Application of Plasma Activation in Flame-Retardant Treatment for Cotton Fabric
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
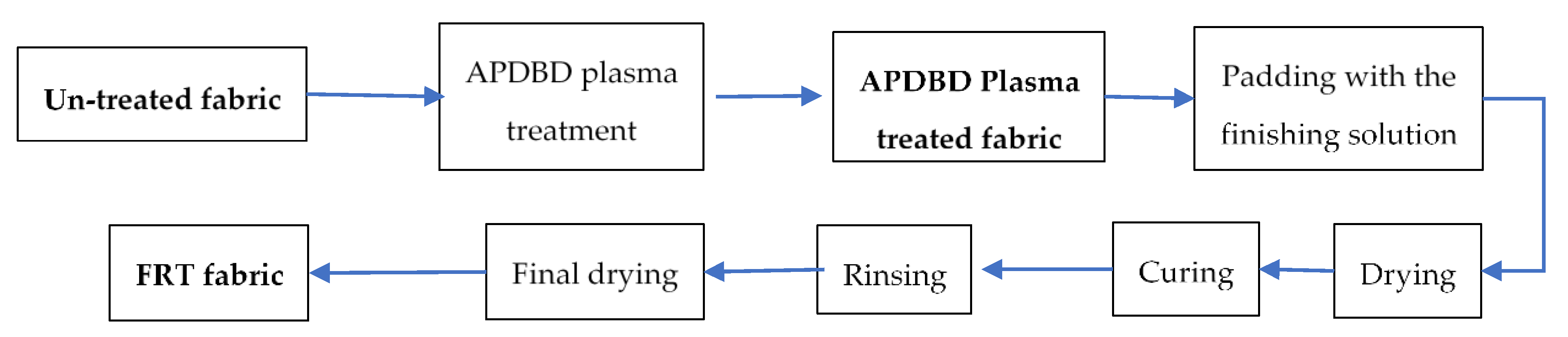
2.2.1. APDBD Plasma Treatment for Fabric
2.2.2. Flame-Retardant Treatment for the APDBD Plasma-Treated Cotton Fabric
2.2.3. Characterization of the Control and Treated Cotton Fabric
2.2.4. Statistical Processing of the Experimental Results
3. Results
3.1. The Experimental Results
3.2. Model Determination and Analysis of Fitting Model
3.3. Effect of the Curing Conditions on the Properties of the Finished Fabric
3.3.1. Effect of the Curing Conditions on the LOI Values and the Optimal Solutions
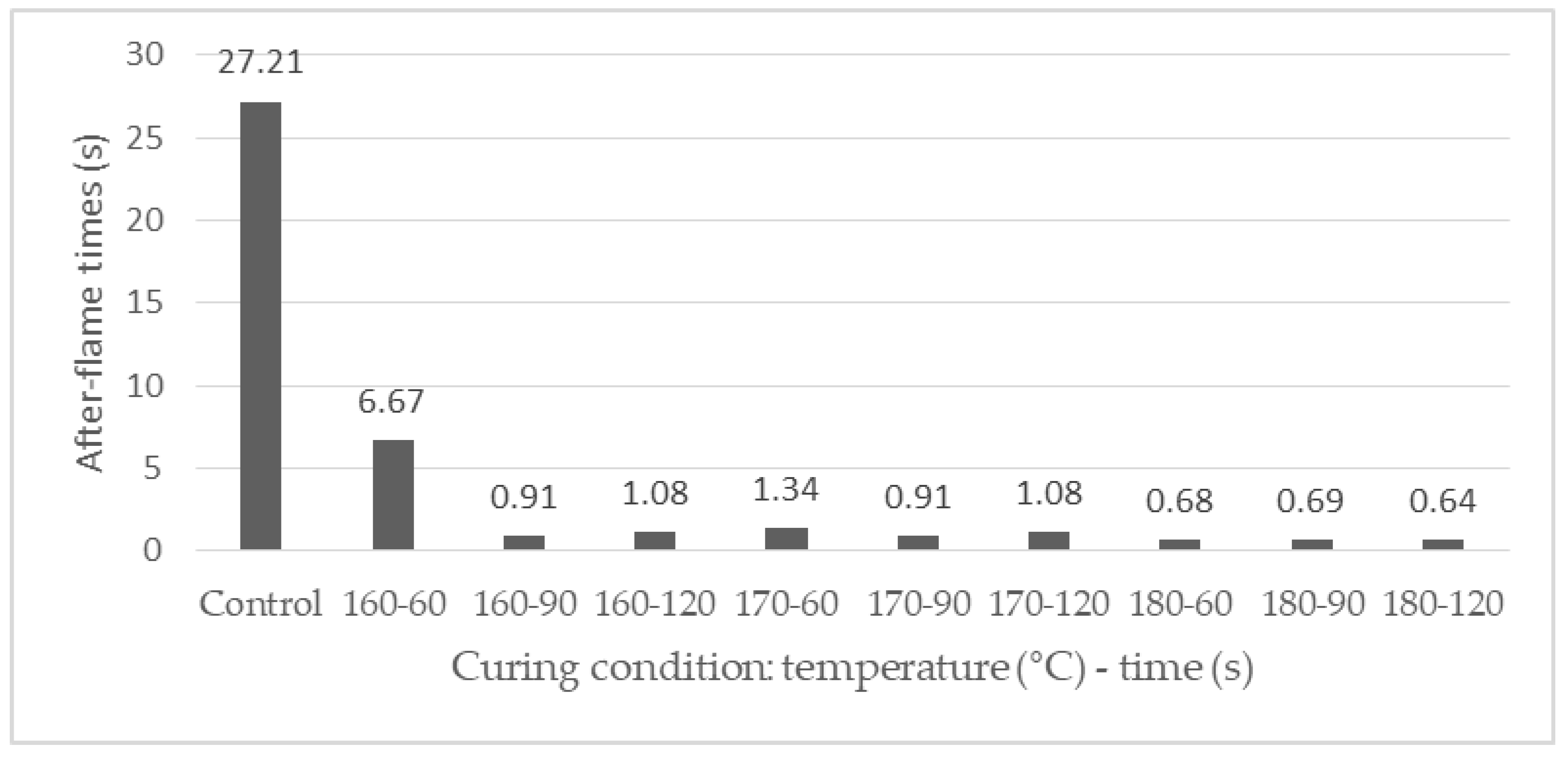
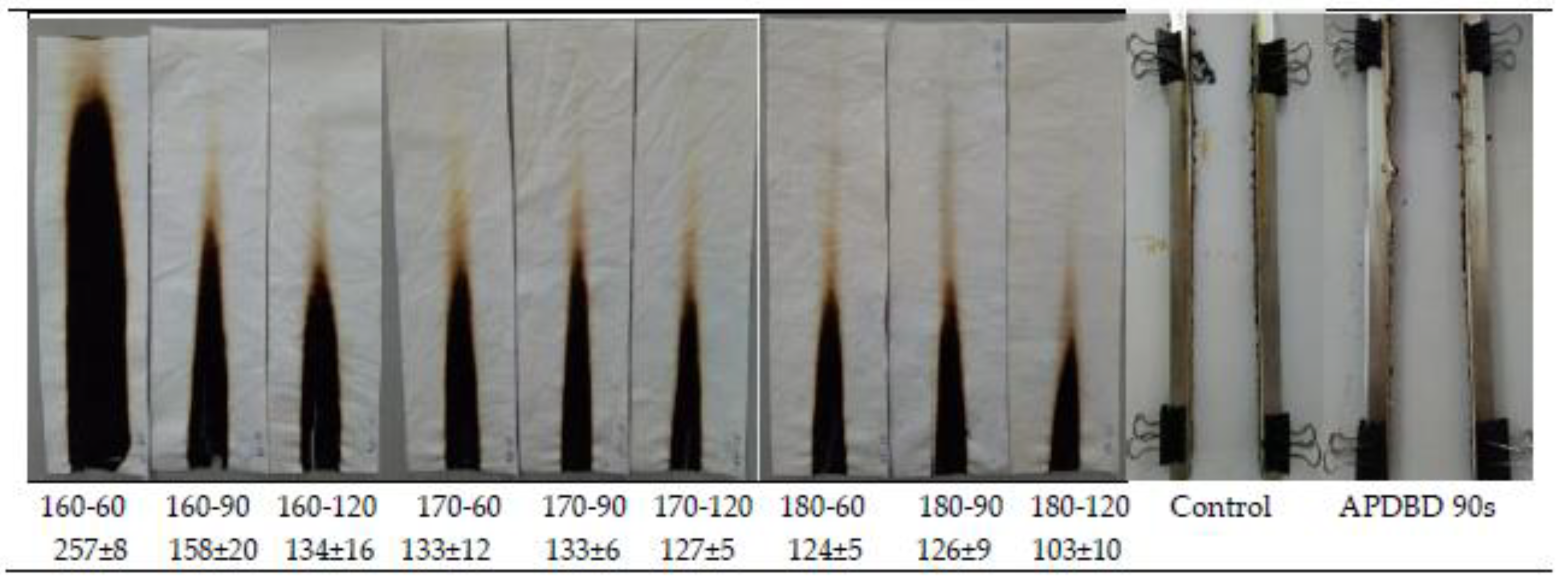
3.3.2. Effect of the Curing Conditions on the Vertical Flammability Characteristics of the FRT Samples
3.3.3. Mechanical Strength Loss of FRT Cotton Fabric
3.3.4. Optimizing the Temperature and Time of the Curing Step
3.4. Results of the Fiber Surface Analysis.
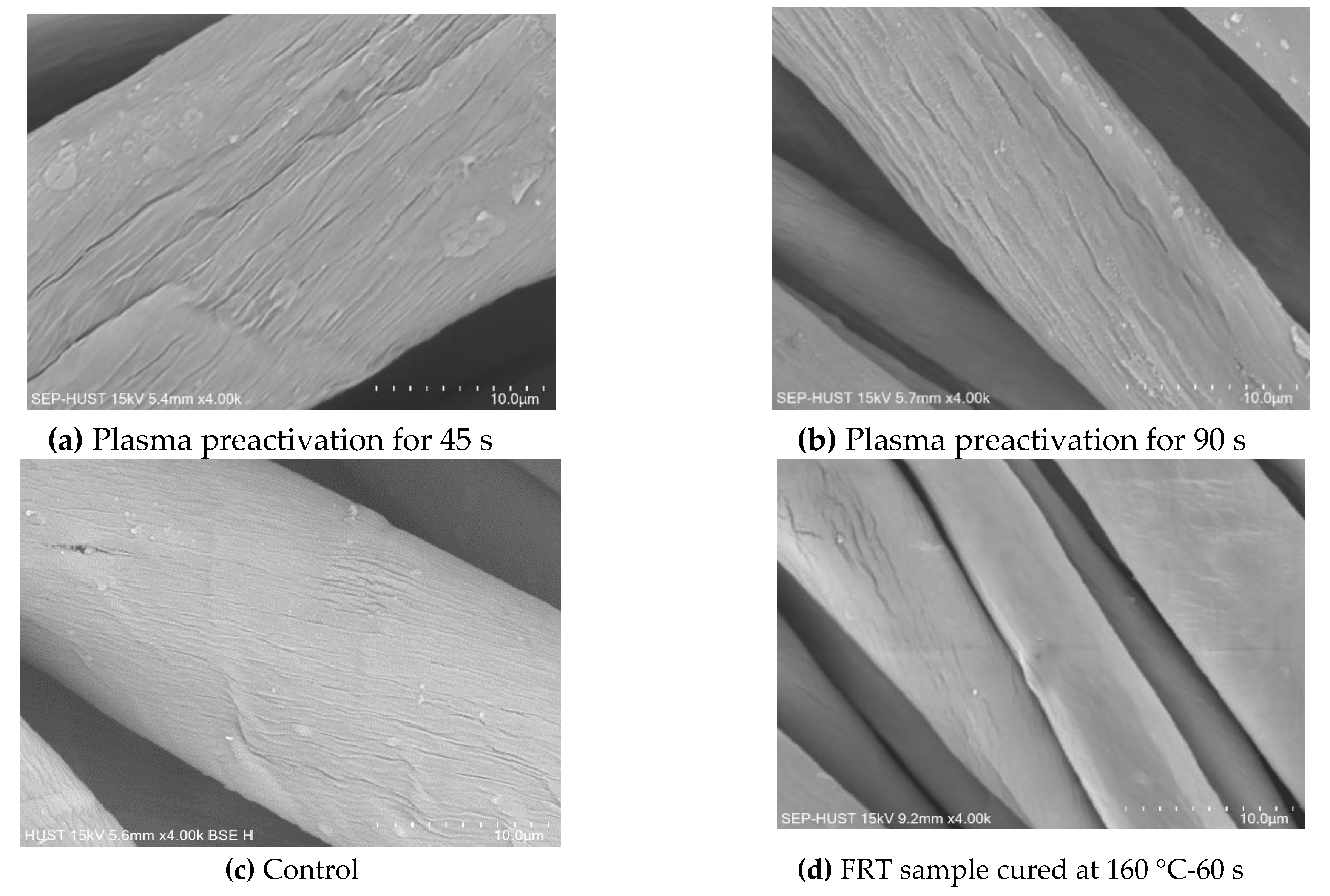
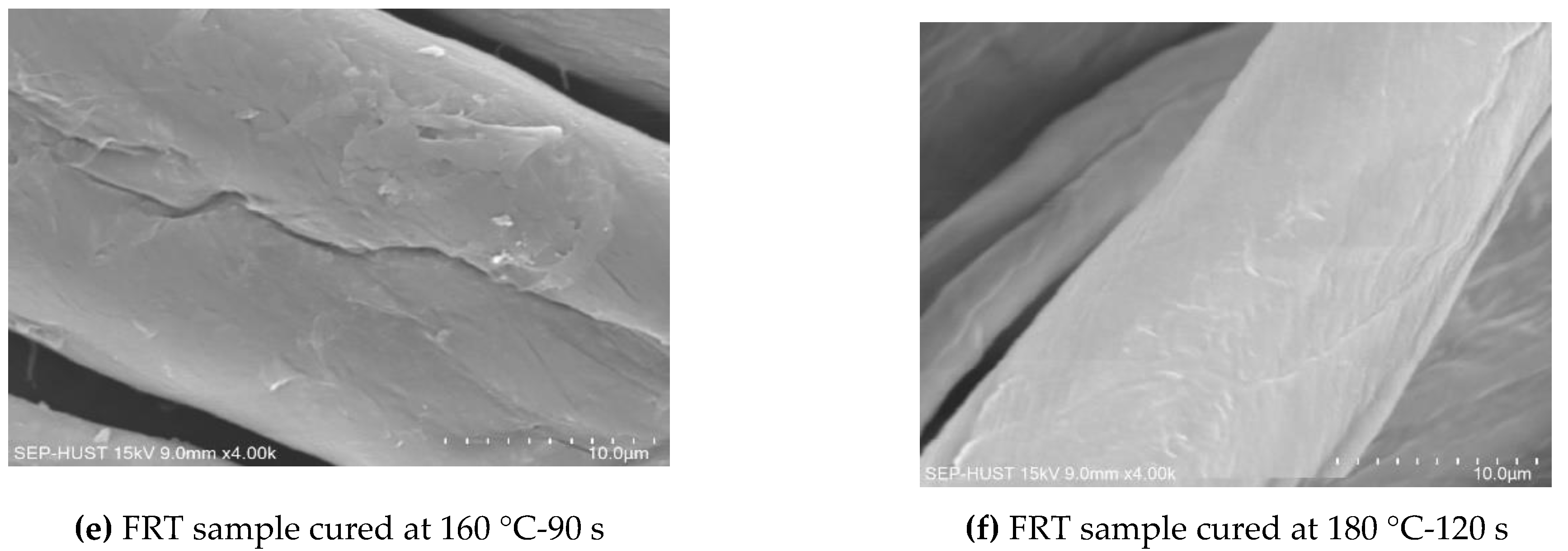
3.4.1. Scanning Electron Microscopy Images
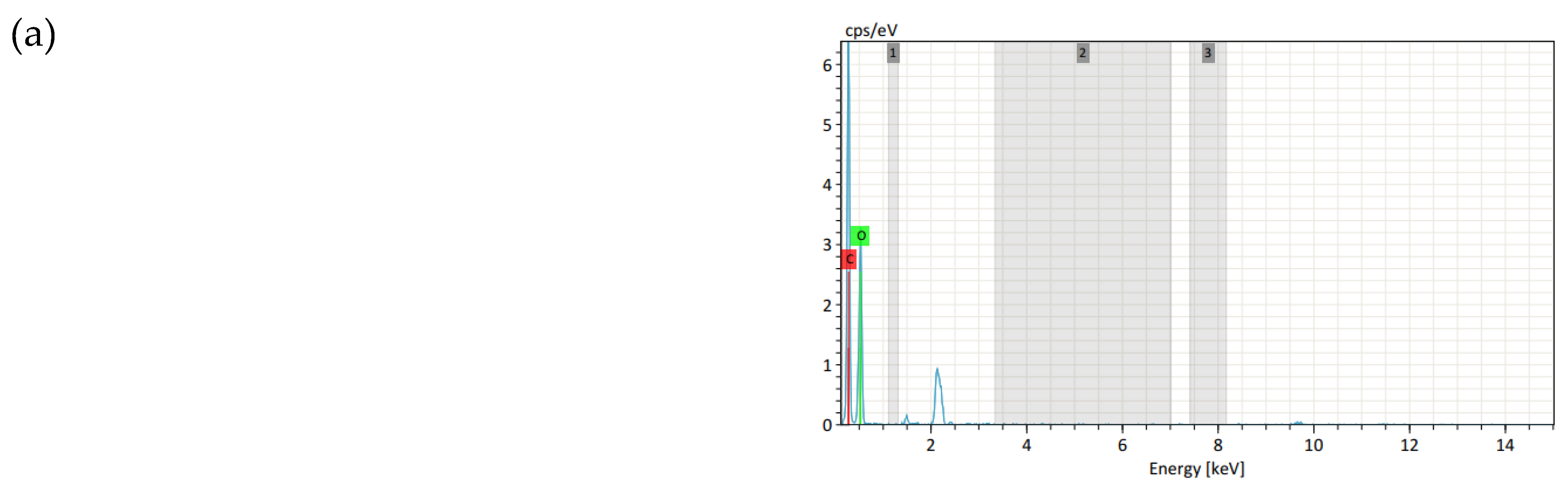
3.4.2. Energy-Dispersive Spectroscopy
3.4.3. X-ray Photoelectron Spectrometer
4. Discussion
5. Conclusions
- (1)
- Applying APDBD plasma with the power of one watt per square centimeter to cotton fabric for 90 s before it is treated with FR can allow a significant reduction in temperature and time of curing step. However, the FRT fabric is always flame retardant fabric. This could allow to minimize the mechanical strength losses to cotton fabrics caused by the curing step. Nevertheless, the plasma power of one watt per square