Part. B Polym. Phys. 2019, 57, 524–534. [Google Scholar] [CrossRef]
- Jing, X.; Huang, Z.; Lu, H.; Wang, B. Use of a Hydrophobic Associative Four-Armed Star Anionic Polymer to Create a Saline Aqueous Solution of CO2-Switchability. J. Dispers. Sci. Technol. 2017, 38, 1698–1704. [Google Scholar] [CrossRef]
- McCullough, L.A.; Dufour, B.; Matyjaszewski, K. Incorporation of Poly(2-Acrylamido-2-Methyl-N-Propanesulfonic Acid) Segments into Block and Brush Copolymers by ATRP. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5386–5396. [Google Scholar] [CrossRef]
- Gonzalez-Diaz, M.O.; Morales, S.L.; Lagadec, R.L.; Alexandrova, L. Homogeneous Radical Polymerization of 2-Hydroxyethyl Methacrylate Mediated by Cyclometalated Cationic Ruthenium(II) Complexes with PF6− and Cl− in Protic Media. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4562–4577. [Google Scholar] [CrossRef]
- Das, K.; Dutta, M.; Das, B.; Srivastava, H.K.; Kumar, A. Efficient Pincer-Ruthenium Catalysts for Kharasch Addition of Carbon Tetrachloride to Styrene. Adv. Synth. Catal. 2019, 361, 2965–2980. [Google Scholar] [CrossRef]
- Nishizawa, K.; Ouchi, M.; Sawamoto, M. Design of a Hydrophilic Ruthenium Catalyst for Metal-Catalyzed Living Radical Polymerization: Highly Active Catalysis in Water. Rsc Adv. 2016, 6, 6577–6582. [Google Scholar] [CrossRef]
- Alfredo, N.V.; Jalapa, N.E.; Morales, S.L.; Ryabov, A.D.; Le Lagadec, R.; Alexandrova, L. Light-Driven Living/Controlled Radical Polymerization of Hydrophobic Monomers Catalyzed by Ruthenium(II) Metalacycles. Macromolecules 2012, 45, 8135–8146. [Google Scholar] [CrossRef]
- Martínez Cornejo, V.; Olvera Mancilla, J.; López Morales, S.; Oviedo Fortino, J.A.; Hernández-Ortega, S.; Alexandrova, L.; Le Lagadec, R. Synthesis and Comparative Behavior of Ruthena(II)Cycles Bearing Benzene Ligand in the Radical Polymerization of Styrene and Vinyl Acetate. J. Organomet. Chem. 2015, 799–800, 299–310. [Google Scholar]
