Interpolymer Complexes of Eudragit® Copolymers as Novel Carriers for Colon-Specific Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solid IPCs
2.3. Elemental Analysis
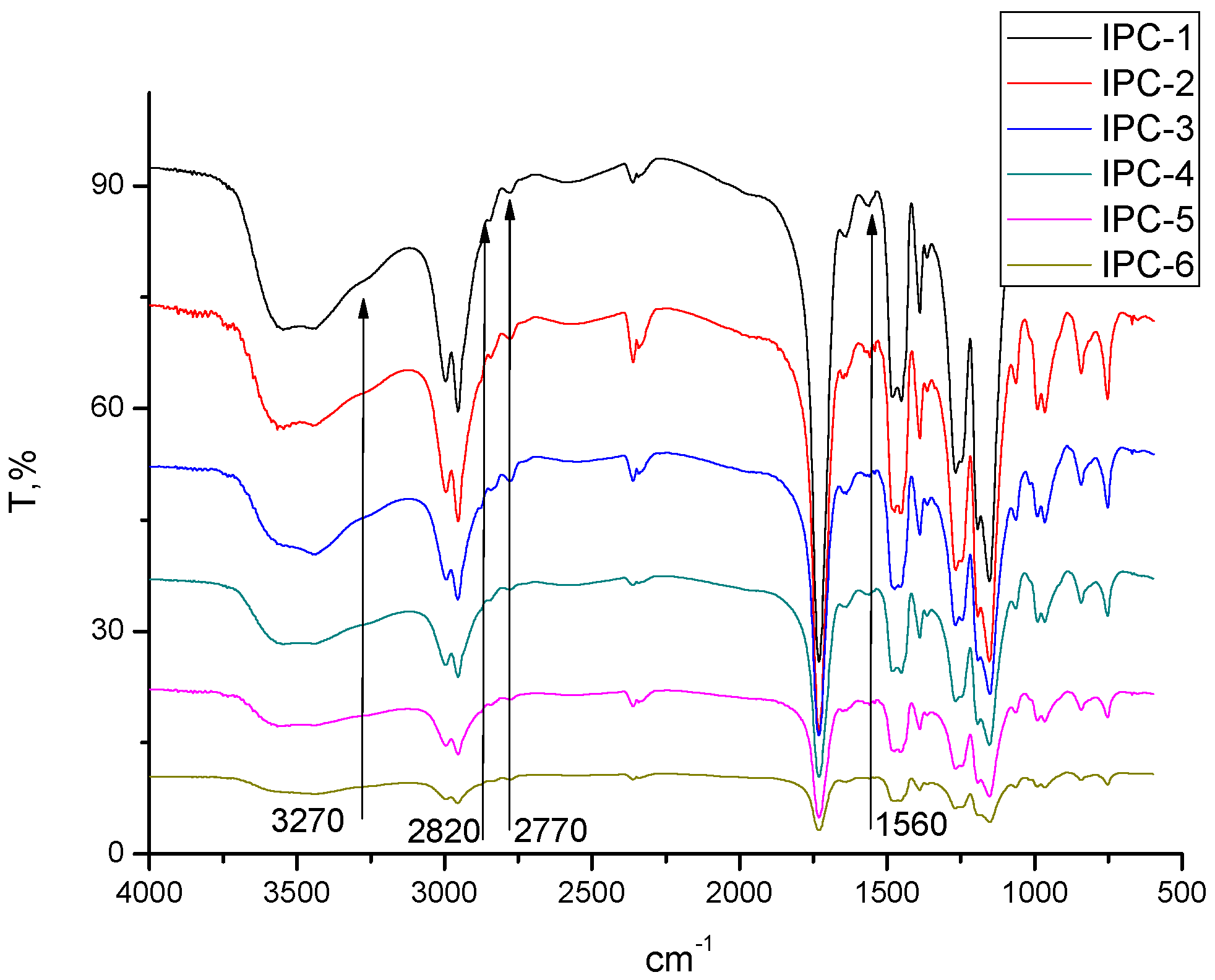
2.4. Fourier Transformed Infrared (ATR-FTIR) Spectroscopy
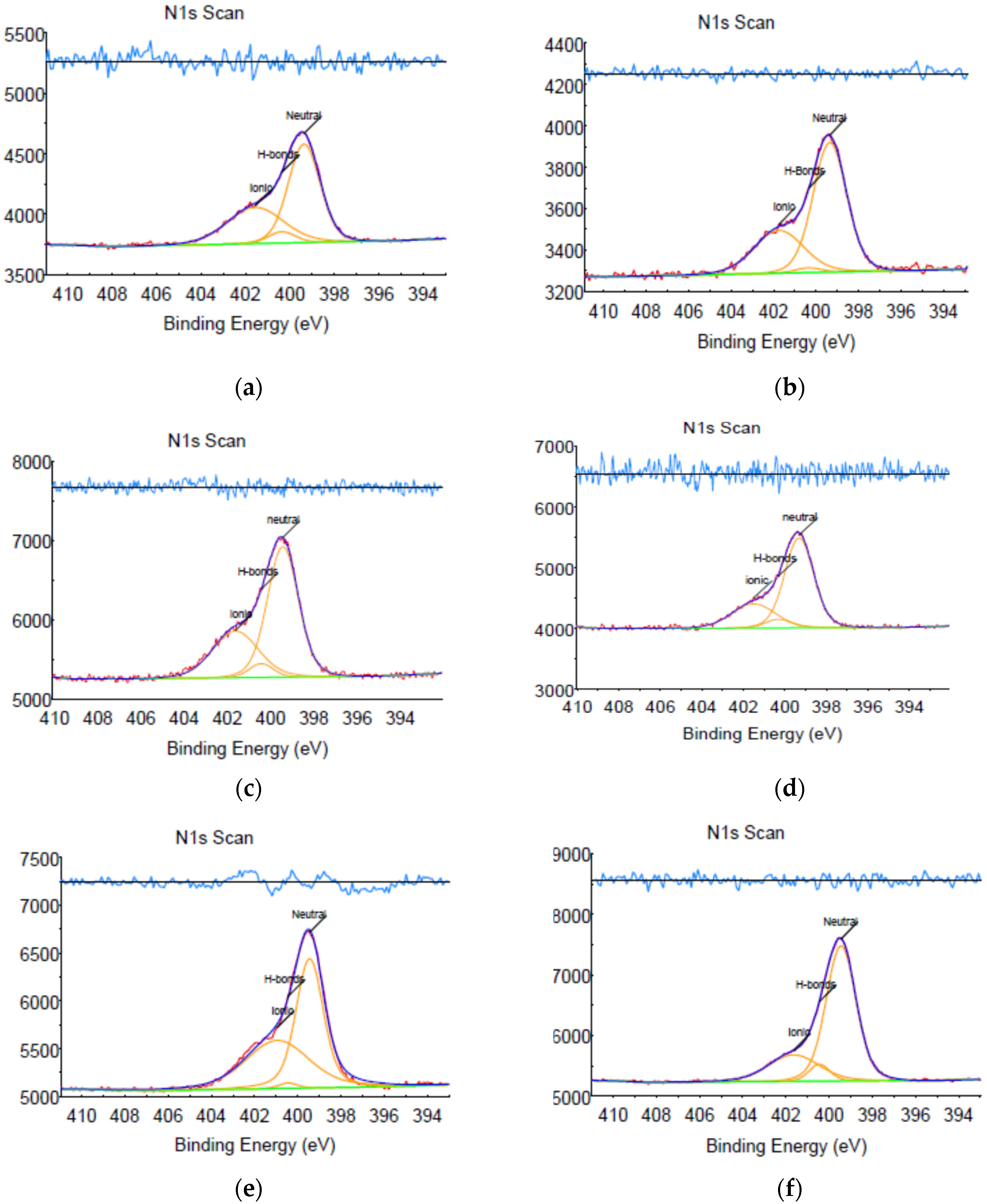
2.5. X-ray Photoelectron Spectroscopy (XPS)
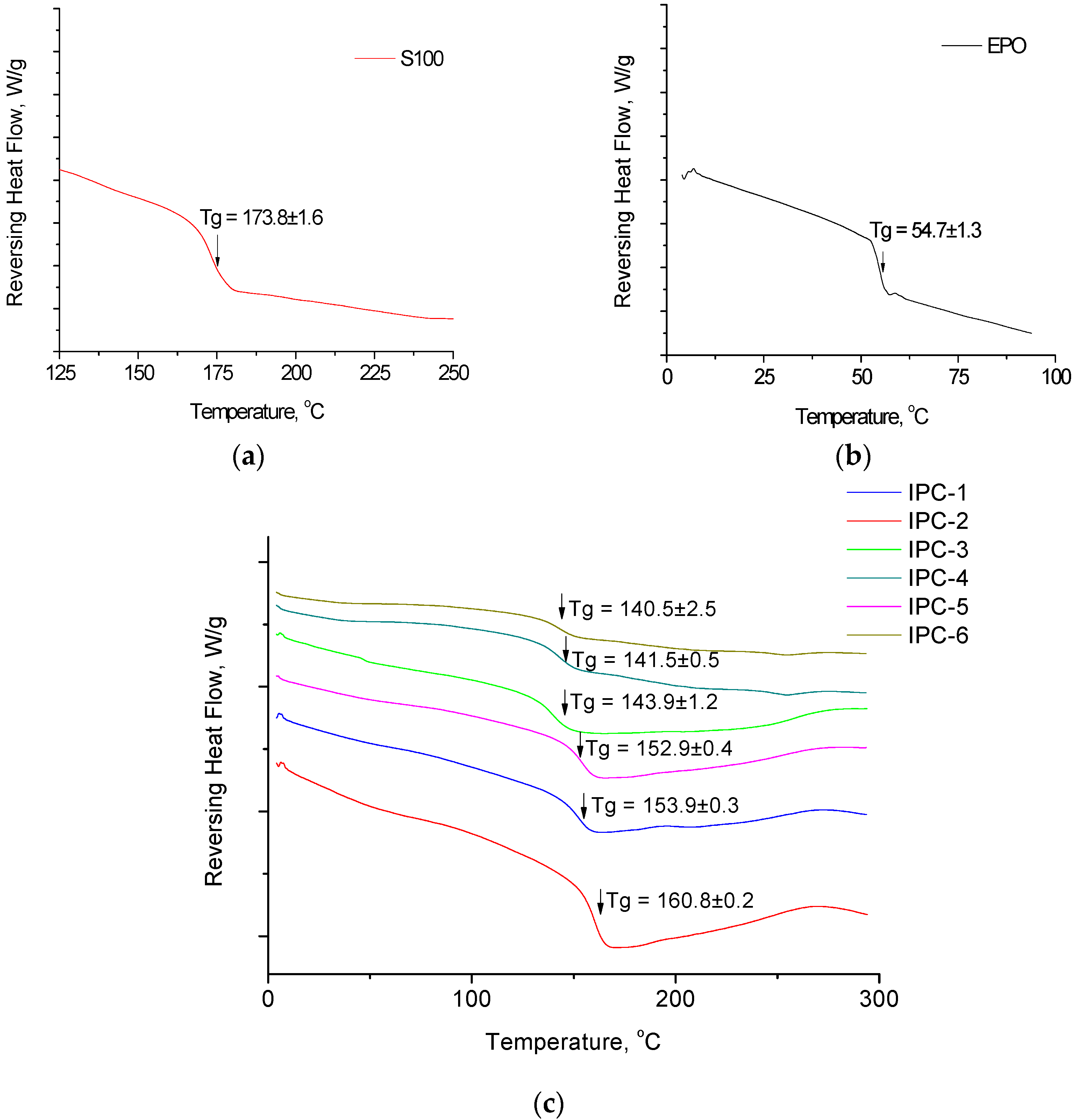
2.6. Thermal Analysis
2.7. Preparation of IPC Compacts
2.8. Swelling Studies
2.9. Preparation of Biorelevant Media
2.10. Indomethacin Release Studies
3. Results
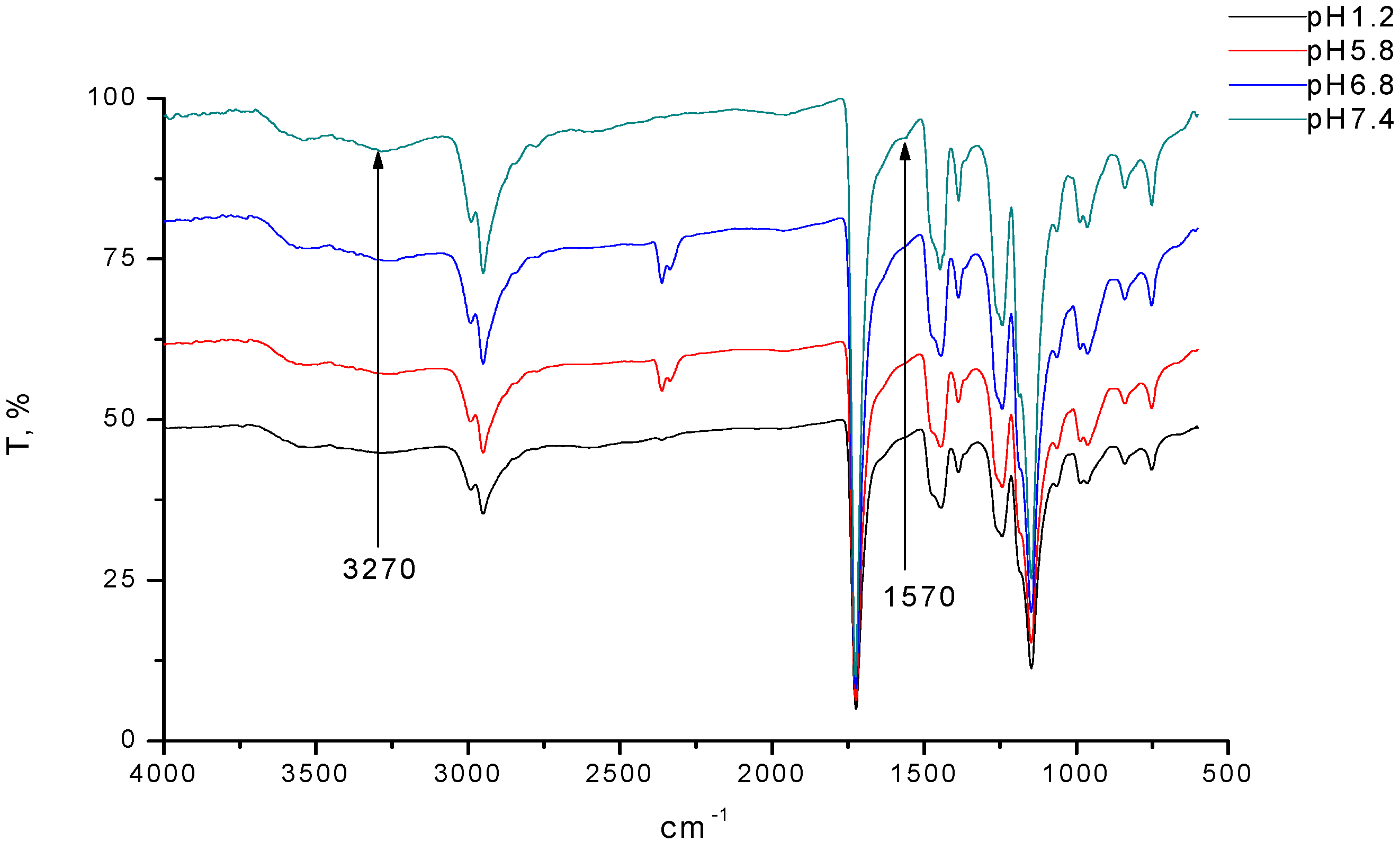
3.1. Evaluation of Structure and Composition of IPC
3.2. Thermal Analysis
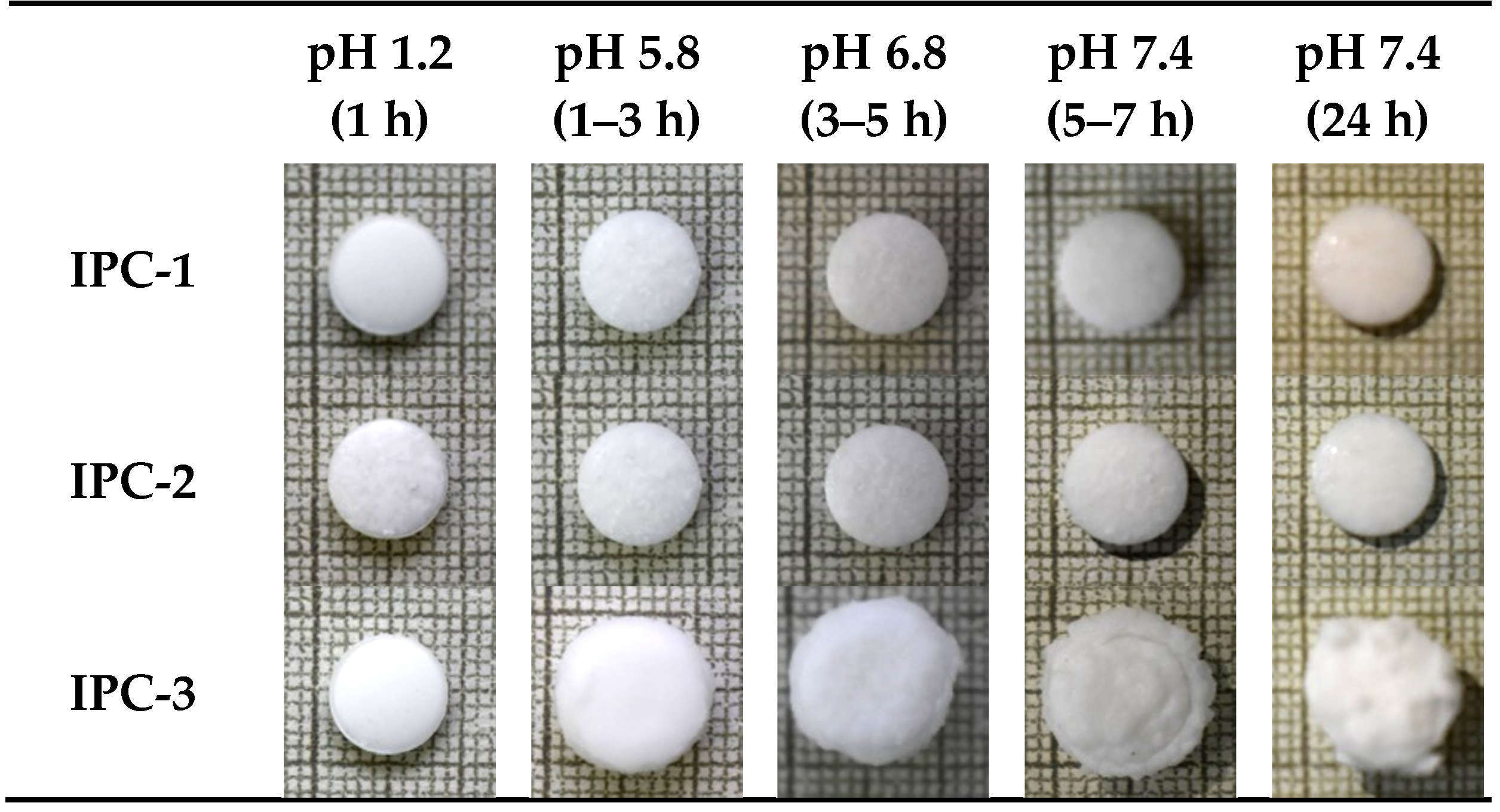
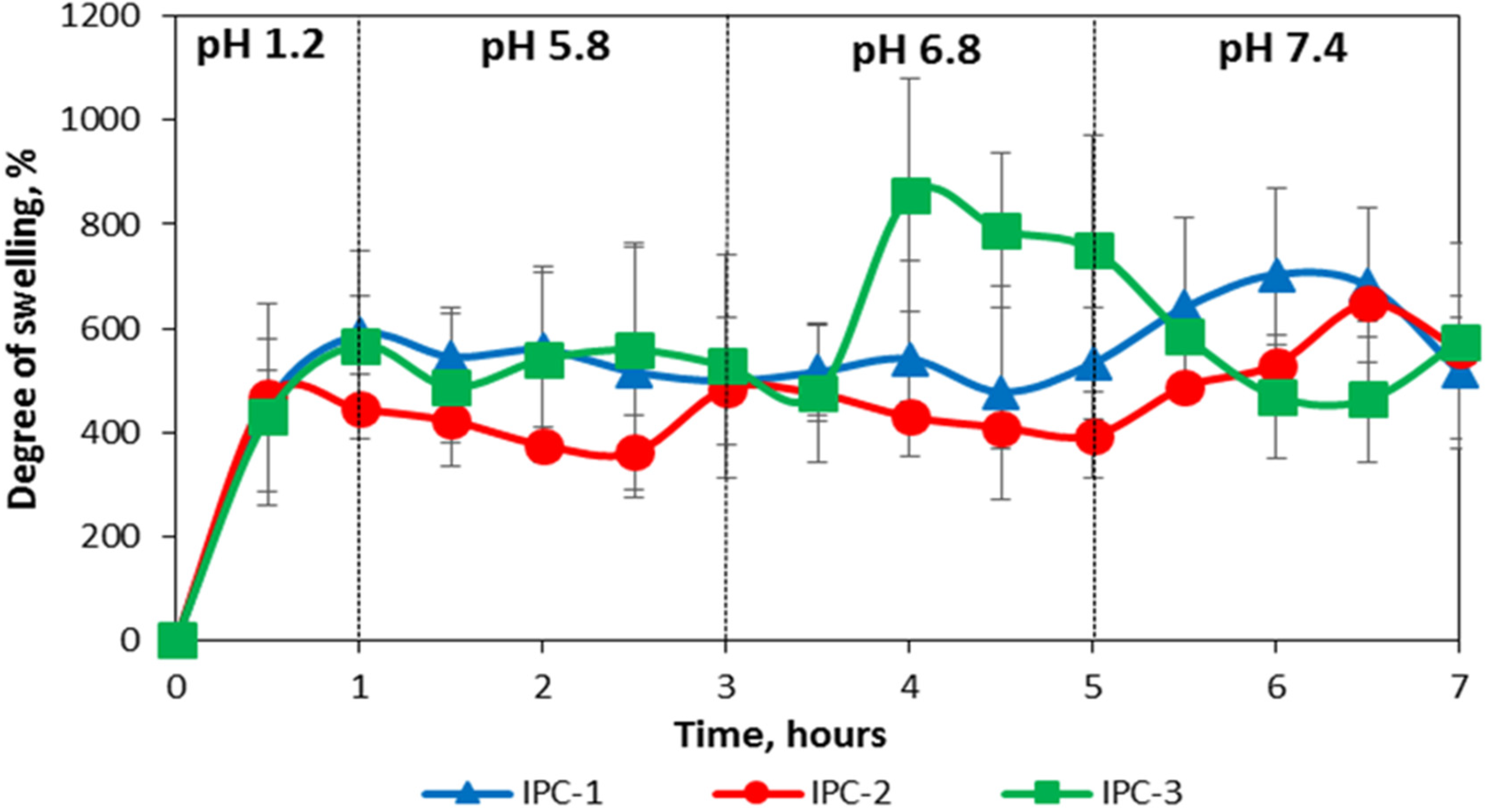
3.3. Swelling Properties
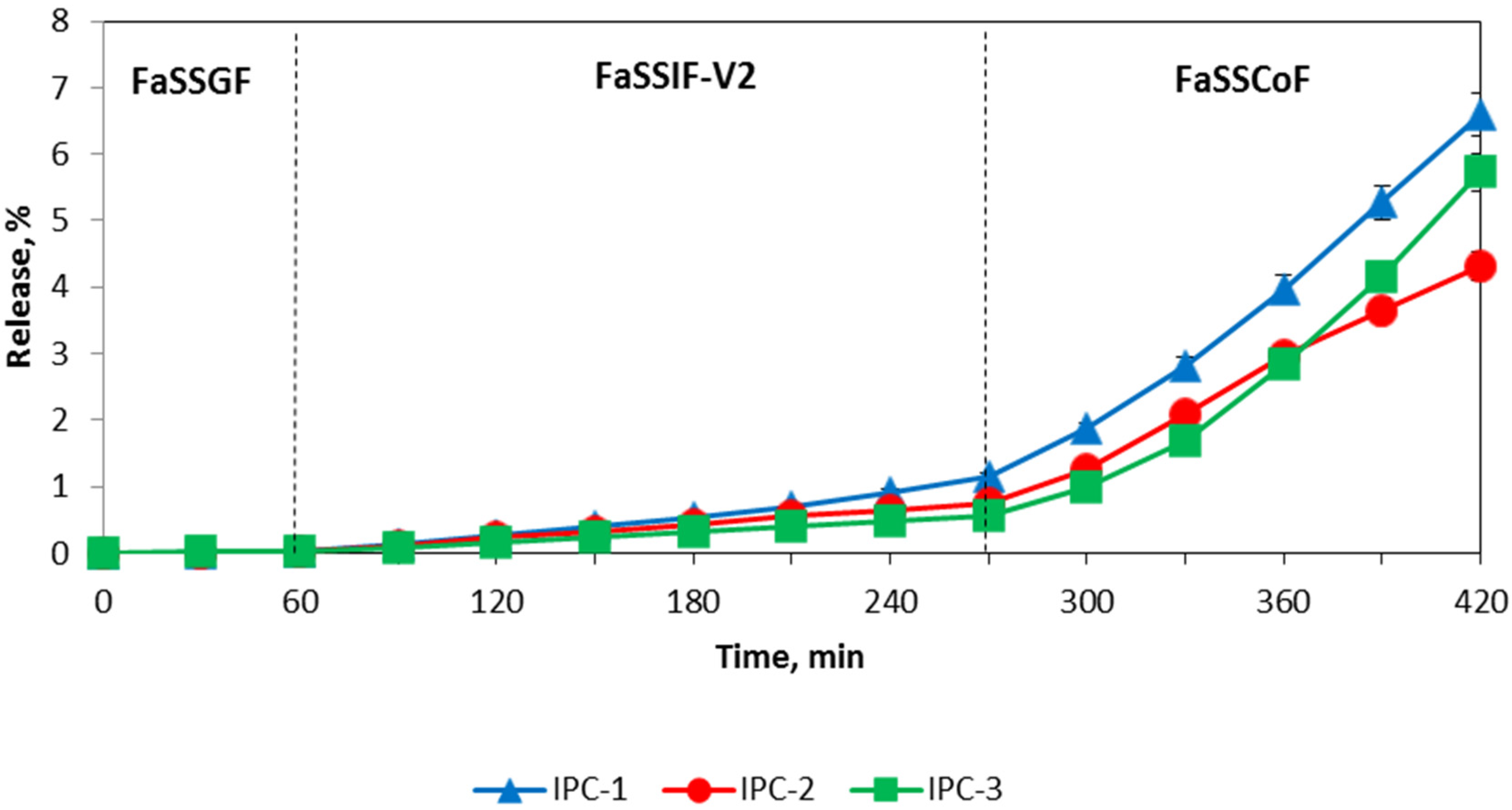
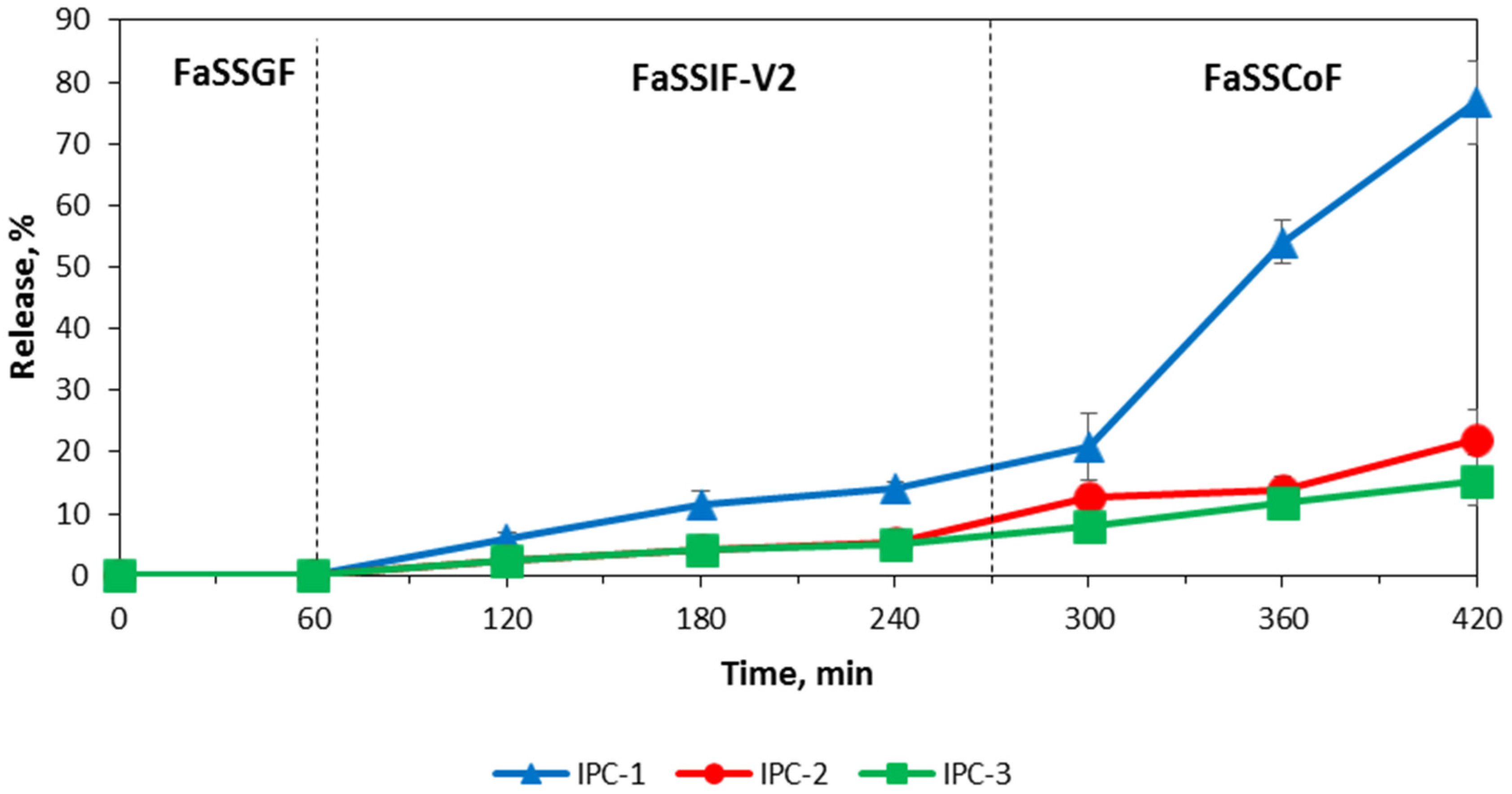
3.4. Release of Indomethacin from IPC Compacts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallardo, D.; Skalsky, B.; Kleinebudde, P. Characterization of combinations between anionic-cationic poly(methyl methacrylate) copolymers. Pharm. Ind. 2011, 73, 1875–1884. [Google Scholar]
- Mustafin, R.I. Interpolymer combinations of chemically complementary grades of Eudragit copolymers: A new direction in the design of peroral solid dosage forms of drug delivery systems with controlled release (review). Pharm. Chem. J. 2011, 45, 285–295. [Google Scholar] [CrossRef]
- Evonik Pharma Polymers. Eudragit Application Guidelines, 12th ed.; Evonik Pharma Polymers: Darmstadt, Germany, 2013; 308p. [Google Scholar]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Exp. Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Zezin, A.B.; Rogacheva, V.B. Polyelectrolyte complexes. In Achievements of the Physics and Chemistry of Polymers; Khimiya: Moscow, Russia, 1973; pp. 3–30. (In Russian) [Google Scholar]
- Tsuchida, E.; Abe, K. Interactions between macromolecules in solution and intermacromolecular complexes. Adv. Polym. Sci. 1982, 45, 1–119. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Smyslov, R.Y.; Yakimansky, A.V. Modern methods for studying polymer complexes in aqueous and organic solutions. Polym. Sci. Ser. A 2018, 60, 553–576. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Mussabaeva, B.K.; Kassymova, Z.S.; Klivenko, A.N.; Orazzhanova, L.K. Interpolyelectrolyte complexes: Advances and prospects of application. Russ. Chem. Rev. 2019, 88, 1046–1062. [Google Scholar] [CrossRef]
