Characterization and Performance Evaluation of Cellulose Acetate–Polyurethane Film for Lead II Ion Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Acetate–Polyurethane (CA–PU) Film
2.3. Characterization of the Film
2.4. Batch Adsorption Performance Evaluation of the Film
3. Results and Discussion
3.1. Reaction and Functional Group Analysis
3.2. Mechanical Properties
3.3. Thermal Properties
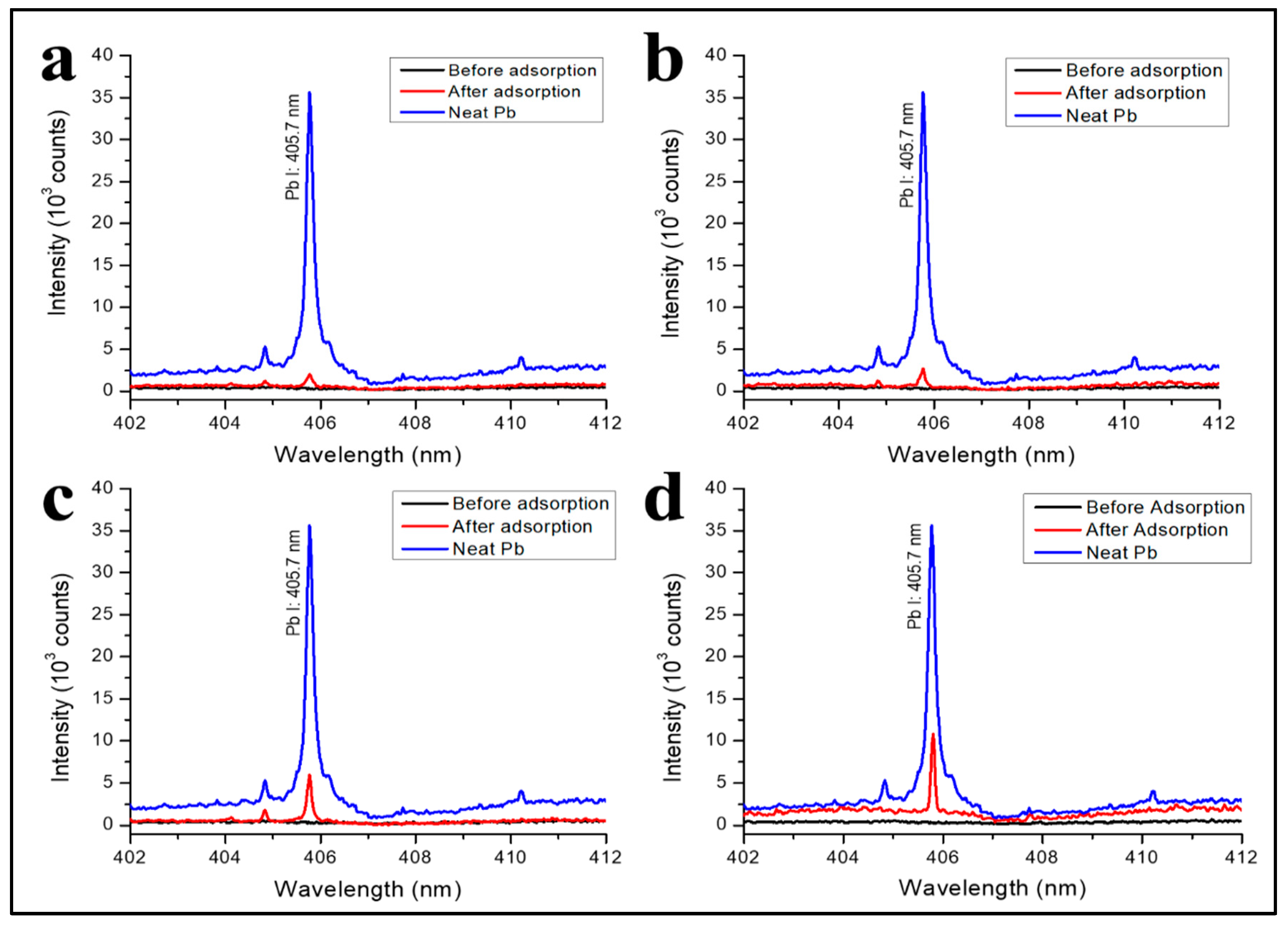
3.4. Pb2+ Ions Adsorption Detected with Laser-Induced Breakdown Spectroscopy (LIBS)
3.5. Effect of Contact Time, pH, and Initial Concentration
3.6. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process–a systematic review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavilà, L.; Esposito, D. Cellulose acetate as a convenient intermediate for the preparation of 5-acetoxymethylfurfural from biomass. Green Chem. 2017, 19, 2496–2500. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, M.; Duan, C.; Sun, J.; Xu, Y. Preparation and characterization of cellulose-based adsorbent and its application in heavy metal ions removal. Carbohydr. Polym. 2019, 206, 837–843. [Google Scholar] [CrossRef]
- El Azhari, A.; Rhoujjati, A.; El Hachimi, M.L.; Ambrosi, J.-P. Pollution and ecological risk assessment of heavy metals in the soil-plant system and the sediment-water column around a former Pb/Zn-mining area in NE Morocco. Ecotoxicol. Environ. Saf. 2017, 144, 464–474. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibullah-Al-Mamun, M.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Liu, Y.; Zhang, X.; Ravikumar, B.; Bai, G.; Li, X. Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 2018, 191, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Armid, A.; Shinjo, R.; Ruslan, R. Distributions and pollution assessment of heavy metals Pb, Cd and Cr in the water system of Kendari Bay. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012002. [Google Scholar]
