Copolymerization of Ethylene and Vinyl Fluoride by Self-Assembled Multinuclear Palladium Catalysts
Abstract
:1. Introduction
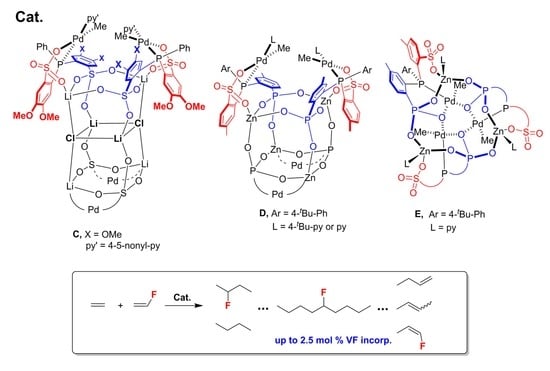
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boffa, L.S.; Novak, B.M. Copolymerization of polar monomers with olefins using transition-metal complexes. Chem. Rev. 2000, 100, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef]
- Berkefeld, A.; Mecking, S. Coordination copolymerization of polar vinyl monomers H2C=CHX. Angew. Chem. Int. Ed. 2008, 47, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination−insertion copolymerization of fundamental polar monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.-X. Coordination polymerization of polar vinyl monomers by single-site metal catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef]
- Nakamura, A.; Anselment, T.M.J.; Claverie, J.; Goodall, B.; Jordan, R.F.; Mecking, S.; Rieger, B.; Sen, A.; van Leeuwen, P.W.N.M.; Nozaki, K. Ortho-phosphinobenzenesulfonate: A superb ligand for palladium-catalyzed coordination–insertion copolymerzation of polar vinyl monomers. Acc. Chem. Res. 2013, 46, 1438–1449. [Google Scholar] [CrossRef]
- Mu, H.L.; Pan, L.; Song, D.; Li, Y.S. Neutral nickel catalysts for olefin homo- and copolymerization: Relationships between catalyst structures and catalytic properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef]
- Chen, C. Designing catalysts for olefin polymerization and copolymerization: Beyond electronic and steric tuning. Nat. Rev. Chem. 2018, 2, 6–14. [Google Scholar] [CrossRef]
- Kang, M.; Sen, A.; Zakharov, L.; Rheingold, A.L. Diametrically opposite trends in alkene insertion in late and early transition metal compounds: Relevance to transition-metal-catalyzed polymerization of polar vinyl monomers. J. Am. Chem. Soc. 2002, 124, 12080–12081. [Google Scholar] [CrossRef]
- Zhao, H.; Ariafard, A.; Lin, Z. In-depth insight into metal–alkene bonding interactions. Inorg. Chim. Acta 2006, 359, 3527–3534. [Google Scholar] [CrossRef]
- Stockland, R.A., Jr.; Jordan, R.F. Reaction of vinyl chloride with a prototypical metallocene catalyst: Stoichiometric insertion and β-Cl elimination reactions with rac-(EBI)ZrMe+ and catalytic dechlorination/oligomerization to oligopropylene by rac-(EBI)ZrMe2/MAO. J. Am. Chem. Soc. 2000, 122, 6315–6316. [Google Scholar] [CrossRef]