- Mustafin, R.I.; Kabanova, T.V.; Zhdanova, E.R.; Bukhovets, A.V.; Garipova, V.R.; Nasibullin, S.F.; Kemenova, V.A. Synthesis and physicochemical evaluation of new carrier based on interpolyelectrolyte complexes formed by Eudragit® EPO and Carbomer 940. Pharm. Chem. J. 2010, 44, 271–273. [Google Scholar] [CrossRef]
- Mustafin, R.I.; Kabanova, T.V.; Zhdanova, E.R.; Bukhovets, A.V.; Garipova, V.R.; Nasibullin, S.F.; Kemenova, V.A. Diffusion-transport properties of a polycomplex matrix system based on Eudragit® EPO and Carbomer 940. Pharm. Chem. J. 2010, 44, 147–150. [Google Scholar] [CrossRef]
- Mustafin, R.I.; Kabanova, T.V.; Semina, I.I.; Bukhovets, A.V.; Garipova, V.R.; Shilovskaya, E.V.; Nasibullin, S.F.; Sitenkov, A.Y.; Kazakova, R.R.; Kemenova, V.A. Biopharmaceutical assessment of polycomplex matrix system based on Carbomer 940 and Eudragit® EPO for colon-specific drug delivery. Pharm. Chem. J. 2011, 45, 491–494. [Google Scholar] [CrossRef]
- Mustafin, R.I.; Semina, I.I.; Garipova, V.R.; Bukhovets, A.V.; Sitenkov, A.Y.; Salakhova, A.R.; Gennari, C.G.M.; Cilurzo, F. Comparative study of polycomplexes based on Carbopol® and oppositely charged polyelectrolytes as a new oral drug delivery system. Pharm. Chem. J. 2015, 49, 1–6. [Google Scholar] [CrossRef]
- Garipova, V.R.; Gennari, C.G.M.; Selmin, F.; Cilurzo, F.; Moustafine, R.I. Mucoadhesive interpolyelectrolyte complexes for the buccal delivery of clobetasol. Polymers 2018, 10, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustafine, R.; Kemenova, V.; Van den Mooter, G. Characteristics of interpolyelectrolyte complexes of Eudragit E100 with sodium alginate. Int. J. Pharm. 2005, 294, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Moustafine, R.I.; Salachova, A.R.; Frolova, E.S.; Kemenova, V.A.; Van den Mooter, G. Interpolyelectrolyte complexes of Eudragit® EPO with sodium alginate as potential carriers for colonic drug delivery: Monitoring of structural transformation and composition changes during swellability and release evaluating. Drug Dev. Ind. Pharm. 