- Sikka, R.; Nayyar, V. Monitoring of lead (Pb) pollution in soils and plants irrigated with untreated sewage water in some industrialized cities of Punjab, India. Bull. Environ. Contam. Toxicol. 2016, 96, 443–448. [Google Scholar] [CrossRef]
- Okorie, A.; Entwistle, J.; Dean, J.R. Estimation of daily intake of potentially toxic elements from urban street dust and the role of oral bioaccessibility testing. Chemosphere 2012, 86, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Enniya, I.; Rghioui, L.; Jourani, A. Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain. Chem. Pharm. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd (II) and Pb (II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef] [Green Version]
- Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Long, J.R. Metal− organic frameworks as adsorbents for hydrogen purification and precombustion carbon dioxide capture. J. Am. Chem. Soc. 2011, 133, 5664–5667. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Liu, R.; Li, Y.; Wang, D.; Tan, J.; Wu, R.; Huang, Y. Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment. Carbohydr. Polym. 2011, 83, 743–748. [Google Scholar] [CrossRef]
- Salama, A.; Mohamed, A.; Aboamera, N.M.; Osman, T.; Khattab, A. Characterization and mechanical properties of cellulose acetate/carbon nanotube composite nanofibers. Adv. Polym. Technol. 2018, 37, 2446–2451. [Google Scholar] [CrossRef] [Green Version]
- Kamal, H.; Abd-Elrahim, F.; Lotfy, S. Characterization and some properties of cellulose acetate-co-polyethylene oxide blends prepared by the use of gamma irradiation. J. Radiat. Res. Appl. Sci. 2014, 7, 146–153. [Google Scholar] [CrossRef]