- García Vargas, M.; Mendoza Aquino, G.; Aguilar Lugo, C.; López Morales, S.; Torres González, J.E.; Le Lagadec, R.; Alexandrova, L. Living Radical Polymerization of Hydrophobic Monomers Catalyzed by Cyclometalated Ruthenium(II) Complexes: Improved Control and Formation of Block Co-Polymers. Eur. Polym. J. 2018, 108, 171–181. [Google Scholar] [CrossRef]
- Barbosa, A.S.L.; Werlé, C.; Colunga, C.O.O.; Rodríguez, C.F.; Toscano, R.A.; Le Lagadec, R.; Pfeffer, M. Further Insight into the Lability of MeCN Ligands of Cytotoxic Cycloruthenated Compounds: Evidence for the Antisymbiotic Effect Trans to the Carbon Atom at the Ru Center. Inorg. Chem. 2015, 54, 7617–7626. [Google Scholar] [CrossRef]
- Ryabov, A.D.; Sukharev, V.S.; Alexandrova, L.; Le Lagadec, R.; Pfeffer, M. New Synthesis and New Bio-Application of Cyclometalated Ruthenium(II) Complexes for Fast Mediated Electron Transfer with Peroxidase and Glucose Oxidase. Inorg. Chem. 2001, 40, 6529–6532. [Google Scholar] [CrossRef]
- Nadim, E.; Bouhendi, H.; Ziaee, F.; Nouri, A. Kinetic Study of the Aqueous Free-Radical Polymerization of 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid via an Online Proton Nuclear Magnetic Resonance Technique. J. Appl. Polym. Sci. 2012, 126, 156–161. [Google Scholar] [CrossRef]
- Mincheva, R.; Paneva, D.; Mespouille, L.; Manolova, N.; Rashkov, I.; Dubois, P. Optimized Water-Based ATRP of an Anionic Monomer: Comprehension and Properties Characterization. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1108–1119. [Google Scholar] [CrossRef]
- Kazantsev, O.A.; Igolkin, A.V.; Shirshin, K.V.; Kuznetsova, N.A.; Spirina, A.N.; Malyshev, A.P. Spontaneous Polymerization of 2-Acrylamido-2-Methylpropanesulfonic Acid in Acidic Aqueous Solutions. Russ. J. Appl. Chem. 2002, 75, 465–469. [Google Scholar]
