Enhanced Hydrophilic and Electrophilic Properties of Polyvinyl Chloride (PVC) Biofilm Carrier
Abstract
:1. Introduction
2. Experimental
2.1. Materials
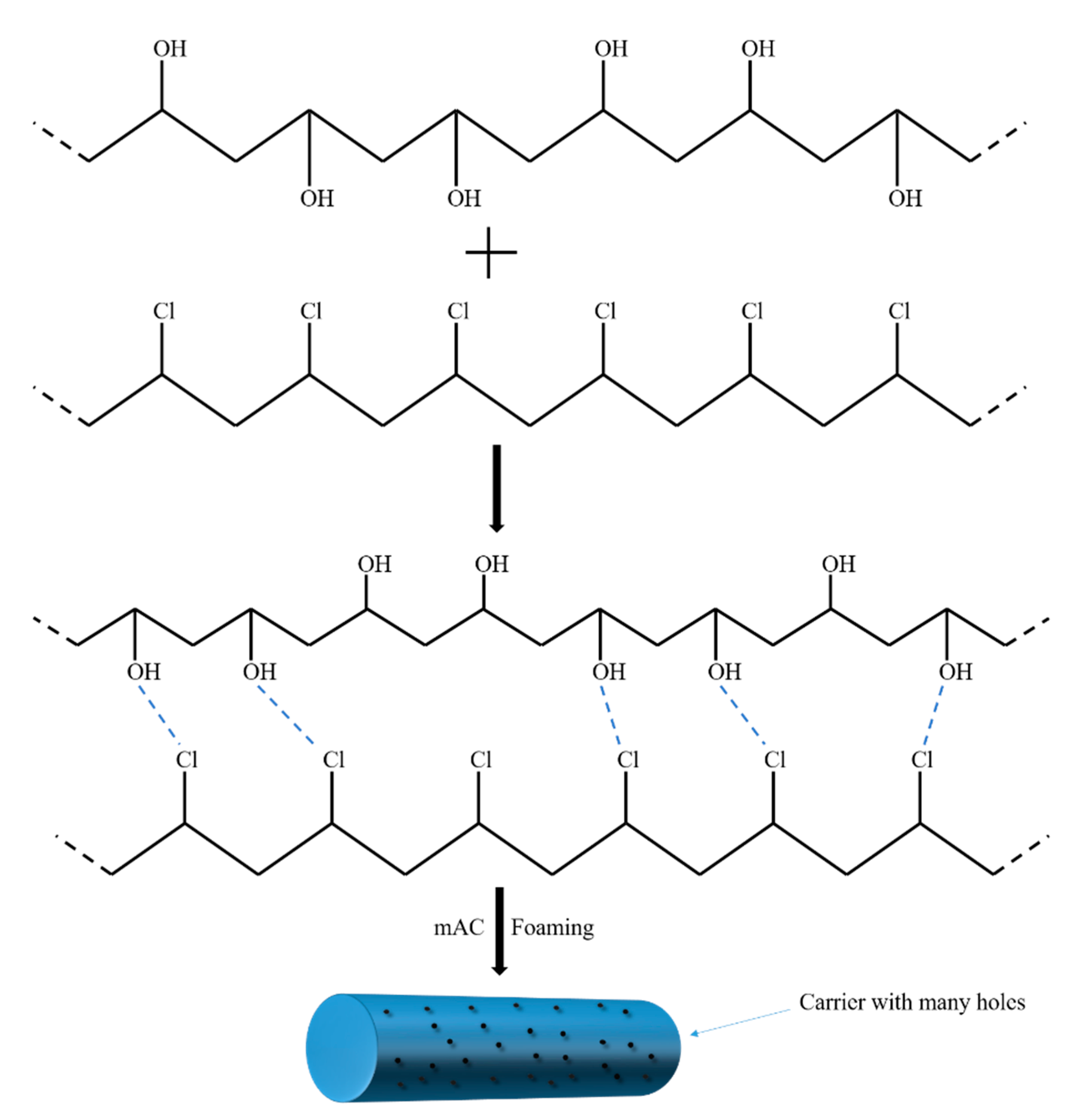
2.2. Preparation of PVC Biofilm Carrier