- Sánchez-Márquez, J.; Fuentes-Ramírez, R.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Rubio-Rosas, E.; Kenny, J.M.; Rescignano, N. Membrane made of cellulose acetate with polyacrylic acid reinforced with carbon nanotubes and its applicability for chromium removal. Int. J. Polym. Sci. 2015, 320631, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Riaz, T.; Ahmad, A.; Saleemi, S.; Adrees, M.; Jamshed, F.; Hai, A.M.; Jamil, T. Synthesis and characterization of polyurethane-cellulose acetate blend membrane for chromium (VI) removal. Carbohydr. Polym. 2016, 153, 582–591. [Google Scholar] [CrossRef]
- Hong, H.-J.; Lim, J.S.; Hwang, J.Y.; Kim, M.; Jeong, H.S.; Park, M.S. Carboxymethlyated cellulose nanofibrils (CMCNFs) embedded in polyurethane foam as a modular adsorbent of heavy metal ions. Carbohydr. Polym. 2018, 195, 136–142. [Google Scholar] [CrossRef]
- Fischer, F.; Rigacci, A.; Pirard, R.; Berthon-Fabry, S.; Achard, P. Cellulose-based aerogels. Polymer 2006, 47, 7636–7645. [Google Scholar] [CrossRef]
- Kumari, S.; Chauhan, G.S.; Ahn, J.H. Novel cellulose nanowhiskers-based polyurethane foam for rapid and persistent removal of methylene blue from its aqueous solutions. Chem. Eng. J. 2016, 304, 728–736. [Google Scholar] [CrossRef]
- Li, G.; Chai, K.; Zhou, L.; Tong, Z.; Ji, H. Easy fabrication of aromatic-rich cellulose-urethane polymer for preferential adsorption of acetophenone over 1-phenylethanol. Carbohydr. Polym. 2019, 206, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Cui, M.; Yoon, Y.; Khim, J.; Jang, M. Passive treatment of arsenic and heavy metals contaminated circumneutral mine drainage using granular polyurethane impregnated by coal mine drainage sludge. J. Clean. Prod. 2018, 186, 282–292. [Google Scholar] [CrossRef]