centimeter and the exposure time of 90 s also caused the considerable mechanical damage to the cotton fabric. In this case, the mechanical damage to the fabric of both causes may be greater than in the absence of plasma pretreatment. Therefore, it is necessary to find the optimal plasma parameters so that it can support the bond between the chemicals and the fabric, but its damage to the mechanical strength of the fabric is as low as possible. Thus, plasma pretreatment exposure time should be so short that the plasma pretreatment can only support the creation of chemical bonds between cellulose and chemicals without damaging their mechanical strength.
- (2)
- How to reduce the plasma exposure time and no need to increase the temperature and time of the curing step, but the FRT fabrics always have the LOI ≥ 25? The content of this study will be detailed in our next work.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kilinc, F.S. Handbook of Fire Resistant Textiles, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Khanh, V.T.H.; Huong, N.T. Influence of crosslinking agent on the effectiveness of flame retardant treatment for cotton fabric. Ind. Text. 2019, 70, 413–420. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Effect of CaAlCO3-LDHs on fire resistant properties of intumescent fireproof coatings for steel structure. Appl. Surf. Sci. 2018, 457, 164–169. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Efficient flame-retardant and smoke-suppression properties of MgAlCO3-LDHs on the intumescent fire retardant coating for steel structures. Prog. Org. Coat. 2019, 135, 291–298. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Montmorillonite-synergized water-based intumescent flame retardant coating for plywood. Coatings 2020, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Katović, D.; Grgac, S.F.; Bischof-Vukušić, S.; Katović, A. Formaldehyde free binding system for flame retardant finishing of cotton fabrics. Fibers Text. East. Eur. 2012, 1, 94–98. [Google Scholar]
- Yang, Z.; Wang, X.; Lei, D.; Fei, B.; Xin, J.H. A durable flame retardant for cellulosic fabrics. Polym. Degrad. Stab. 2012, 97, 2467–2472. [Google Scholar] [CrossRef]
- Mengal, N.; Syed, U.; Malik, S.A.; Sahito, I.A.; Jeong, S.H. Citric acid based durable and sustainable flame retardant treatment for lyocell fabric. Carbohydr. Polym. 2016, 153, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Behary, N.; Giraud, S.; Perwuelz, A. In situ degradation of organophosphorus flame retardant on cellulosic fabric using advanced oxidation process: A study on degradation and characterization. Polym. Degrad. Stab. 2016, 126, 1–8. [Google Scholar] [CrossRef]
- Sohail, Y.; Parag, B.; Nemeshwaree, B.; Giorgio, R. Optimizing Organophosphorus Fire Resistant Finish for Cotton Fabric Using Box-Behnken Design. Int. J. Environ. Res. 2016, 10, 313–320. [Google Scholar]
- Poon, C.K.; Kan, C.W. Effects of TiO2 and curing temperatures on flame retardant finishing of cotton. Carbohydr. Polym. 2015, 121, 457–467. [Google Scholar] [CrossRef]
- Poon, C.K.; Kan, C.W. Relationship between curing temperature and low stress mechanical properties of titanium dioxide catalyzed flame retardant finished cotton fabric. Fibers Polym. 2016, 17, 380–388. [Google Scholar] [CrossRef]
- Mohsin, M.; Ahmad, S.W.; Khatri, A.; Zahid, B. Performance enhancement of fire retardant finish with environment friendly bio cross-linker for cotton. J. Clean. Prod. 2013, 51, 191–195. [Google Scholar] [CrossRef]