- Foley, S.R.; Stockland, R.A.; Shen, H.; Jordan, R.F. Reaction of vinyl chloride with late transition metal olefin polymerization catalysts. J. Am. Chem. Soc. 2003, 125, 4350–4361. [Google Scholar] [CrossRef] [PubMed]
- Strazisar, S.A.; Wolczanski, P.T. Insertion of H2CCHX (X = F, Cl, Br, OiPr) into (tBu3SiO)3TaH2 and β-X-Elimination from (tBu3SiO)3HTaCH2CH2X (X = OR): Relevance to Ziegler−Natta copolymerizations. J. Am. Chem. Soc. 2001, 123, 4728–4740. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, S.G. Vinyl Chloride as a Chain Transfer agent in olefin polymerizations: Preparation of highly branched and end functional polyolefins. Macromolecules 2003, 36, 4692–4698. [Google Scholar] [CrossRef]
- Watson, L.A.; Yandulov, D.V.; Caulton, K.G. C−D0 (D0 = π-donor, F) cleavage in H2C=CH(D0) by (Cp2ZrHCl)n: Mechanism, agostic fluorines, and a carbene of Zr(IV). J. Am. Chem. Soc. 2001, 123, 603–611. [Google Scholar] [CrossRef]
- Kilyanek, S.M.; Stoebenau, E.J., III; Vinayavekhin, N.; Jordan, R.F. Mechanism of the reaction of vinyl chloride with (α-diimine)PdMe+ species. Organometallics 2010, 29, 1750–1760. [Google Scholar] [CrossRef]
- Stockland, R.A., Jr.; Foley, S.R.; Jordan, R.F. Reaction of vinyl chloride with group 4 metal olefin polymerization catalysts. J. Am. Chem. Soc. 2003, 125, 796–809. [Google Scholar] [CrossRef]
- Boone, H.W.; Athey, P.S.; Mullins, M.J.; Philipp, D.; Muller, R.; Goddard, W.A. Copolymerization studies of vinyl chloride and vinyl acetate with ethylene using a transition-metal catalyst. J. Am. Chem. Soc. 2002, 124, 8790–8791. [Google Scholar] [CrossRef] [Green Version]
- Leicht, H.; Gottker-Schnetmann, I.; Mecking, S. Incorporation of vinyl chloride in insertion polymerization. Angew. Chem. Int. Ed. 2013, 52, 3963–3966. [Google Scholar] [CrossRef]
- Clot, E.; Mégret, C.; Kraft, B.M.; Eisenstein, O.; Jones, W.D. Defluorination of perfluoropropene using Cp*2ZrH2 and Cp2ZrHF: A mechanism investigation from a joint experimental-theoretical perspective. J. Am. Chem. Soc. 2004, 126, 5647–5653. [Google Scholar] [CrossRef]