- Góes, M.M.; Keller, M.; Masiero Oliveira, V.; Villalobos, L.D.G.; Moraes, J.C.G.; Carvalho, G.M. Polyurethane foams synthesized from cellulose-based wastes: Kinetics studies of dye adsorption. Ind. Crops Prod. 2016, 85, 149–158. [Google Scholar]
- Ikhwan, F.; Ilmiati, S.; Adi, H.K.; Arumsari, R.; Chalid, M. Novel route of synthesis for cellulose fiber-based hybrid polyurethane. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Medan, Indonesia, 7–10 November 2016; p. 012019. [Google Scholar]
- Rivera-Armenta, J.; Heinze, T.; Mendoza-Martínez, A. New polyurethane foams modified with cellulose derivatives. Eur. Polym. J. 2004, 40, 2803–2812. [Google Scholar] [CrossRef]
- Sun, P.; Yang, S.; Sun, X.; Wang, Y.; Pan, L.; Wang, H.; Wang, X.; Guo, J.; Nie, C. Functional porous carboxymethyl cellulose/cellulose acetate composite microspheres: Preparation, characterization, and application in the effective removal of HCN from cigarette smoke. Polymers 2019, 11, 181. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhou, S.; Zhang, L.; You, T.; Xu, F. Adsorption of heavy metals by graphene oxide/cellulose hydrogel prepared from NaOH/urea aqueous solution. Materials 2016, 9, 582. [Google Scholar] [CrossRef]
- Yang, J.; Kubota, F.; Baba, Y.; Kamiya, N.; Goto, M. Application of cellulose acetate to the selective adsorption and recovery of Au (III). Carbohydr. Polym. 2014, 111, 768–774. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Synthesis and mechanical properties of bio-sourced polyurethane adhesives obtained from castor oil and MDI-modified cellulose acetate: Influence of cellulose acetate modification. Int. J. Adhes. Adhes. 2019, 95, 102404. [Google Scholar] [CrossRef]