- Ouchi, M.; Terashima, T.; Sawamoto, M. Transition Metal-Catalyzed Living Radical Polymerization: Toward Perfection in Catalyst and Precision Polymer Synthesis. Chem. Rev. 2009, 109, 4963–5050. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Ivan, B. Designed Polymers by Carbocationic Macromolecular Engineering: Theory and Practice; Hanser Publishers: Munich, Germany; New York, NY, USA, 1992. [Google Scholar]
- Iván, B. Macromolecular Nomenclature Note No. 19—Terminology and Classification of Quasiliving Polymerization and Ideal Living Polymerization on the Basis of the Logic Elementary Polymerization Reactions, and Comments on Using the Term “Controlled”. Polym. Prepr. 2000, 41, 6–13. [Google Scholar]
- Robinson, K.L.; Khan, M.A.; De Paz Báñez, M.V.; Wang, X.S.; Armes, S.P. Controlled Polymerization of 2-Hydroxyethyl Methacrylate by ATRP at Ambient Temperature. Macromolecules 2001, 34, 3155–3158. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, H.J.; Joe, B. Water Soluble Polymers as Proton Exchange Membranes for Fuel Cells. Polymers 2012, 4, 913–963. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, E.K.; Bethani, A.; Bokias, G.; Kallitsis, J.K. Poly(Sodium Styrene Sulfonate)-b-Poly(Methyl Methacrylate) Diblock Copolymers through Direct Atom Transfer Radical Polymerization: Influence of Hydrophilic–Hydrophobic Balance on Self-Organization in Aqueous Solution. Eur. Polym. J. 2011, 47, 752–761. [Google Scholar] [CrossRef]
- Peng, C.H.; Kong, J.; Seeliger, F.; Matyjaszewski, K. Mechanism of Halogen Exchange in ATRP. Macromolecules 2011, 44, 7546–7557. [Google Scholar] [CrossRef]
- Datta, H.; Singha, N.K.; Bhowmick, A.K. Beneficial Effect of Nanoclay in Atom Transfer Radical Polymerization of Ethyl Acrylate: A One Pot Preparation of Tailor-Made Polymer Nanocomposite. Macromolecules 2008, 41, 50–57. [Google Scholar] [CrossRef]