2.3. Surface Contact Angle
2.4. Fourier Transform Infrared Spectra
2.5. Zeta Potential
2.6. Field Emission Scanning Electron Microscope
2.7. Mechanical Properties
3. Results and Discussion
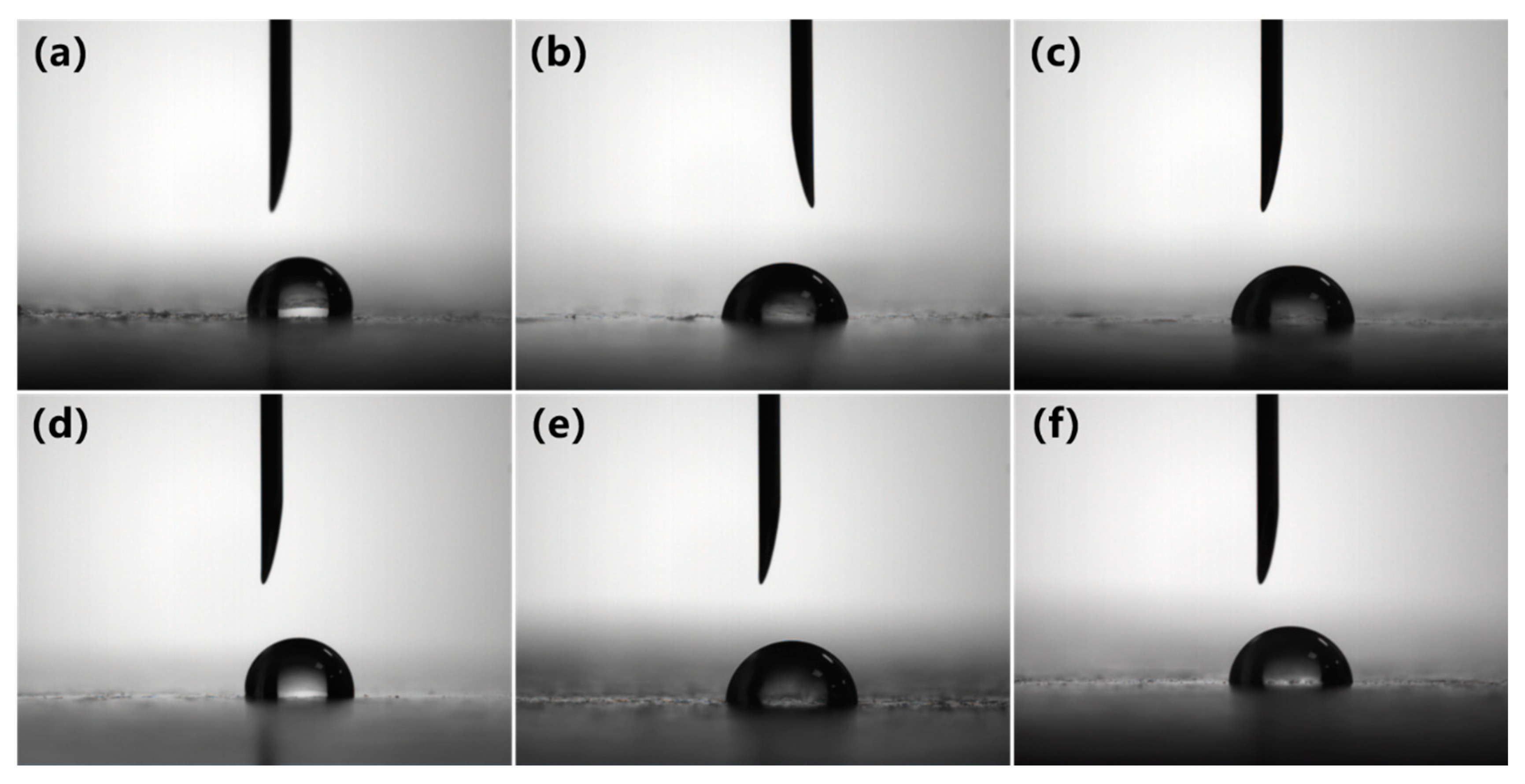
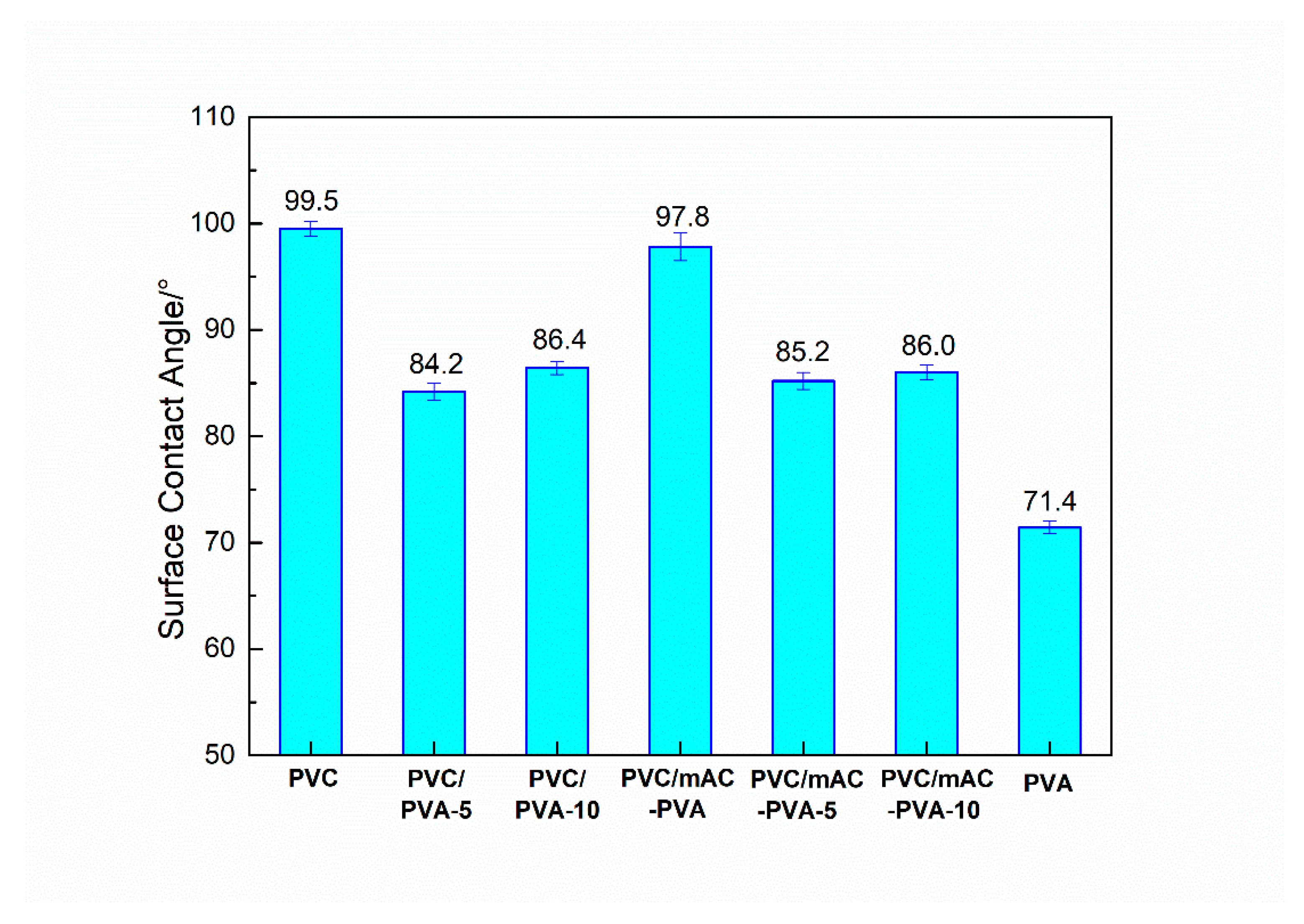
3.1. Hydrophilicity of PVC Biofilm Carrier