- Nurfatimah, R. Preparation of polyethylene glycol diglycidyl ether (PEDGE) crosslinked chitosan/activated carbon composite film for Cd2+ removal. Carbohydr. Polym. 2018, 199, 499–505. [Google Scholar]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Preparation, characterization and mechanical properties of bio-based polyurethane adhesives from isocyanate-functionalized cellulose acetate and castor oil for bonding wood. Polymers 2017, 9, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.; Lai, T.K.; Chong, E.W.N.; Gopakumar, D.A.; Rizal, S.; Hossain, M.S.; Fazita, M.R.N.; Haafiz, M.K.M.; Paridah, M.T.; Khalil, H.A. Organic and Inorganic Fillers’ Role on the Amelioration of Kappaphycus spp.-based Biopolymer Films’ Performance. BioResources 2019, 14, 9198–9213. [Google Scholar]
- Hasan, M.; Lai, T.K.; Gopakumar, D.A.; Jawaid, M.; Owolabi, F.A.T.; Mistar, E.M.; Alfatah, T.; Noriman, N.Z.; Haafiz, M.K.M.; Abdul Khalil, H.P.S. Micro Crystalline Bamboo Cellulose Based Seaweed Biodegradable Composite Films for Sustainable Packaging Material. J. Polym. Environ. 2019, 27, 1602–1612. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb(II): From surface properties to sorption mechanism. Desalin. Water Treat. 2016, 57, 10730–10744. [Google Scholar] [CrossRef]
- Marlina, M.; Iqhrammullah, M.; Darmadi, D.; Mustafa, I.; Rahmi, R. The application of chitosan modified polyurethane foam adsorbent. Rasayan J. Chem. 2019, 12, 494–501. [Google Scholar] [CrossRef]