2009, 35, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Rivas, S.; Fritz, H.F.; Valenzuela, C.; Santiviago, C.A.; Morales, J.O. Development of novel EE/alginate polyelectrolyte complex nanoparticles for lysozyme delivery: Physicochemical properties and in vitro safety. Pharmaceutics 2019, 11, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choiri, S.; Sulaiman, T.N.S.; Rohman, A. Characterization of Eudragit types and Kollidon SR inter-polymer complexes and their effects on the drug release. J. Appl. Polym. Sci. 2019, 9, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, D.; Skalsky, B.; Kleinebudde, P. Controlled release solid dosage forms using combinations of (meth)acrylate copolymer. Pharm. Dev. Technol. 2008, 13, 413–423. [Google Scholar] [CrossRef]
- Mustafin, R.I.; Bukhovets, A.V.; Sitenkov, A.Y.; Garipova, V.R.; Kemenova, V.A.; Rombaut, P.; Van den Mooter, G. Synthesis and characterization of a new carrier based on Eudragit® EPO/S100 interpolyelectrolyte complex for controlled colon-specific drug delivery. Pharm. Chem. J. 2011, 45, 568–574. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Bobyleva, V.L.; Bukhovets, A.V.; Garipova, V.R.; Kabanova, T.V.; Kemenova, V.A.; Van den Mooter, G. Structural transformations during swelling of polycomplex matrices based on countercharged (meth)acrylate copolymers (Eudragit® EPO/Eudragit® L100-55). J. Pharm. Sci. 2011, 100, 874–885. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Bodrov, A.V.; Kemenova, V.A.; Rombaut, P.; Van den Mooter, G. Drug release modification by interpolymer interaction between countercharged types of Eudragit® RL 30D and FS 30D in double layer films. Int. J. Pharm. 2012, 439, 17–21. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Bukhovets, A.V.; Sitenkov, A.Y.; Kemenova, V.A.; Rombaut, P.; Van den Mooter, G. Eudragit® E PO as a complementary material for designing oral drug delivery systems with controlled release properties: Comparative evaluation of new interpolyelectrolyte complexes with countercharged Eudragit® L100 copolymers. Mol. Pharm. 2013, 10, 2630–2641. [Google Scholar] [CrossRef]