- Huong, N.T.; Khanh, V.T.H. Optimizing curing conditions in flame retardant treatment for cotton fabric. In Texteh 9 International Conference Proceedings; Certex Publishing House: Bucharest, Romania, 2019. [Google Scholar]
- Huong, N.T.; Khanh., V.T.H. Study on contents of Pyrovatex CP New and Knittex FFRC in flame retardant treatment for cotton fabrics. In Proceedings of the Indonesian Textile Conference, Bandung, Indonesia, 27 July 2019. [Google Scholar]
- John, M.J.; Anandjiwala, R.D. Surface modification and preparation techniques for textile materials. In Surface Modification of Textiles; Woodhead Publishing: Amsterdam, The Netherlands, 2009; pp. 1–25. [Google Scholar]
- Shahidi, S.; Wiener, J.; Ghoranneviss, M. Surface modification methods for improving the dyeability of textile fabrics. In Eco-Friendly Textile Dyeing and Finishing; IntechOpen Limited: Londod, UK, 2013; pp. 34–50. [Google Scholar]
- Sun, D. Surface modification of natural fibers using plasma treatment. In Biodegradable Green Composites; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 18–39. [Google Scholar]
- Karahan, H.A.; Özdoğan, E.; Demir, A.; Aydin, H.; Seventekin, N. Effects of atmospheric pressure plasma treatments on certain properties of cotton fabrics. Fibers Text. East. Eur. 2009, 73, 19–22. [Google Scholar]
- Kale, K.H.; Desai, A.N. Atmospheric pressure plasma treatment of textiles using non-polymerising gases. Indian J. Fibre Text. Res. 2011, 36, 289–299. [Google Scholar]
- Gotoh, K.; Yasukawa, A. Atmospheric pressure plasma modification of polyester fabric for improvement of textile-specific properties. Text. Res. J. 2011, 81, 368–378. [Google Scholar] [CrossRef]