- Darmadi, D.; Irfan, M.; Iqhramullah, M.; Marlina, M.; Lubis, M.R. Syhnthesis of Chitosan Modified Polyurethane Foam for Adsoprtion of Mercury (II) Ions. Jurnal Bahan Alam Terbarukan 2018, 7, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.R.; De, S. Preparation, characterization and antifouling properties of polyacrylonitrile/polyurethane blend membranes for water purification. RSC Adv. 2015, 5, 23599–23612. [Google Scholar] [CrossRef]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Hedwig, R.; Karnadi, I.; Kurniawan, K.; Olaiya, N.; Mohamad Haafiz, M.; Abdul Khalil, H.; Abdulmadjid, S. Filler-Modified Castor Oil-Based Polyurethane Foam for the Removal of Aqueous Heavy Metals Detected Using Laser-Induced Breakdown Spectroscopy (LIBS) Technique. Polymers 2020, 12, 903. [Google Scholar] [CrossRef] [Green Version]
- Saranya, M.; Srinivasan, L.; Gopal, R.M.R.; Gomathi, T.; Sudha, P.N.; Sukumaran, A. Adsorption Studies of Lead(II) from aqueous solution onto Nanochitosan /Polyurethane /Polypropylene glycol ternary blends. Int. J. Biol. Macromol. 2017, 104, 1436–1448. [Google Scholar]
- Chen, Q.; Zheng, J.; Wen, L.; Yang, C.; Zhang, L. A multi-functional-group modified cellulose for enhanced heavy metal cadmium adsorption: Performance and quantum chemical mechanism. Chemosphere 2019, 224, 509–518. [Google Scholar] [CrossRef]
- Mustapha, S.; Shuaib, D.T.; Ndamitso, M.M.; Etsuyankpa, M.B.; Sumaila, A.; Mohammed, U.M.; Nasirudeen, M.B. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions using Albizia lebbeck pods. Appl. Water Sci. 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interfaces Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Park, M.H.; Jeong, S.; Kim, J.Y. Adsorption of NH3-N onto rice straw-derived biochar. J. Environ. Chem. Eng. 2019, 7, 103039. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 3039817, 1–11. [Google Scholar] [CrossRef]