- Moustafine, R.I.; Sitenkov, A.Y.; Bukhovets, A.V.; Nasibullin, S.F.; Appeltans, B.; Kabanova, T.V.; Khutoryanskiy, V.V.; Van den Mooter, G. Indomethacin-containing interpolyelectrolyte complexes based on Eudragit® E PO/S 100 copolymers as a novel drug delivery system. Int. J. Pharm. 2017, 524, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.I.; Prebeg, Ž.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation of drug release using Eudragit® L100-55 and Eudragit® S100 combinations. J. Conrol. Rel. 1999, 58, 215–222. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Štedul, H.P.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation of drug release using Eudragit® L100 and Eudragit® S100 combinations. Drug Dev. Ind. Pharm. 2000, 26, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, D.A.; Manzo, R.H.; Allemandi, D.A. Design of a colonic delivery system based on cationic polymethacrylate (E100)-mesalamine complexes. Drug. Deliv 2010, 17, 208–213. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Khan, M.A. Strategies of targeting oral drug delivery systems to the colon and their potential use for the treatment of colorectal cancer. Pharm. Dev. Tech. 2012, 17, 521–540. [Google Scholar] [CrossRef]
- Sardo, H.S.; Saremnejad, F.; Bagheri, S.; Akhgari, A.; Garekani, H.A.; Sadeghi, F. A review on 5-aminosalicylic acid colon-targeted oral drug delivery systems. Int. J. Pharm. 2019, 558, 367–379. [Google Scholar] [CrossRef]
- Friend, D.R. New oral delivery systems for treatment of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2005, 57, 247–265. [Google Scholar] [CrossRef] [PubMed]
- García-Couce, J.; Bada-Rivero, N.; Hernández, D.L.; Nogueira, A.; Caracciolo, P.C.; Abraham, G.A.; Hernández, J.A.R.; Peniche, C. Dexamethasone-Loaded chitosan beads coated with a ph-dependent interpolymer complexes for colon-specific drug delivery. Int. J. Pol. Sci. 2019, 2019, 4204375. [Google Scholar] [CrossRef] [Green Version]
- Joseph, S.K.; Sabitha, M.; Nair, S.C. Stimuli-responsive polymeric nanosystem for colon specific drug delivery. Adv. Pharm. Bul. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Watts, P.J.; Illum, L. Colonic drug delivery. Drug Dev. Ind. Pharm. 1997, 23, 893–913. [Google Scholar] [CrossRef]