- Zille, A.; Oliveira, F.R.; Souto, A.P. Plasma Treatment in Textile Industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef] [Green Version]
- Sohbatzadeh, F.; Farhadi, M.; Shakerinasab, E. A new DBD apparatus for super-hydrophobic coating deposition on cotton fabric. Surf. Coat. Technol. 2019, 374, 944–956. [Google Scholar] [CrossRef]
- Raslan, W.M.; Rashed, U.S.; El-Sayad, H.; El-Halwagy, A.A. Ultraviolet protection, flame retardancy and antibacterial properties of treated polyester fabric using plasma-nano technology. Mater. Sci. Appl. 2011, 2, 1432. [Google Scholar] [CrossRef] [Green Version]
- Demir, A.G.; Oliveira, F.R.; Gulumser, T.; Souto, A.P. New Possibilities of Raw Cotton Pre-treatment before reactive dyeing. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Istanbul, Turkey, 20–22 June 2018; Volume 460, p. 012026. [Google Scholar]
- Fan, Z.; Di, L.; Zhang, X.; Wang, H. A Surface Dielectric Barrier Discharge Plasma for Preparing Cotton-Fabric-Supported Silver Nanoparticles. Nanomaterials 2019, 9, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Cui, N.; Brown, N.M.; Meenan, B.J. Effects of DBD plasma operating parameters on the polymer surface modification. Surf. Coat. Technol. 2004, 185, 311–320. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Rusu, G.B.; Topala, I.; Chiper, A.S.; Popa, G. Surface modifications of polymer induced by atmospheric DBD plasma in different configurations. J. Optoelectron. Adv. Mater. 2008, 10, 2038–2042. [Google Scholar]
- Carneiro, N.; Souto, A.P.; Foster, F.; Fernandes, F.; Dias, P.; Oliveira, F.R. A DBD plasma machine in textile wet processing. In Proceedings of the 21st IFATCC International Congress, Barcelona, Spain, 6–8 May 2008. [Google Scholar]