- Utomo, H.D.; Salim, M.R. Adsorption of Heavy Metals from Water and Waste Water Using Low Cost Adsorbents from Agricultural By-Products. Asian J. Water Environ. Pollut. 2009, 6, 73–80. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, M.A. Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems. Polymers 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Medellin-Castillo, N.A.; Padilla-Ortega, E.; Regules-Martínez, M.C.; Leyva-Ramos, R.; Ocampo-Pérez, R.; Carranza-Alvarez, C. Single and competitive adsorption of Cd(II) and Pb(II) ions from aqueous solutions onto industrial chili seeds (Capsicum annuum) waste. Sustain. Environ. Res. 2017, 27, 61–69. [Google Scholar] [CrossRef]
- Chen, L.; Bromberg, L.; Hatton, T.A.; Rutledge, G.C. Electrospun cellulose acetate fibers containing chlorhexidine as a bactericide. Polymer 2008, 49, 1266–1275. [Google Scholar] [CrossRef]
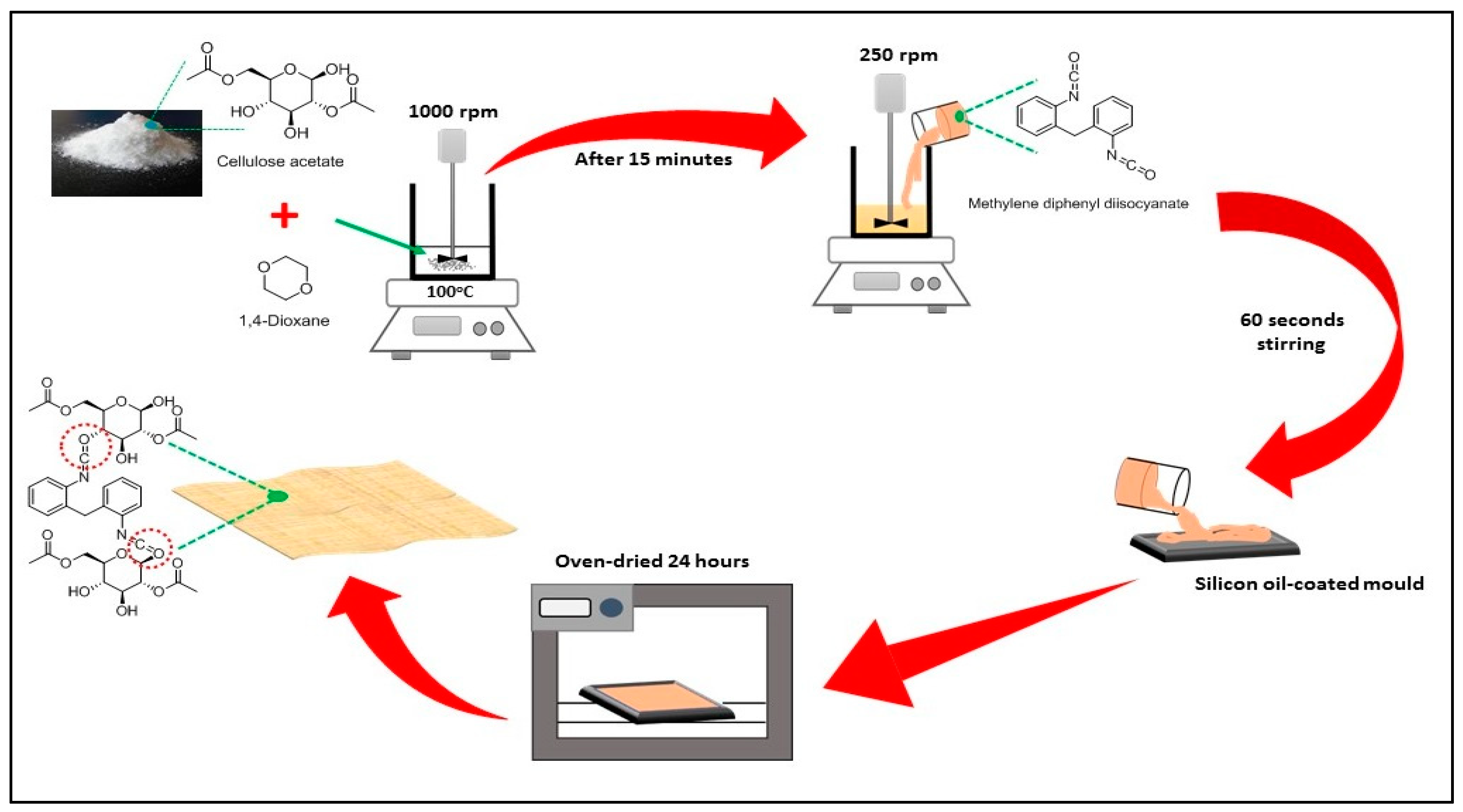
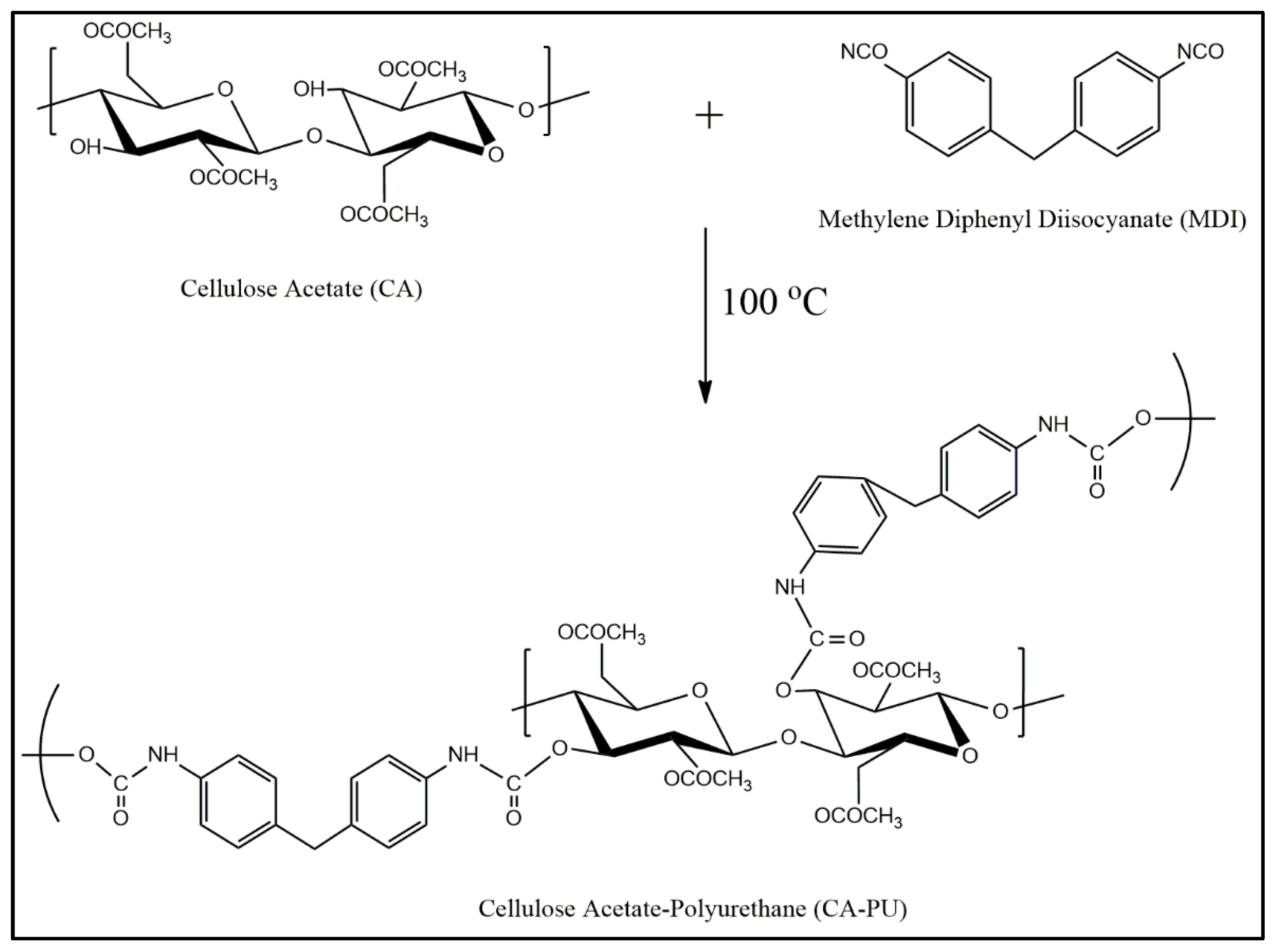


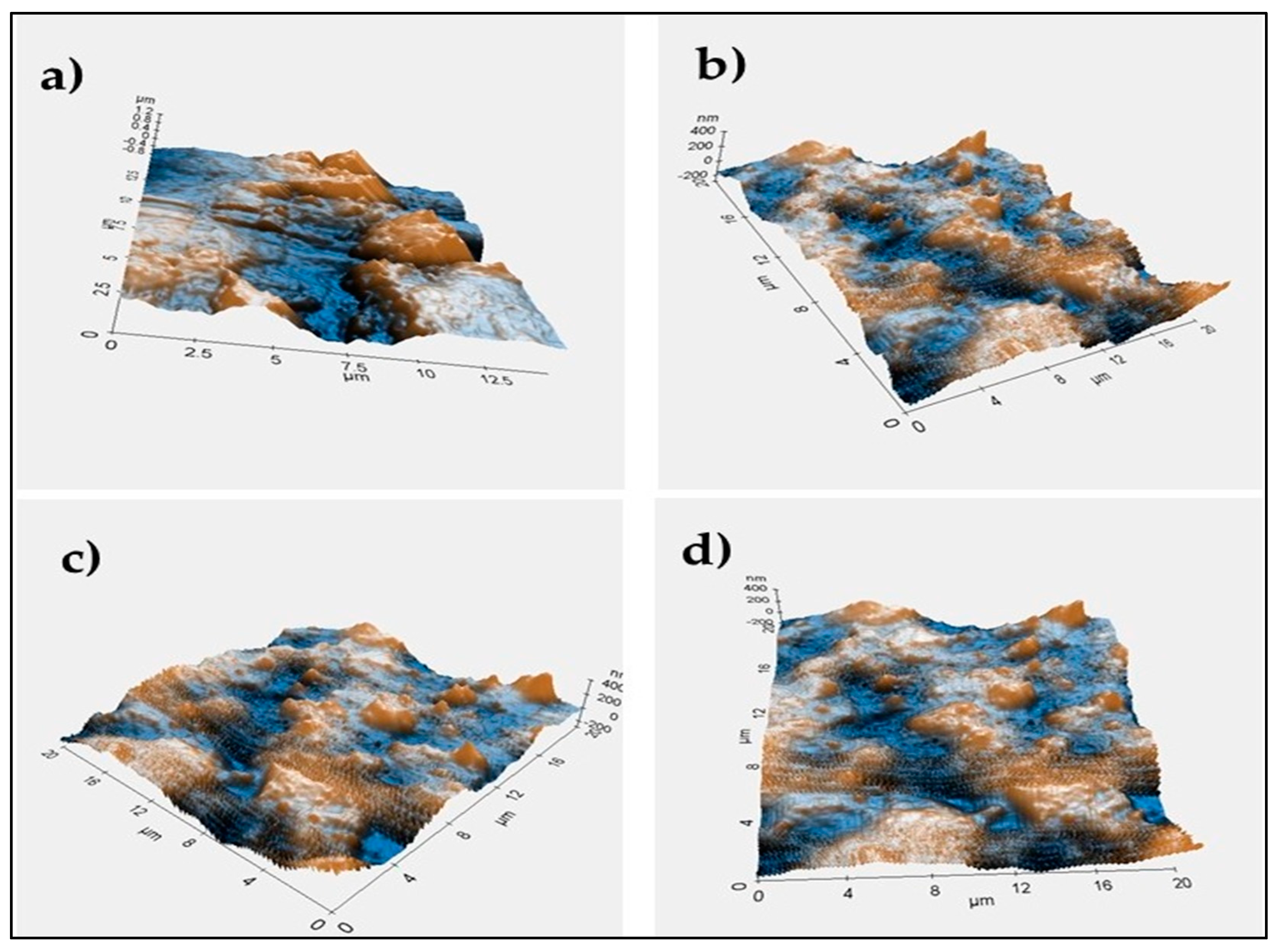
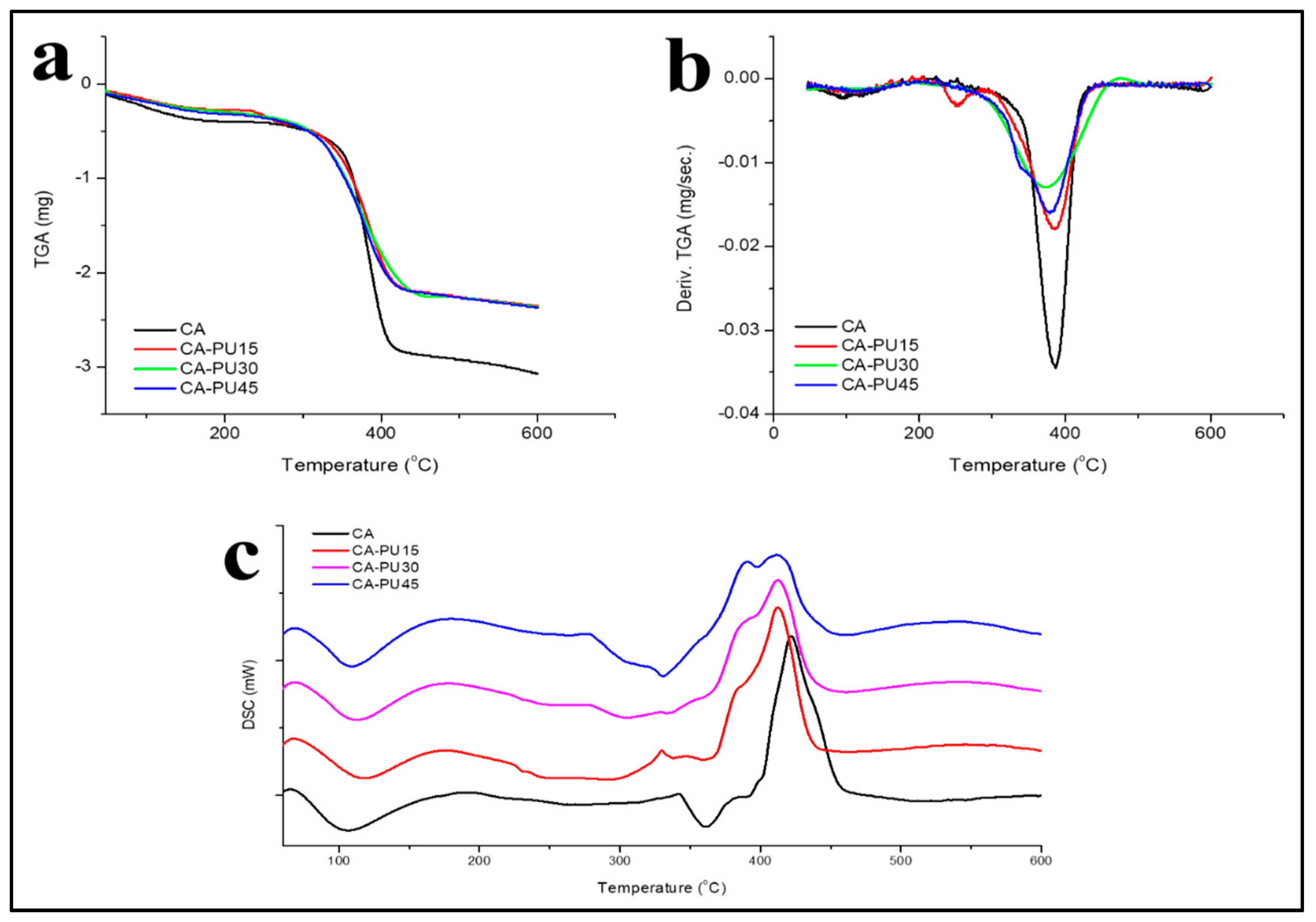



| Label | Cellulose Acetate Powder (g) | 1,4-Dioxane (mL) | MDI (% w/w Cellulose Acetate) |
|---|---|---|---|
| CA | 1 | 10 | - |
| CA–PU15 | 1 | 10 | 15 |
| CA–PU30 | 1 | 10 | 30 |
| CA–PU | 1 | 10 | 45 |
| MDI (%) | Droplet Image | Contact Angle | θ |
|---|---|---|---|
| 0 |  | 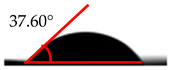 | 37.60° ± 1.2° |
| 15 |  |  | 45.30° ± 2.3° |
| 30 |  | 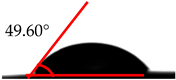 | 49.60° ± 1.7° |
| 45 |  |  | 61.70° ± 1.6° |
| No. | MDI (% w/w) | Tensile Strength (MPa) | Elongation (%) | Tensile Modulus (MPa) |
|---|---|---|---|---|
| 1 | 0 | 17.3 ± 0.3 | 12.2 | 140.0 ± 2.1 |
| 2 | 15 | 17.5 ± 0.2 | 9.1 | 123.0 ± 1.5 |
| 3 | 30 | 20.4 ± 0.6 | 11.9 | 168.0 ± 1.2 |
| 4 | 45 | 24.1 ±0.9 | 15.6 | 213.0 ± 1.7 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| R2 | Qm (mg/g) | KL (dm3/mg) | R2 | KF (mg/g)(dm3/g) | 1/n |