- Linares, V.; Casas, M.; Caraballo, I. Prinfills: 3D printed systems combining fused deposition modeling and injection volume filling. Application to colon-specific drug delivery. Eur. J. Pharm. Biopharm. 2019, 134, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ma, R.; Wang, X.; Gao, J.; Zheng, Y.; Sun, Z. Enzyme and PH responsive 5-flurouracil (5-FU) loaded hydrogels based on olsalazine derivatives for colon-specific drug delivery. Eur. Polym. J. 2019, 118, 64–70. [Google Scholar] [CrossRef]
- Zhu, W.; Han, C.; Dong, Y.; Jian, B. Enzyme-responsive mechanism based on multi-walled carbon nanotubes and pectin complex tablets for oral colon-specific drug delivery system. J. Radioanal. Nucl. Chem. 2019, 320, 503–512. [Google Scholar] [CrossRef]
- Lamprecht, A.; Yamamoto, H.; Takeuchi, H.; Kaashima, Y. Microsphere design for the colonic delivery of 5-fluouracil. J. Control. Release 2003, 90, 313–322. [Google Scholar] [CrossRef]
- Barba, A.A.; Dalmoro, A.; d’Amore, M.; Lamberti, G. In-vitro dissolution of pH sensitive microparticles for colon-specific drug delivery. Pharm. Dev. Technol. 2013, 18, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; Sitenkov, A.Y.; Cascone, S.; Lamberti, G.; Barba, A.A.; Moustafine, R.I. Hydrophilc drug encapsulation in shell-core microcarriers by two stage polyelectrolyte complexation method. Int. J. Pharm. 2017, 518, 50–58. [Google Scholar] [CrossRef]
- Wang, K.; Wen, H.-F.; Yu, D.-G.; Yang, Y.; Zhang, D.-F. Electrosprayed hydrophilic nanocomposites coated with shellac for colon-specific delayed drug delivery. Mater. Des. 2018, 143, 248–255. [Google Scholar] [CrossRef]
- Iglesias, N.; Galbis, E.; Díaz-Blanco, M.J.; Lucas, R.; Benito, E.; de-Paz, M.V. Nanostructured chitosan-based biomaterials for sustained and colon-specific resveratrol release. Int. J. Mol. Sci. 2019, 20, 398. [Google Scholar] [CrossRef] [Green Version]
- Bisharat, L.; Barker, S.A.; Narbad, A.; Craig, D.Q.M. In vitro drug release from acetylated high amylose starch-zein films for oral colon-specific drug delivery. Int. J. Pharm. 2019, 556, 311–319. [Google Scholar] [CrossRef]
- Laxmi, G.R.P.; Srikanth, G. Formulation and evaluation of colon specific drug delivery of press coated esomeprazole tablets. J. Drug Deliv Ther. 2019, 9, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.N.U.; Tran, P.H.L.; Tran, T.T.D. A single-layer film coating for colon-targeted oral delivery. Int. J. Pharm. 