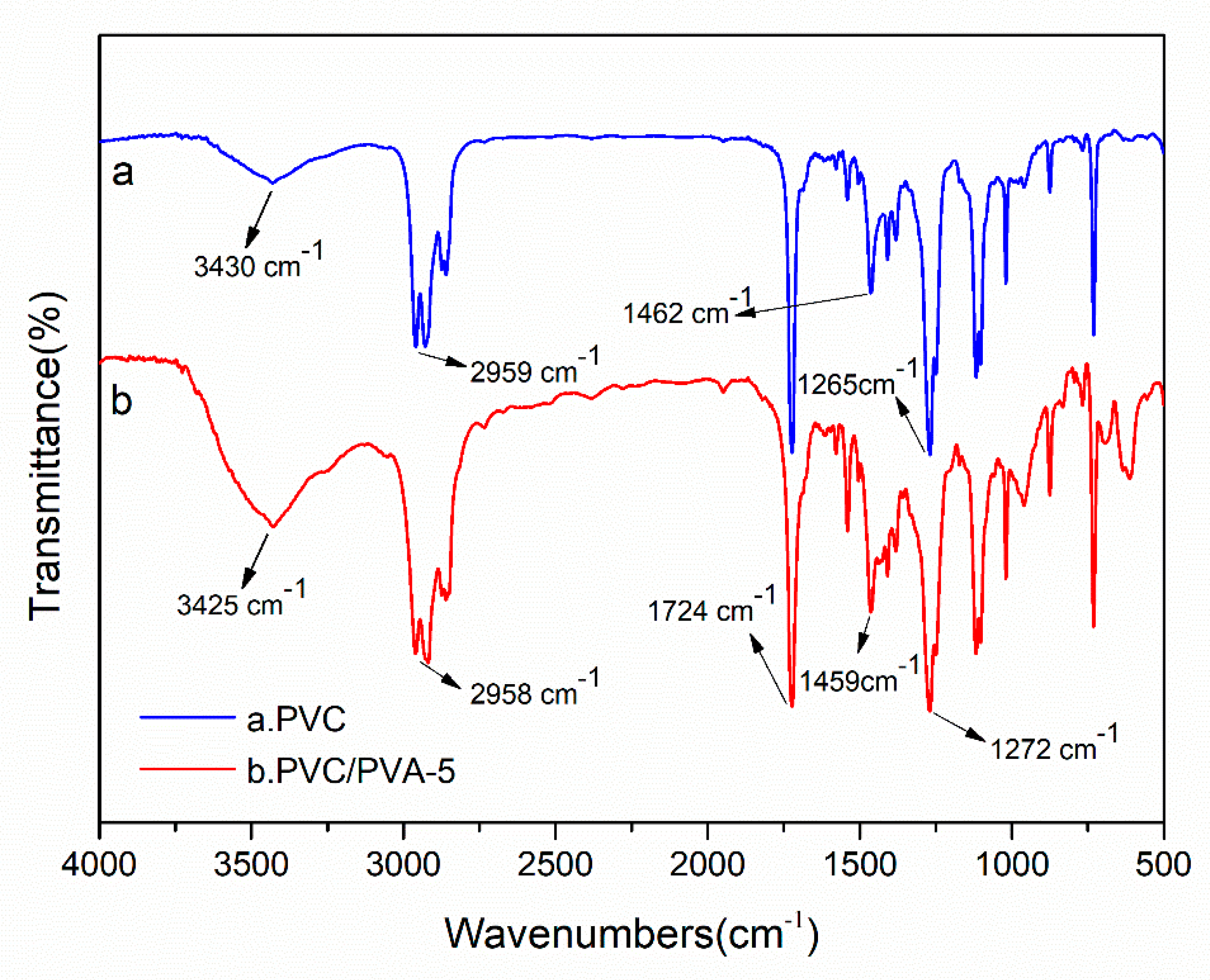
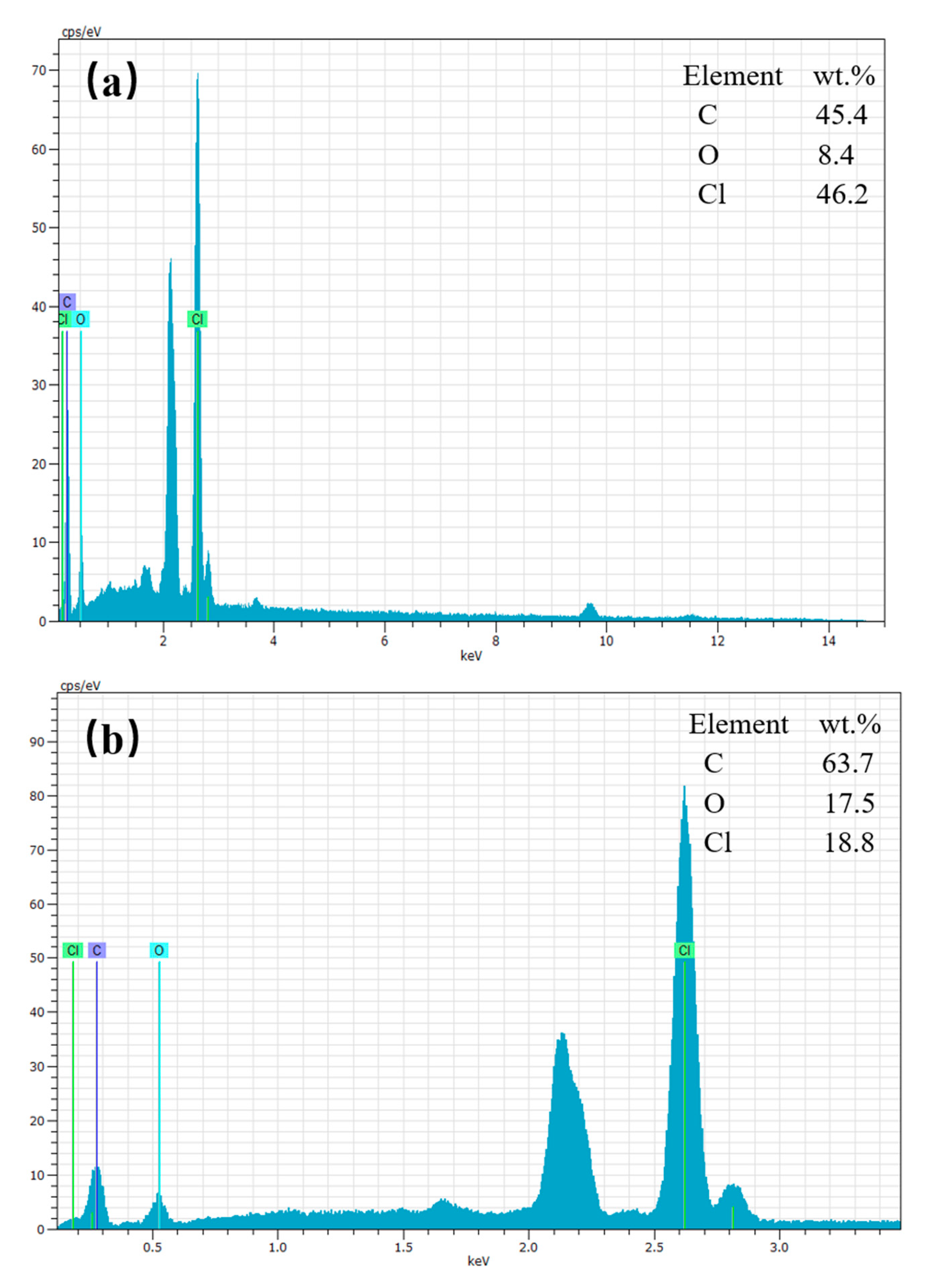
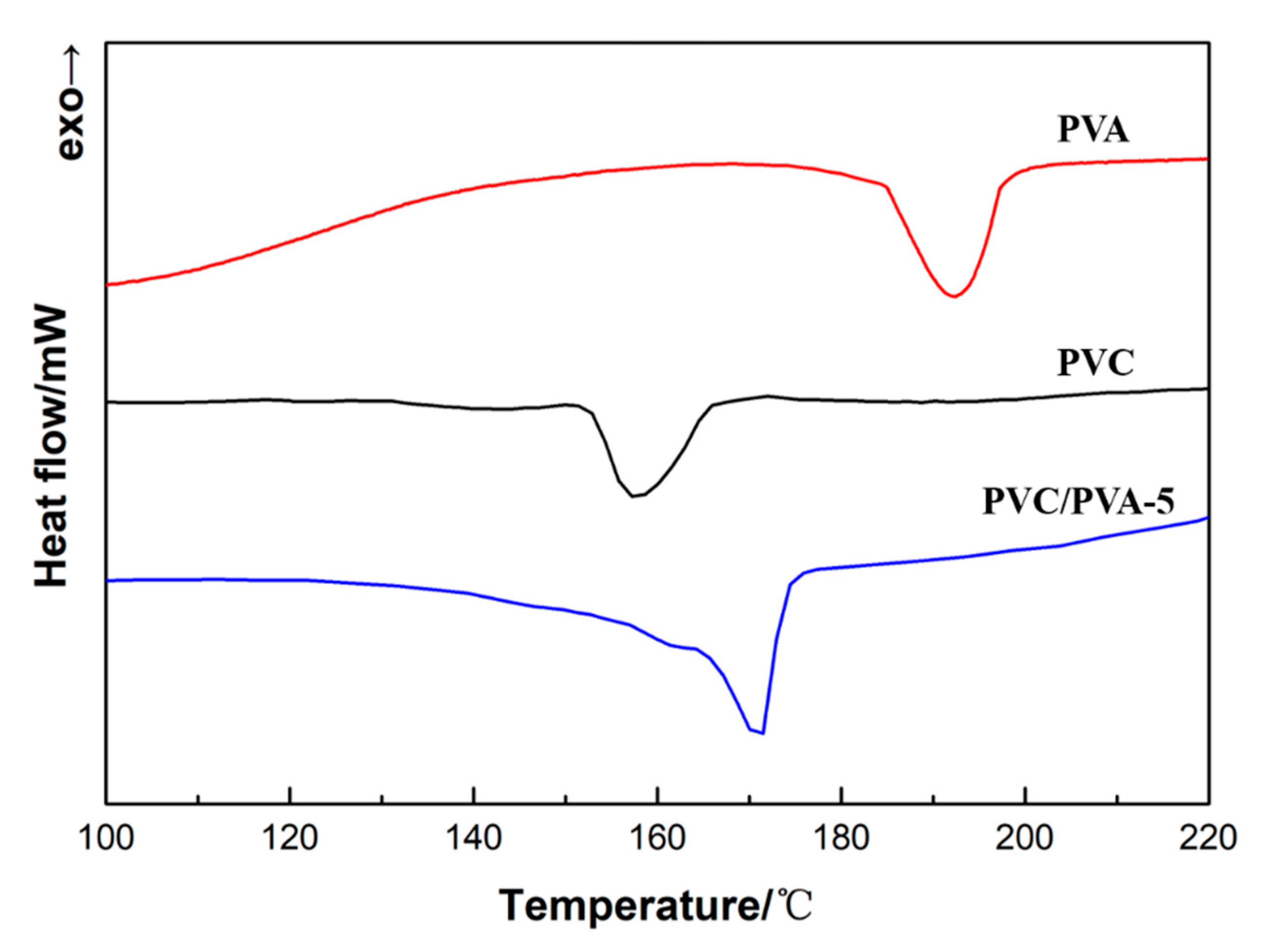