- Wang, X.S.; Armes, S.P. Facile Atom Transfer Radical Polymerization of Methoxy-Capped Oligo(Ethylene Glycol) Methacrylate in Aqueous Media at Ambient Temperature. Macromolecules 2000, 33, 6640–6647. [Google Scholar] [CrossRef]
- Reining, B.; Keul, H.; Höcker, H. Block Copolymers Comprising Poly(Ethylene Oxide) and Poly(Hydroxyethyl Methacrylate) Blocks: Synthesis and Characterization. Polymer 2002, 43, 3139–3145. [Google Scholar] [CrossRef]
- Szabó, Á.; Wacha, A.; Thomann, R.; Szarka, G.; Bóta, A.; Iván, B. Synthesis of Poly(Methyl Methacrylate)-Poly(Poly(Ethylene Glycol) Methacrylate)-Polyisobutylene ABCBA Pentablock Copolymers by Combining Quasiliving Carbocationic and Atom Transfer Radical Polymerizations and Characterization Thereof. J. Macromol. Sci. Part A Pure Appl. Chem. 2015, 52, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Sumerlin, B.S.; Donovan, M.S.; Mitsukami, Y.; Lowe, A.B.; McCormick, C.L. Water-Soluble Polymers. 84. Controlled Polymerization in Aqueous Media of Anionic Acrylamido Monomers via RAFT. Macromolecules 2001, 34, 6561–6564. [Google Scholar] [CrossRef]
- Bucholz, T.L.; Loo, Y.-L.L. Polar Aprotic Solvents Disrupt Interblock Hydrogen Bonding and Induce Microphase Separation in Double Hydrophilic Block Copolymers of PEGMA and PAAMPSA. Macromolecules 2008, 41, 4069–4070. [Google Scholar] [CrossRef]
- Agut, W.; Brûlet, A.; Taton, D.; Lecommandoux, S. Thermoresponsive Micelles from Jeffamine-b-Poly(L-Glutamic Acid) Double Hydrophilic Block Copolymers. Langmuir 2007, 23, 11526–11533. [Google Scholar] [CrossRef] [PubMed]
- Hajibeygi, M.; Shafiei-Navid, S.; Shabanian, M.; Vahabi, H. Novel Poly(Amide-Azomethine) Nanocomposites Reinforced with Polyacrylic Acid-Co-2-Acrylamido-2-Methylpropanesulfonic Acid Modified LDH: Synthesis and Properties. Appl. Clay Sci. 2018, 157, 165–176. [Google Scholar] [CrossRef]
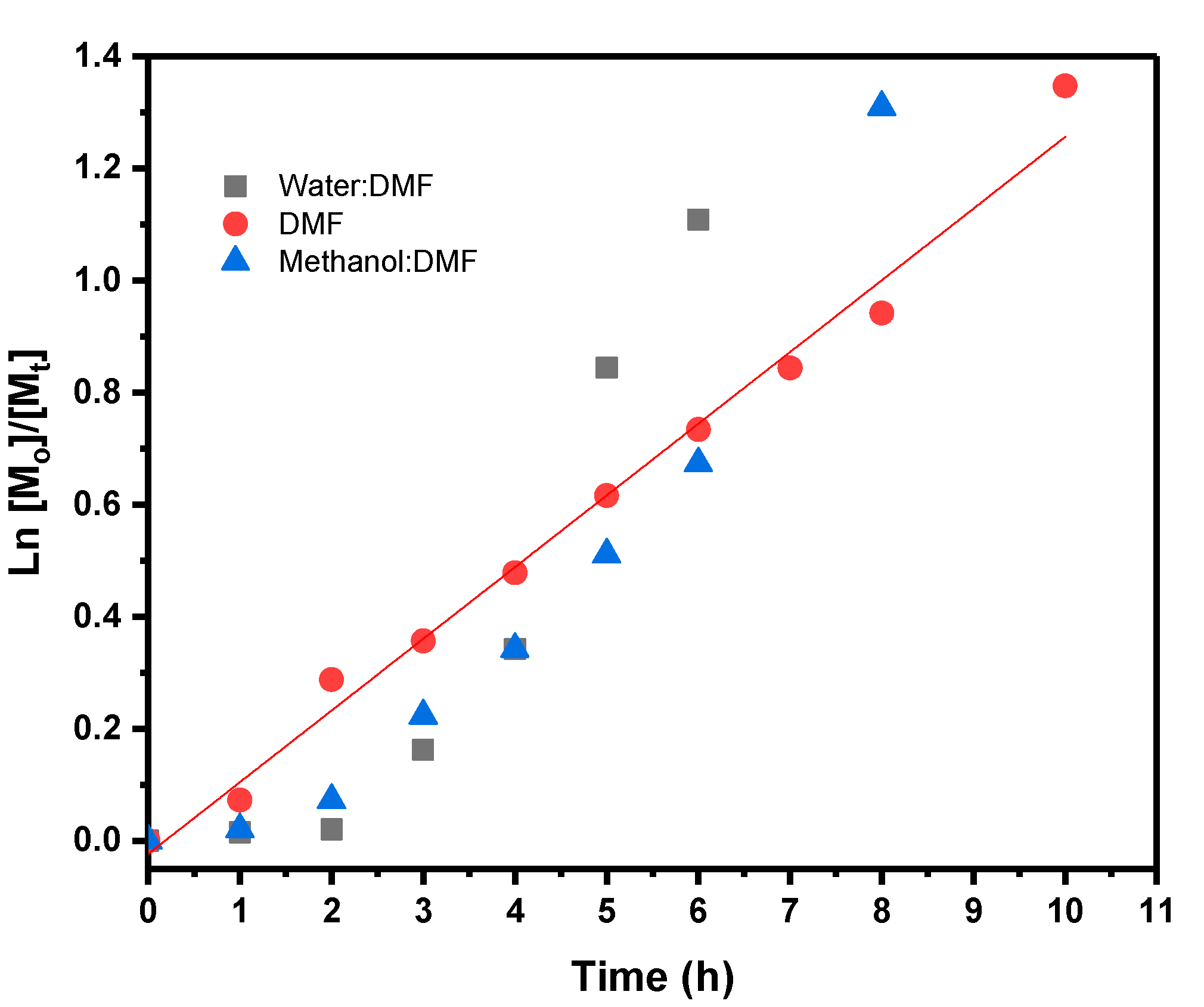
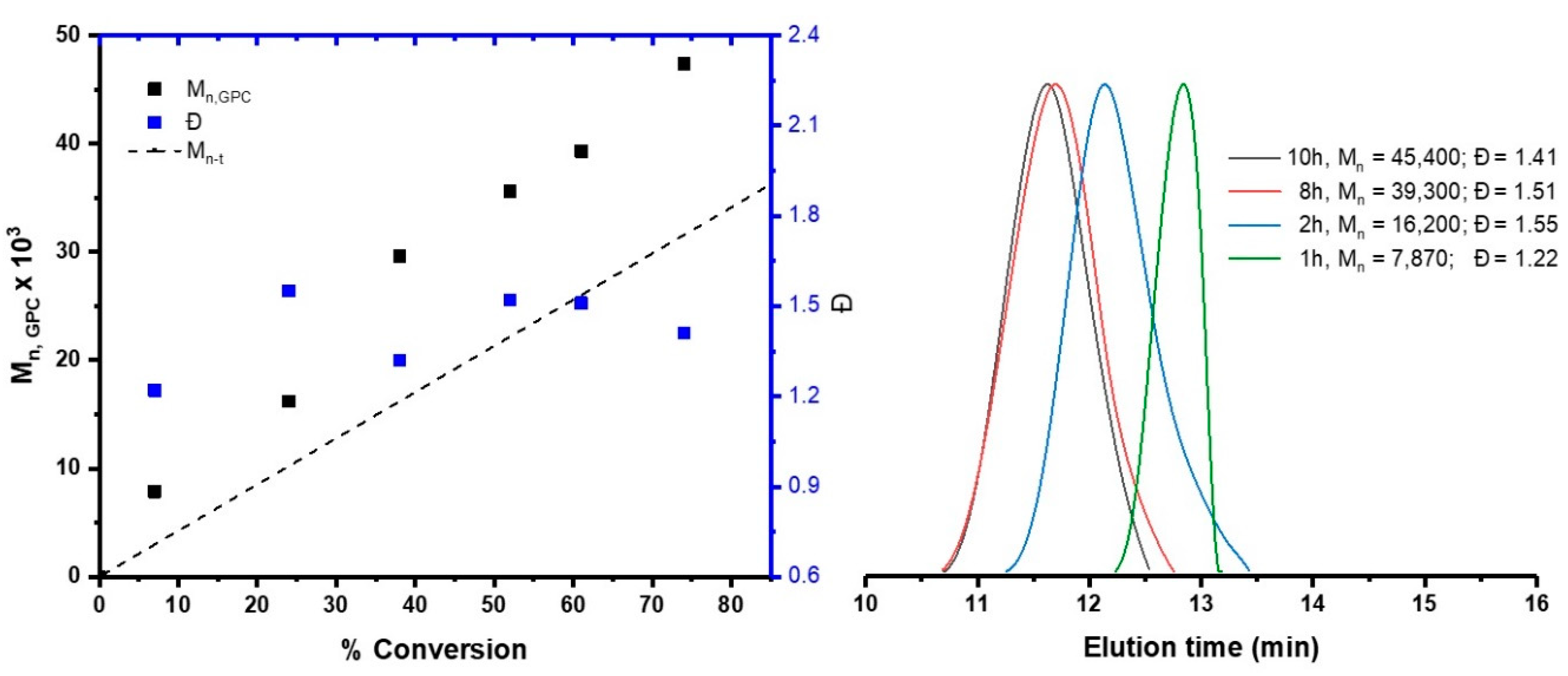