2019, 559, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Rijo, J.; Sabitha, M. Guargum and Eudragit® coated curcumin liquid solid tablets for colon specific drug delivery. Int. J. Biol. Macromol. 2018, 110, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, G.; Jain, S.K. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials 2015, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Yadav, A.K.; Jain, N.K.; Agrawal, G.P. Eudragit-coated dextran microspheres of 5-fluorouracil for site-specific delivery to colon. Drug Deliv. 2016, 23, 328–337. [Google Scholar] [CrossRef]
- Kumari, A.; Jain, A.; Hurkat, P.; Tiwari, A. Eudragit S100 coated microsponges for Colon targeting of prednisolone. Drug Dev. Ind. Pharm. 2018, 44, 902–913. [Google Scholar] [CrossRef]
- Dupeyron, D.; Kawakami, M.; Ferreira, A.M.; Caceres-Velez, P.R.; Rieumont, J.; Azevedo, R.B.; Carvalho, J.C.T. Design of indomethacin-loaded nanoparticles: Effect of polymer matrix and surfactant. Int. J. Nanomed. 2013, 8, 3467–3477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.L.; Ying, G.; Li, Z.C.; Lin, J. Screening for novel protein targets of indomethacin in HCT116 human colon cancer cells using proteomics. Oncol. Lett. 2013, 6, 1222–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.M.; Zhang, G.Y. Indomethacin suppresses growth of colon cancer via inhibition of angiogenesis in vivo. World J. Gastroenterol. 2005, 11, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Lamoza, M.L.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Design of microencapsulated chitosan microspheres for colonic drug delivery. J. Control. Release 1998, 52, 109–118. [Google Scholar] [CrossRef]
- Jantraid, E.; Janssen, N.; Reppas, C.; Dressman, J.B. Dissolution media simulating conditions in the proximal human gastrointestinal tract: An update. Pharm. Res. 2008, 25, 1663. [Google Scholar] [CrossRef]
- Vertzoni, M.; Diakidou, A.; Chatzilias, M.; Söderlind, E.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Biorelevant media to simulate fluids in the ascending colon of humans and their usefulness in predicting intracolonic drug solubility. Pharm. Res. 2010, 27, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Docler, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Bekturov, E.A.; Bimendina, L.A. Interpolymer complexes. Adv. Polym. Sci. 1981, 41, 99–147. [Google Scholar]