- Ghimire, B.; Subedi, D.P.; Khanal, R. Improvement of wettability and absorbancy of textile using atmospheric pressure dielectric barrier discharge. AIP Adv. 2017, 7, 085213. [Google Scholar] [CrossRef] [Green Version]
- Dutka, M.; Ditaranto, M.; Løvås, T. Application of a central composite design for the study of NOx emission performance of a low NOx burner. Energies 2015, 8, 3606–3627. [Google Scholar] [CrossRef] [Green Version]
- Pransilp, P.; Pruettiphap, M.; Bhanthumnavin, W.; Paosawatyanyong, B.; Kiatkamjornwong, S. Surface modification of cotton fabrics by gas plasmas for color strength and adhesion by inkjet ink printing. Appl. Surf. Sci. 2016, 364, 208–220. [Google Scholar] [CrossRef]
- Ražić, S.E.; Čunko, R.; Bautista, L.; Bukošek, V. Plasma effect on the chemical structure of cellulose fabric for modification of some functional properties. Procedia Eng. 2017, 200, 333–340. [Google Scholar] [CrossRef]
- Rani, K.V.; Sarma, B.; Sarma, A. Plasma treatment on cotton fabrics to enhance the adhesion of Reduced Graphene Oxide for electro-conductive properties. Diam. Relat. Mater. 2018, 84, 77–85. [Google Scholar] [CrossRef]



| Exp. No | A | B | X1 (°C) | X2 (s) |
|---|---|---|---|---|
| 1 | +1 | −1 | 180 | 60 |
| 2 | +1 | +1 | 180 | 120 |
| 3 | 0 | −1 | 170 | 60 |
| 4 | −1 | 0 | 160 | 90 |
| 5 | 0 | 0 | 170 | 90 |
| 6 | +1 | 0 | 180 | 90 |
| 7 | 0 | 0 | 170 | 90 |
| 8 | 0 | +1 | 170 | 120 |
| 9 | −1 | +1 | 160 | 120 |
| 10 | −1 | −1 | 160 | 60 |
| Sample | Variable Factor | LOI (%) | Characteristics of Vertical Flammability Test | Warp Fmax (N) | Mechanical Strength Loss (%) | |||
|---|---|---|---|---|---|---|---|---|