- Foley, S.R.; Shen, H.; Qadeer, U.A.; Jordan, R.F. Generation and insertion reactivity of cationic palladium complexes that contain halogenated alkyl ligands. Organometallics 2004, 23, 600–609. [Google Scholar] [CrossRef]
- Weng, W.; Shen, Z.; Jordan, R.F. Copolymerization of ethylene and vinyl fluoride by (Phosphine-Sulfonate)Pd(Me)(py)catalysts. J. Am. Chem. Soc. 2007, 129, 15450–15451. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Jordan, R.F. Olefin insertion into a Pd-F bond: Catalyst reactivation following β-F elimination in ethylene/vinyl fluoride copolymerization. Angew. Chem. Int. Ed. 2017, 129, 1846–1850. [Google Scholar] [CrossRef]
- Liu, Q.; Jordan, R.F. Synthesis and reactivity of phosphine-arenesulfonate palladium(II) alkyl complexes that contain methoxy substituents. J. Organomet. Chem. 2019, 896, 207–214. [Google Scholar] [CrossRef]
- Black, R.E.; Kilyanek, S.M.; Reinhart, E.D.; Jordan, R.F. Olefin insertion reactivity of a (phosphine-arenesulfonate) palladium(II) fluoride complex. Organometallics 2019, 38, 4250–4260. [Google Scholar] [CrossRef]
- Drent, E.; van Dijk, R.; van Ginkel, R.; van Oort, B.; Pugh, R.I. Palladium catalysed copolymerisation of ethene with alkylacrylates: Polar comonomer built into the linear polymer chain. Chem. Commun. 2002, 7, 744–745. [Google Scholar] [CrossRef]
- Luo, S.; Vela, J.; Lief, G.R.; Jordan, R.F. Copolymerization of ethylene and alkyl vinyl ethers by a (phosphine- sulfonate)PdMe catalyst. J. Am. Chem. Soc. 2007, 129, 8946–8947. [Google Scholar] [CrossRef]
- Guironnet, D.; Roesle, P.; Runzi, T.; Gottker-Schnetmann, I.; Mecking, S. Insertion polymerization of acrylate. J. Am. Chem. Soc. 2009, 131, 422–423. [Google Scholar] [CrossRef] [Green Version]
- Kochi, T.; Noda, S.; Yoshimura, K.; Nozaki, K. Formation of linear copolymers of ethylene and acrylonitrile catalyzed by phosphine sulfonate palladium Complexes. J. Am. Chem. Soc. 2007, 129, 8948–8949. [Google Scholar] [CrossRef]
- Ito, S.; Munakata, K.; Nakamura, A.; Nozaki, K. Copolymerization of vinyl acetate with ethylene by palladium/alkylphosphine−sulfonate catalysts. J. Am. Chem. Soc. 2009, 131, 14606–14607. [Google Scholar] [CrossRef]