- Nurkeeva, Z.S.; Mun, G.A.; Khutoryanskiy, V.V.; Mangazbaeva, R.A.; Zotov, A.A. Interpolymer complexes of polyvinyl ether of ethyleneglycol with poly(carboxylic acids) in aqueous, alcohol and mixed solutions. Polymer 2000, 41, 7647–7651. [Google Scholar] [CrossRef]
- Mun, G.A.; Nurkeeva, Z.S.; Khutoryanskiy, V.V.; Sergaziyev, A.D. Interpolymer complexes of copolymers of vinyl ether of diethyleneglycol with poly(acrylic acid). Colloid Polym. Sci. 2002, 280, 282–289. [Google Scholar] [CrossRef]
- Zhunuspayev, D.E.; Mun, G.A.; Hole, P.; Khutoryanskiy, V.V. Solvent effects on the formation of nanoparticles and multilayered coatings based on hydrogen-bonded interpolymer complexes of poly(acrylic acid) with homo- and copolymers of N-vinyl pyrrolidone. Langmuir 2008, 24, 13742–13747. [Google Scholar] [CrossRef]
- Nurkeeva, Z.S.; Mun, G.A.; Khutoryanskiy, V.V.; Sergaziyev, A.D. Complex formation of poly(vinyl ether of diethyleneglycol) with polyacrylic acid. II. Effect of molecular weight of polyacrylic acid and solvent nature. Eur. Polym. J. 2002, 38, 313–316. [Google Scholar] [CrossRef]
- Jie, D.A.I.; GoH, S.H.; LEE, S.Y.; Smw, K.S. Interpolymer complexation and blend formation between poly(N-vinyl-2-pyrrolidone) and aliphatic hydroxyl-containing polymers. Polym. J. 1995, 27, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Cesteros, L.C.; Meaurio, E.; Katime, L. Formation of interpolymer complexes between poly(monoethyl itaconate) and poly(n-vinyl-2-pyrrolidone). Polym. Int. 1994, 34, 97–103. [Google Scholar] [CrossRef]
- Bou-Chacra, N.; Melo, K.J.C.; Morales, I.A.C.; Stippler, E.S.; Kesisoglou, F.; Yazdanian, M.; Löbenberg, R. Evolution of choice of solubility and dissolution media after two decades of biopharmaceutical classification system. AAPS J. 2017, 19, 989–1001. [Google Scholar] [CrossRef] [Green Version]
- Yazdanian, M.; Briggs, K.; Jankovsky, C.; Hawi, A. The “high solubility” definition of the current FDA guidance on biopharmaceutical classification system may be too strict for acidic drugs. Pharm. Res. 2004, 21, 293–299. [Google Scholar] [CrossRef] [PubMed]


| IPC | Solvent | Polymer Ratio in Mixture [EPO]:[S100], (mol/mol) | Order of Mixing | Composition of IPC [EPO]:[S100], (mol/mol) | Tg, °C |