| Curing Temp. (°C) | Curing Time (s) | After-Flame Times (s) | After-Glow Times (s) | Char Length (mm) | ||||
| Control (CS) | – | – | 17.1 | 27.21 | 36.19 | Completely burned | 1507 ± 43 | |
| Plasma-treated (PS) | – | – | 28.03 | 39.35 | Completely burned | 1097 ± 71 | 27.18 | |
| 1 | 180 | 60 | 27.6 | 0.68 | 0 | 124 ± 5 | 1018 ± 39 | 32.44 |
| 2 | 180 | 120 | 28.0 | 0.64 | 0 | 103 ± 10 | 794 ± 47 | 47.32 |
| 3 | 170 | 60 | 25.0 | 1.34 | 0 | 133 ± 12 | 936 ± 77 | 37.90 |
| 4 | 160 | 90 | 26.8 | 0.91 | 0 | 158 ± 20 | 981 ± 52 | 34.91 |
| 5 | 170 | 90 | 26.8 | 0.91 | 0 | 133 ± 6 | 952 ± 69 | 36.82 |
| 6 | 180 | 90 | 28.1 | 0.69 | 0 | 126 ± 9 | 890 ± 66 | 40.96 |
| 7 | 170 | 90 | 26.8 | – | – | – | 854 ± 112 | 43.35 |
| 8 | 170 | 120 | 27.2 | 1.08 | 0 | 127 ± 5 | 969 ± 13 | 35.71 |
| 9 | 160 | 120 | 25.9 | 1.08 | 0 | 134 ± 16 | 976 ± 39 | 35.22 |
| 10 | 160 | 60 | 22.1 | 6.67 | 0 | 257 ± 8 | 1003 ± 83 | 33.42 |
| Test Response | Model Parameter | Response Equation in Actual and Code Variables | |||
|---|---|---|---|---|---|
| R- Squared | Adj R- Squared | F Value | P- Value | ||
| LOI | 0.6978 | 0.6115 | 8.08 | 0.0152 | |
| Source | F-Value | p-Value Probe > F |
|---|---|---|
| Model | Y1 | |
| -Temperature | 10.66 | 0.0138 |
| -Time | 5.51 | 0.0513 |
| Option | Temperature (°C) | Time (s) |
|---|---|---|
| 1 | 160 | 90 |
| 2 | 167 | 63 |
| Loss in Tensile Strength (%) | |||
|---|---|---|---|
| Curing Time (s) | Curing Temperature (°C) | ||
| 160 | 170 | 180 | |
| 60 | 33.42 | 37.90 | 32.44 |
| 90 | 34.91 | 36.82 | 40.96 |
| 120 | 35.22 | 35.71 | 47.32 |
| Number | Temperature (°C) | Time (s) | LOI | Warp Fmax (N) | Desirability | Goal |
|---|---|---|---|---|---|---|