| 0.96953 | 200 | 0.015 | 0.97044 | 2.380 | 0.867 |
| No. | Adsorbent | KF (mg/g) (dm3/g) | Citation |
|---|---|---|---|
| 1 | Chitosan-iron(III) bio-composite beads | 0.69 | Lapo et al. [51] |
| 2 | Chili seeds (Capsicum annuum) waste | 0.263 | Medellin-Castillo et al. [52] |
| 3 | Apple peel-based activated carbon | 2.2637 | Enniya et al. [12] |
| 4 | Albizia lebbeck pods-based powder | 1.28 | Mustapha et al. [44] |
| 5 | NaOH/urea-based graphene oxide/cellulose hydrogel | 0.92 | Chen et al. [53] |
| 6 | Chitosan–pyromellitic dianhydride modified biochar | 5.0407 | Deng et al. [45] |
| 7 | Cellulose acetate–polyurethane Film | 2.380 | This research |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqhrammullah, M.; Marlina, M.; Khalil, H.P.S.A.; Kurniawan, K.H.; Suyanto, H.; Hedwig, R.; Karnadi, I.; Olaiya, N.G.; Abdullah, C.K.; Abdulmadjid, S.N. Characterization and Performance Evaluation of Cellulose Acetate–Polyurethane Film for Lead II Ion Removal. Polymers 2020, 12, 1317. https://doi.org/10.3390/polym12061317
Iqhrammullah M, Marlina M, Khalil HPSA, Kurniawan KH, Suyanto H, Hedwig R, Karnadi I, Olaiya NG, Abdullah CK, Abdulmadjid SN. Characterization and Performance Evaluation of Cellulose Acetate–Polyurethane Film for Lead II Ion Removal. Polymers. 2020; 12(6):1317. https://doi.org/10.3390/polym12061317
Chicago/Turabian StyleIqhrammullah, M., Marlina Marlina, H. P. S. Abdul Khalil, K. H. Kurniawan, H. Suyanto, R. Hedwig, I. Karnadi, N. G. Olaiya, C. K. Abdullah, and S. N. Abdulmadjid. 2020. "Characterization and Performance Evaluation of Cellulose Acetate–Polyurethane Film for Lead II Ion Removal" Polymers 12, no. 6: 1317. https://doi.org/10.3390/polym12061317