| Solvent | Time (h) | Conversion (%) | Mn × 103 g/mol | Ð |
|---|---|---|---|---|
| Water:DMF | 6 | 68 | 88.3 | 1.83 |
| Methanol:DMF | 8 | 73 | 75.2 | 1.75 |
| DMF | 8 | 61 | 39.3 | 1.51 |
| Copolymer | PAMPS %mol | Theoretical IEC (mmol H+g–1) | Experimental IEC (mmol H+g–1) |
|---|---|---|---|
| PAMPS-b-PMMA | 12 | 0.58 | 0.55 |
| 25 | 1.21 | 1.40 | |
| PAMPS-b-PHEMA | 65 | 3.13 | 3.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cornejo, V.; Velázquez-Roblero, J.; Rosiles-González, V.; Correa-Duran, M.; Avila-Ortega, A.; Hernández-Núñez, E.; Le Lagadec, R.; González-Díaz, M.O. Synthesis of Poly(2-Acrylamido-2-Methylpropane Sulfonic Acid) and its Block Copolymers with Methyl Methacrylate and 2-Hydroxyethyl Methacrylate by Quasiliving Radical Polymerization Catalyzed by a Cyclometalated Ruthenium(II) Complex. Polymers 2020, 12, 1663. https://doi.org/10.3390/polym12081663
Martínez-Cornejo V, Velázquez-Roblero J, Rosiles-González V, Correa-Duran M, Avila-Ortega A, Hernández-Núñez E, Le Lagadec R, González-Díaz MO. Synthesis of Poly(2-Acrylamido-2-Methylpropane Sulfonic Acid) and its Block Copolymers with Methyl Methacrylate and 2-Hydroxyethyl Methacrylate by Quasiliving Radical Polymerization Catalyzed by a Cyclometalated Ruthenium(II) Complex. Polymers. 2020; 12(8):1663. https://doi.org/10.3390/polym12081663
Chicago/Turabian StyleMartínez-Cornejo, Vanessa, Joaquin Velázquez-Roblero, Veronica Rosiles-González, Monica Correa-Duran, Alejandro Avila-Ortega, Emanuel Hernández-Núñez, Ronan Le Lagadec, and Maria Ortencia González-Díaz. 2020. "Synthesis of Poly(2-Acrylamido-2-Methylpropane Sulfonic Acid) and its Block Copolymers with Methyl Methacrylate and 2-Hydroxyethyl Methacrylate by Quasiliving Radical Polymerization Catalyzed by a Cyclometalated Ruthenium(II) Complex" Polymers 12, no. 8: 1663. https://doi.org/10.3390/polym12081663