| 1 | 160 | 90 | 25.0 | 979 | 0.920 | Temperature minimize, time 60->90 s, LOI > 25, Tensile strength maximize |
| Samples | C (%) | O (%) | P (%) | N (%) |
|---|---|---|---|---|
| (a) Control | 56.62 | 43.38 | - | - |
| (b) Plasma-treated for 90 s | 56.51 | 43.49 | - | - |
| (c) Cured at 160 °C-60 s | 54.25 | 40.56 | 1.29 | 3.91 |
| (d) Cured at 160 °C-90 s | 55.60 | 39.93 | 1.49 | 2.98 |
| (e) Cured at 160 °C-120 s | 53.64 | 40.50 | 1.36 | 4.51 |
| (f) Cured at 170 °C-60 s | 54.94 | 40.01 | 1.53 | 3.52 |
| (g) Cured at 170 °C-90 s | 55.31 | 40.48 | 1.51 | 2.70 |
| (h) Cured at 170 °C-120 s | 54.09 | 41.09 | 1.36 | 3.46 |
| (i) Cured at 180 °C-60 s | 53.92 | 39.85 | 1.42 | 4.84 |
| (j) Cured at 180 °C-90 s | 54.58 | 40.33 | 1.48 | 3.61 |
| (k) Cured at 180 °C-120 s | 54.06 | 40.30 | 1.52 | 4.12 |
| Plasma Exposure Time (s) | Functional Group (%) | |||
|---|---|---|---|---|
| C–C/C–H | C–O/C–OH | O–C–O/C=O | O=C–O/COOH | |
| 0 | 42.10 | 40.40 | 17.50 | – |
| 45 | 24.84 | 39.80 | 35.35 | – |
| 90 | 26.05 | 47.34 | 23.68 | 2.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen Thi, H.; Vu Thi Hong, K.; Ngo Ha, T.; Phan, D.-N. Application of Plasma Activation in Flame-Retardant Treatment for Cotton Fabric. Polymers 2020, 12, 1575. https://doi.org/10.3390/polym12071575
Nguyen Thi H, Vu Thi Hong K, Ngo Ha T, Phan D-N. Application of Plasma Activation in Flame-Retardant Treatment for Cotton Fabric. Polymers. 2020; 12(7):1575. https://doi.org/10.3390/polym12071575
Chicago/Turabian StyleNguyen Thi, Huong, Khanh Vu Thi Hong, Thanh Ngo Ha, and Duy-Nam Phan. 2020. "Application of Plasma Activation in Flame-Retardant Treatment for Cotton Fabric" Polymers 12, no. 7: 1575. https://doi.org/10.3390/polym12071575