- Skupov, K.M.; Marella, P.R.; Simard, M.; Yap, G.P.A.; Allen, N.; Conner, D.; Goodall, B.L.; Claverie, J.P. Palladium Aryl sulfonate phosphine catalysts for the copolymerization of acrylates with ethene. Macromol. Rapid Commun. 2007, 28, 2033–2038. [Google Scholar] [CrossRef]
- Skupov, K.M.; Piche, L.; Claverie, J.P. Linear polyethylene with tunable surface properties by catalytic copolymerization of ethylene with N-Vinyl-2-pyrrolidinone and N-Isopropylacrylamide. Macromolecules 2008, 41, 2309–2310. [Google Scholar] [CrossRef]
- Chen, Z.; Brookhart, M. Exploring ethylene/polar vinyl monomer copolymerizations using Ni and Pd α-diimine catalysts. Acc. Chem. Res. 2018, 51, 1831–1839. [Google Scholar] [CrossRef]
- Keyes, A.; Alhan, H.E.B.; Ordonez, E.; Ha, U.; Beezer, D.B.; Dau, H.; Liu, Y.S.; Tsogtgerel, E.; Jones, G.R.; Harth, E. Olefins and vinyl polar monomers: Bridging the gap for next generation materials. Angew. Chem. Int. Ed. 2019, 58, 12370–12391. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Jordan, R.F. Self-assembled tetranuclear palladium catalysts that produce high molecular weight linear polyethylene. J. Am. Chem. Soc. 2010, 132, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Jordan, R.F. Copolymerization of ethylene and vinyl fluoride by (phosphine-bis (arenesulfonate)) PdMe (pyridine) catalysts: Insights into inhibition mechanisms. Macromolecules 2010, 43, 8706–8708. [Google Scholar] [CrossRef]
- Wei, J.; Shen, Z.; Filatov, A.S.; Liu, Q.; Jordan, R.F. Self-assembled cage structures and ethylene polymerization behavior of palladium alkyl complexes that contain phosphine-bis (arenesulfonate) Ligands. Organometallics 2016, 35, 3557–3568. [Google Scholar] [CrossRef]
- Grinshpun, V.; Rudin, A. Measurement of Mark-Houwink constants by size exclusion chromatography with a low angle laser light scattering detector. Die Makromol. Chem. Rapid Commun. 1985, 6, 219–223. [Google Scholar] [CrossRef]
- Liu, Q.; Jordan, R.F. Sterically controlled self-assembly of a robust multinuclear palladium catalyst for ethylene polymerization. J. Am. Chem. Soc. 2019, 141, 6827–6831. [Google Scholar] [CrossRef]