|---|---|---|---|---|---|
| IPC-1 | isopropanol/acetone | 1:2 | EPO to S100 | 1:2.96 | 153.9 ± 0.3 |
| IPC-2 | isopropanol/acetone | 1:3 | S100 to EPO | 1:3.68 | 160.8 ± 0.2 |
| IPC-3 | ethanol | 1:1.5 | EPO to S100 | 1:2.13 | 143.9 ± 1.2 |
| IPC-4 | ethanol | 1:1.5 | S100 to EPO | 1:1.94 | 141.5 ± 0.5 |
| IPC-5 | tetrahydrofuran | 1:2 | EPO to S100 | 1:2.58 | 152.9 ± 0.4 |
| IPC-6 | tetrahydrofuran | 1:1.5 | S100 to EPO | 1:1.90 | 140.5 ± 2.5 |
| Sample | pH | N% | Composition of IPC [EPO]:[S100], (mol/mol) |
|---|---|---|---|
| IPC-1 | 1.2 | 1.60 ± 0.01 | 1:3.15 |
| 5.8 | 1.64 ± 0.01 | 1:3.09 | |
| 6.8 | 1.65 ± 0.02 | 1:3.08 | |
| 7.4 | 1.68 ± 0.02 | 1:2.99 | |
| IPC-2 | 1.2 | 1.44 ± 0.00 | 1:3.73 |
| 5.8 | 1.49 ± 0.02 | 1:3.57 | |
| 6.8 | 1.59 ± 0.01 | 1:3.24 | |
| 7.4 | 1.52 ± 0.02 | 1:3.44 | |
| IPC-3 | 1.2 | 1.92 ± 0.17 | 1:2.44 |
| 5.8 | 1.90 ± 0.01 | 1:2.46 | |
| 6.8 | 1.93 ± 0.05 | 1:2.41 | |
| 7.4 | 1.99 ± 0.01 | 1:2.30 |
| Compact Characteristics | |||
|---|---|---|---|
| IPC-1 | IPC-2 | IPC-3 | |
| Release exponent (n) | 2.67 ± 0.20 | 2.65 ± 0.18 | 2.45 ± 0.24 |
| R2 | 0.9906 | 0.9927 | 0.9846 |
| Transport mechanism | Super Case-II | Super Case-II | Super Case-II |
| Compact Characteristics | |||
|---|---|---|---|
| IPC-1 | IPC-2 | IPC-3 | |
| Release exponent (n) | 2.98 ± 0.37 | 2.08 ± 0.25 | 1.76 ± 0.12 |
| R2 | 0.9736 | 0.9737 | 0.9910 |
| Transport mechanism | Super Case-II | Super Case-II | Super Case-II |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukhovets, A.V.; Fotaki, N.; Khutoryanskiy, V.V.; Moustafine, R.I. Interpolymer Complexes of Eudragit® Copolymers as Novel Carriers for Colon-Specific Drug Delivery. Polymers 2020, 12, 1459. https://doi.org/10.3390/polym12071459
Bukhovets AV, Fotaki N, Khutoryanskiy VV, Moustafine RI. Interpolymer Complexes of Eudragit® Copolymers as Novel Carriers for Colon-Specific Drug Delivery. Polymers. 2020; 12(7):1459. https://doi.org/10.3390/polym12071459
Chicago/Turabian StyleBukhovets, Aleksandra V., Nikoletta Fotaki, Vitaliy V. Khutoryanskiy, and Rouslan I. Moustafine. 2020. "Interpolymer Complexes of Eudragit® Copolymers as Novel Carriers for Colon-Specific Drug Delivery" Polymers 12, no. 7: 1459. https://doi.org/10.3390/polym12071459