- Liu, Q.; Jordan, R.F. Multinuclear palladium olefin polymerization catalysts based on self-assembled zinc phosphonate cages. Organometallics 2018, 37, 4664–4674. [Google Scholar] [CrossRef]
- Geier, S.J.; Gille, A.L.; Gilbert, T.M.; Stephan, D.W. From classical adducts to frustrated lewis pairs: Steric effects in the interactions of pyridines and B(C6F5)3. Inorg. Chem. 2009, 48, 10466–10474. [Google Scholar] [CrossRef]
- Ennan, A.A. Pentacoordinate fluorosilicate anions. Russ. Chem. Rev. 1989, 58, 371. [Google Scholar]
- Pevec, A.; Demšar, A. The variations in hydrogen bonding in hexafluorosilicate salts of protonated methyl substituted pyridines and tetramethylethylenediamine. J. Fluor. Chem. 2008, 129, 707–712. [Google Scholar] [CrossRef]
- Conley, B.D.; Yearwood, B.C.; Parkin, S.; Atwood, D.A. Ammonium hexafluorosilicate salts. J. Fluor. Chem. 2002, 115, 155–160. [Google Scholar] [CrossRef]
- Christe, K.O.; Wilson, W.W. Reaction of the fluoride anion with acetonitrile. Chloroform and methylene chloride. J. Fluor. Chem. 1990, 47, 117. [Google Scholar] [CrossRef]
- Christe, K.O.; Wilson, W.W. Nuclear magnetic resonance spectrum of the fluoride anion. J. Fluor. Chem. 1990, 46, 339. [Google Scholar] [CrossRef]
- Massey, A.G.; Park, A.J. Perfluorophenyl derivatives of the elements: VII. further studies on tris (pentafluorophenyl) boron. J. Organomet. Chem. 1996, 5, 218. [Google Scholar] [CrossRef]
- Grushin, V.V. Palladium fluoride complexes: One more step toward metal-mediated C-F bond formation. Chem. A Eur. J. 2002, 8, 1006–1014. [Google Scholar] [CrossRef]
- Katcher, M.H.; Norrby, P.-O.; Doyle, A.G. Mechanistic investigations of palladium-catalyzed allylic fluorination. Organometallics 2014, 33, 2121–2133. [Google Scholar] [CrossRef]
- Park, H.; Verma, P.; Hong, K.; Yu, J.-Q. Controlling Pd (iv) reductive elimination pathways enables Pd (ii)-catalysed enantioselective C (sp3)−H fluorination. Nat. Chem. 2018, 10, 755–762. [Google Scholar] [CrossRef]
- Smith, D.A.; Beweries, T.; Blasius, C.; Jasim, N.; Nazir, R.; Nazir, S.; Robertson, C.C.; Whitwood, A.C.; Hunter, C.A.; Brammer, L.; et al. The contrasting character of early and late transition metal fluorides as hydrogen bond acceptors. Am. Chem. Soc. 2015, 137, 11820–11831. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, A.; Becker, C. Swimming against the Stream? A discussion of the bonding in d6 and d8 fluoro complexes and its consequences for catalytic applications. Helv. Chim. Acta 2002, 85, 2686–2703. [Google Scholar] [CrossRef]
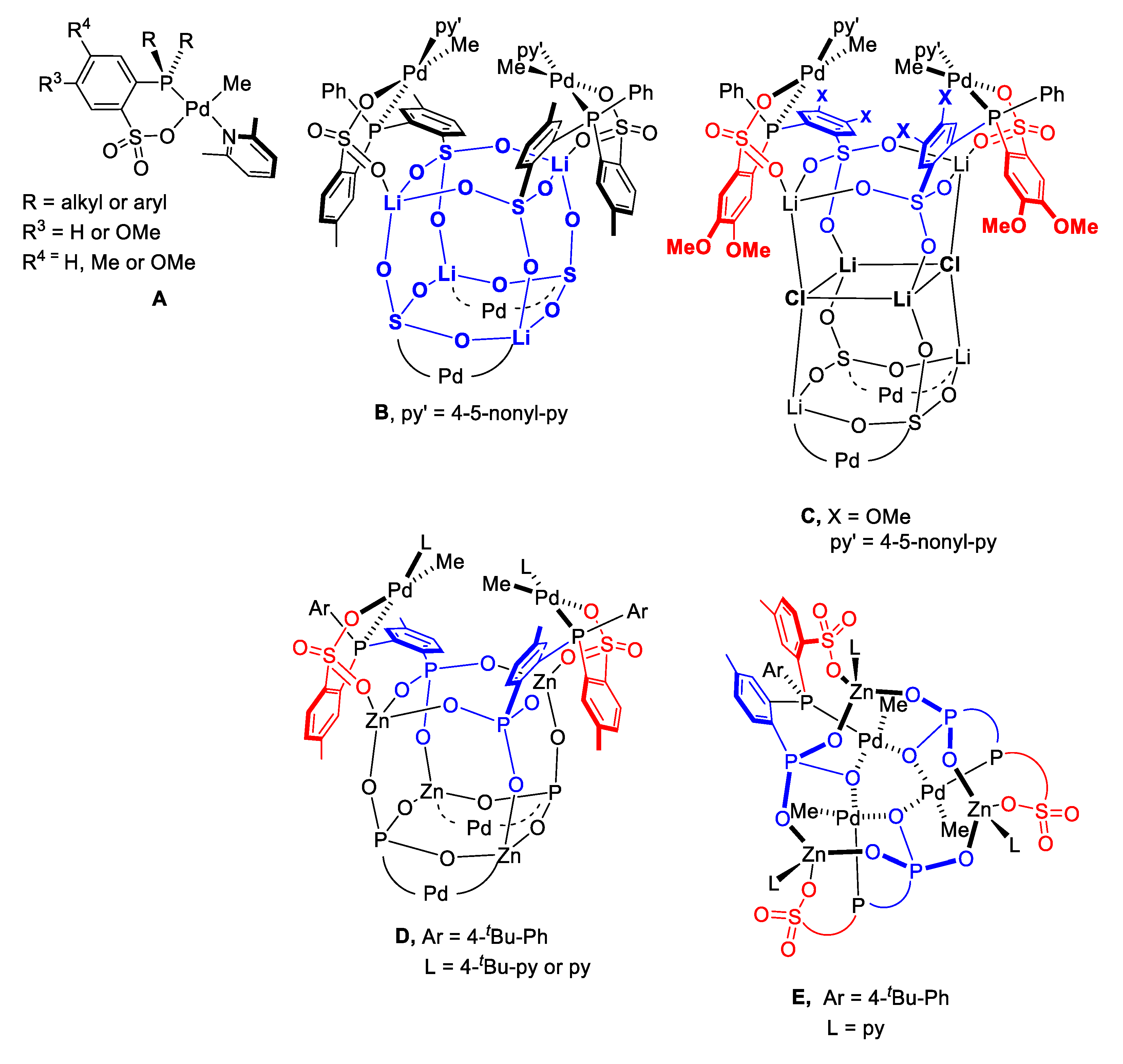


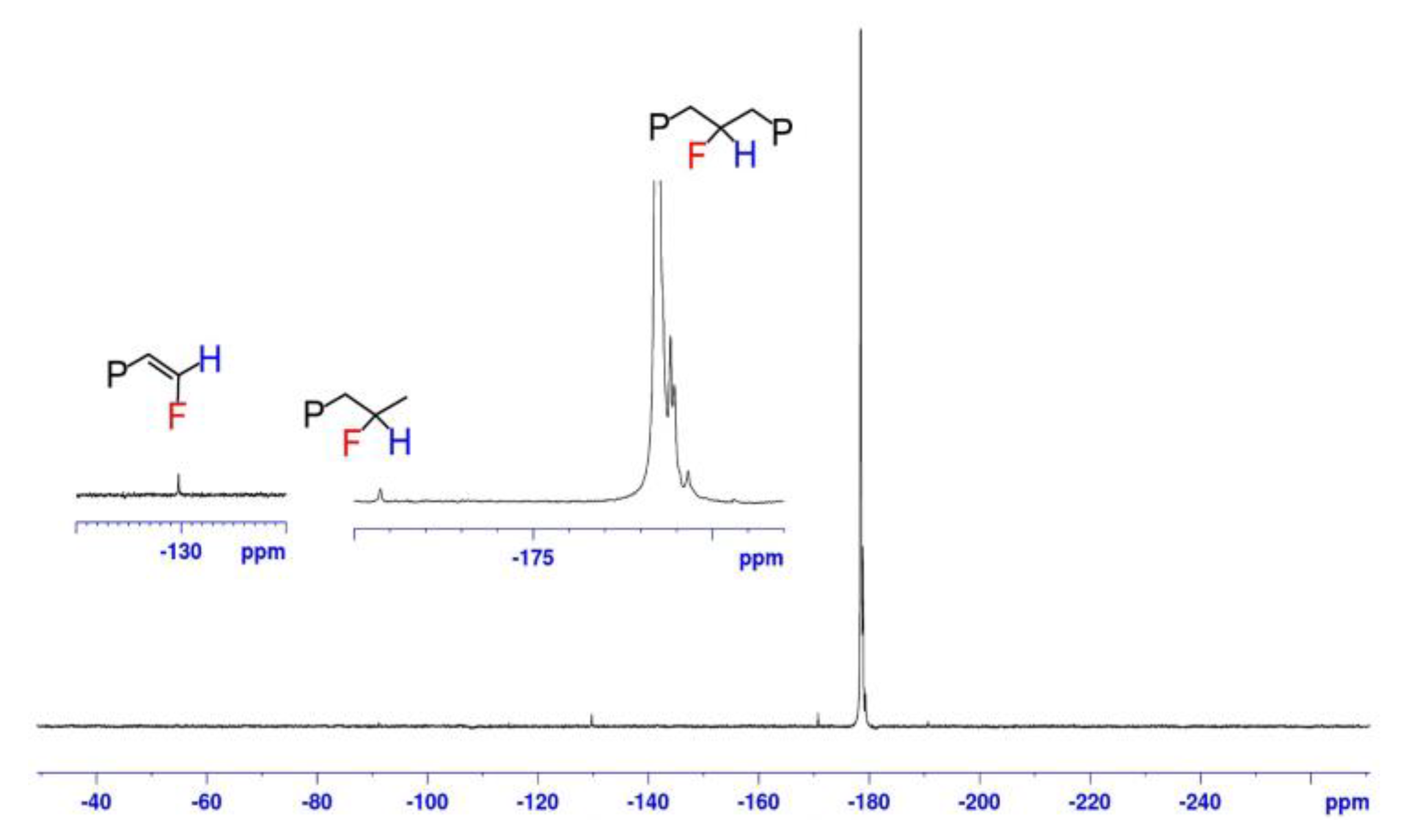
| Entry | Cat. | Solvent | PC2H4 (psi) | PVF (psi) | Acitivity d (kg∙mol−1∙h−1) | Mw e (103) | PDI e | VF Incorp f (mol%) | Tm g (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 a | C | toluene | 220 | 80 | 1.4 | 20.3 | 8.0 | 0.10 | 128.8 |
| 2 a | C | toluene | 130 | 120 | 0.44 | 1.92 | 1.4 | 0.25 | ND h |
| 3 a | C | hexanes | 220 | 80 | 12.0 | 498 | 18.2 | 0.87 | 134.4 |
| 4 a | C | hexanes | 130 | 120 | 3.4 | 419 | 26.2 | 2.5 | 132.8 |
| 5 a,b | D | toluene + PhCl | 130 | 120 | 2.0 | 42.2 | 4.1 | 0.96 | 131.4 |
| 6 a,b | E | toluene + PhCl | 220 | 80 | 4.9 | 50.3 | 2.4 | 0.43 | 134.7 |
| 7 a,b | E | toluene + PhCl | 130 | 120 | 1.0 | 23.0 | 2.3 | 1.1 | 131.6 |
| 8 a,c | B | toluene | 130 | 120 | 1.9 | 4.2 | 2.4 | 2.4 | 127.8 |
| 9 a,c | B | hexanes | 130 | 120 | 1.4 | 494 | 310 | 3.6 | 127.8 |
| Catalyst | Entry in Table 1 | Distribution of VF Incorporation Modes (%) a | ||
|---|---|---|---|---|
 |  |  | ||
| C | 1 | 100 | Not observed | Not observed |
| C | 2 | 85.1 | 5.0 | 9.9 |
| C | 3 | 99.1 | 0.9 | Not observed |
| C | 4 | 99.2 | 0.4 | 0.4 |
| D | 5 | >99 | Trace b | Not observed |
| E | 6 | 99.2 | 0.8 | Not observed |
| E | 7 | 98.8 | 1.2 | Not observed |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Jordan, R.F. Copolymerization of Ethylene and Vinyl Fluoride by Self-Assembled Multinuclear Palladium Catalysts. Polymers 2020, 12, 1609. https://doi.org/10.3390/polym12071609
Liu Q, Jordan RF. Copolymerization of Ethylene and Vinyl Fluoride by Self-Assembled Multinuclear Palladium Catalysts. Polymers. 2020; 12(7):1609. https://doi.org/10.3390/polym12071609
Chicago/Turabian StyleLiu, Qian, and Richard F. Jordan. 2020. "Copolymerization of Ethylene and Vinyl Fluoride by Self-Assembled Multinuclear Palladium Catalysts" Polymers 12, no. 7: 1609. https://doi.org/10.3390/polym12071609