3.2. FTIR Spectra and Energy Dispersive Spectrum (EDS)—The Change of Hydroxyl Groups
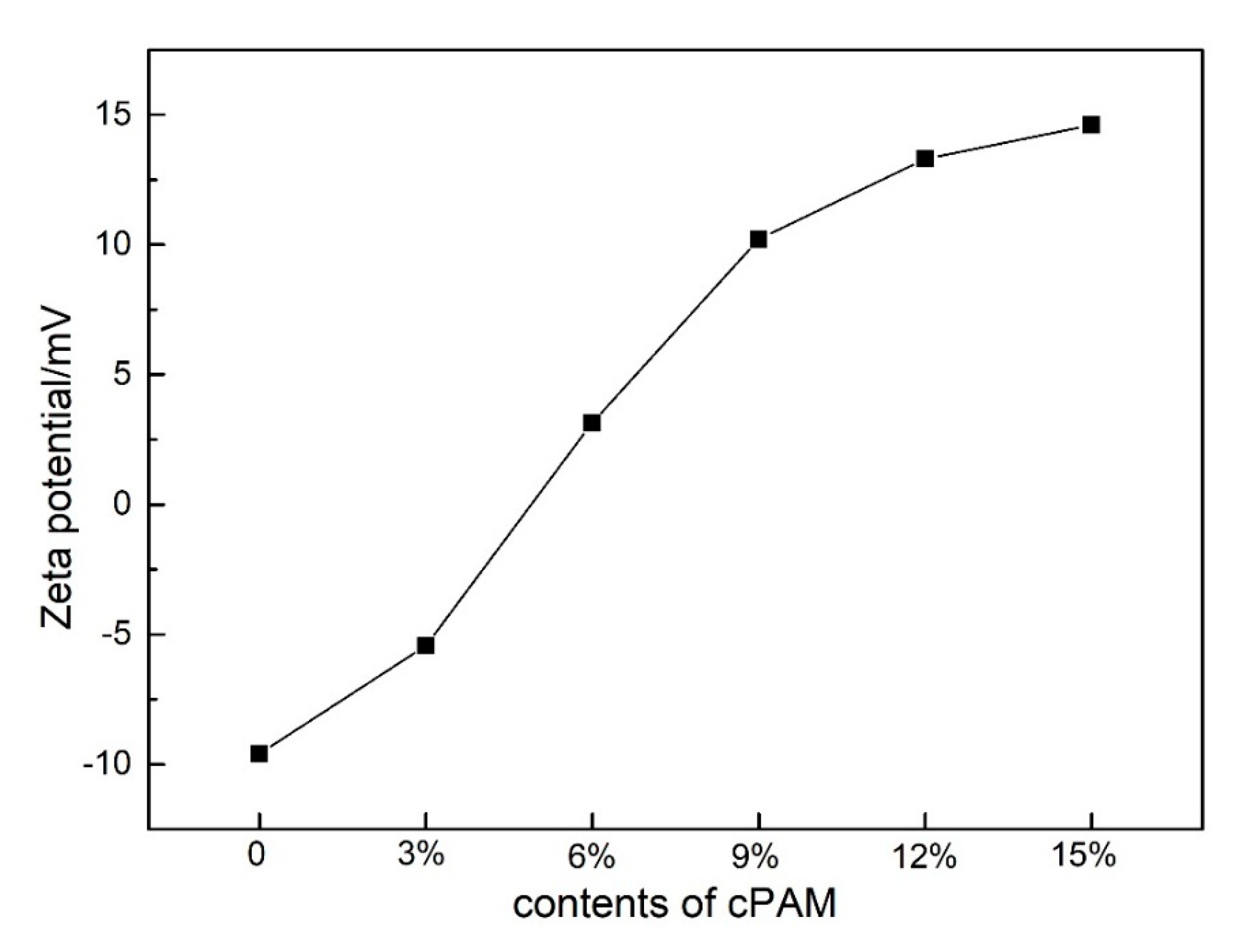
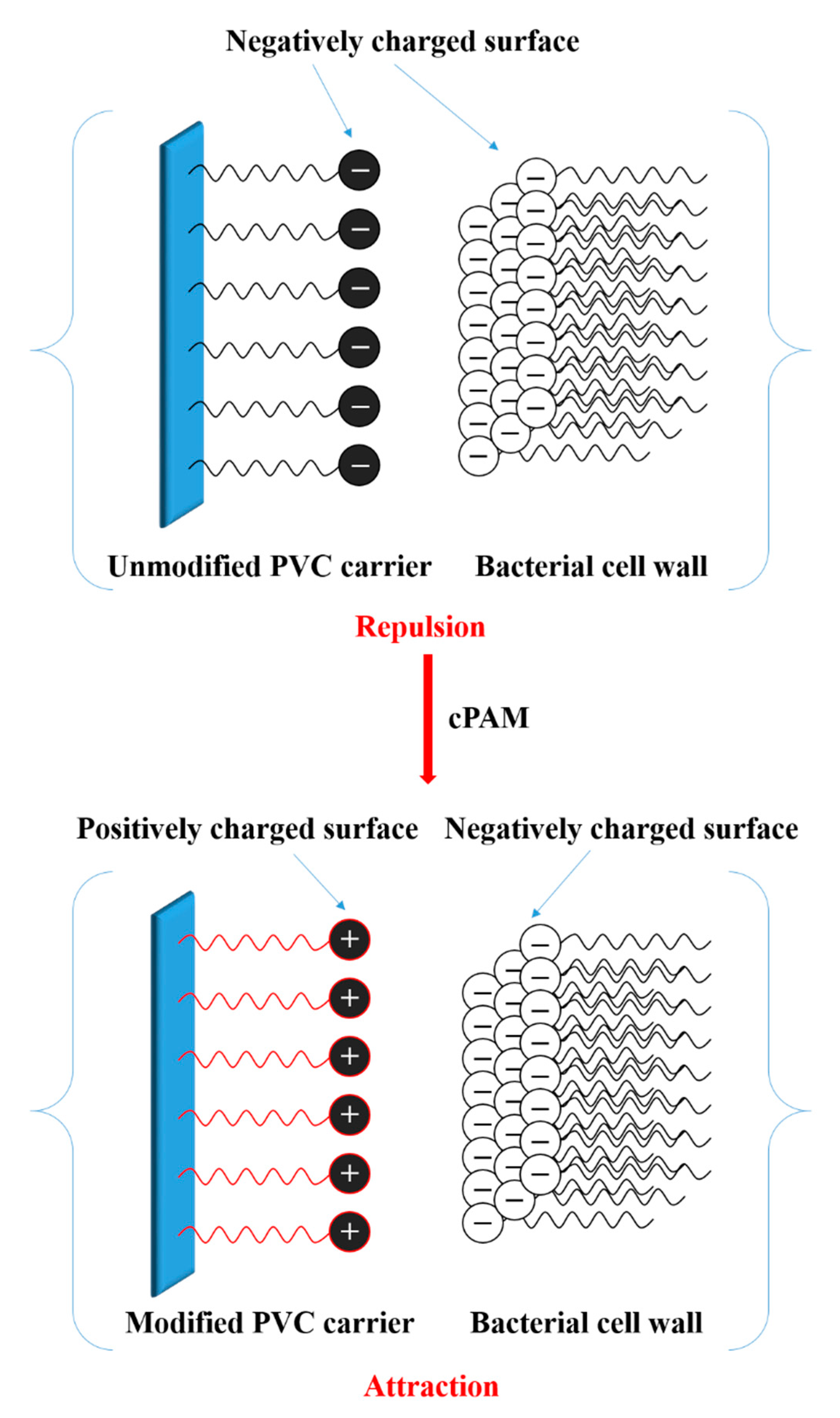
3.3. Electrophilicity of PVC Biofilm Carrier
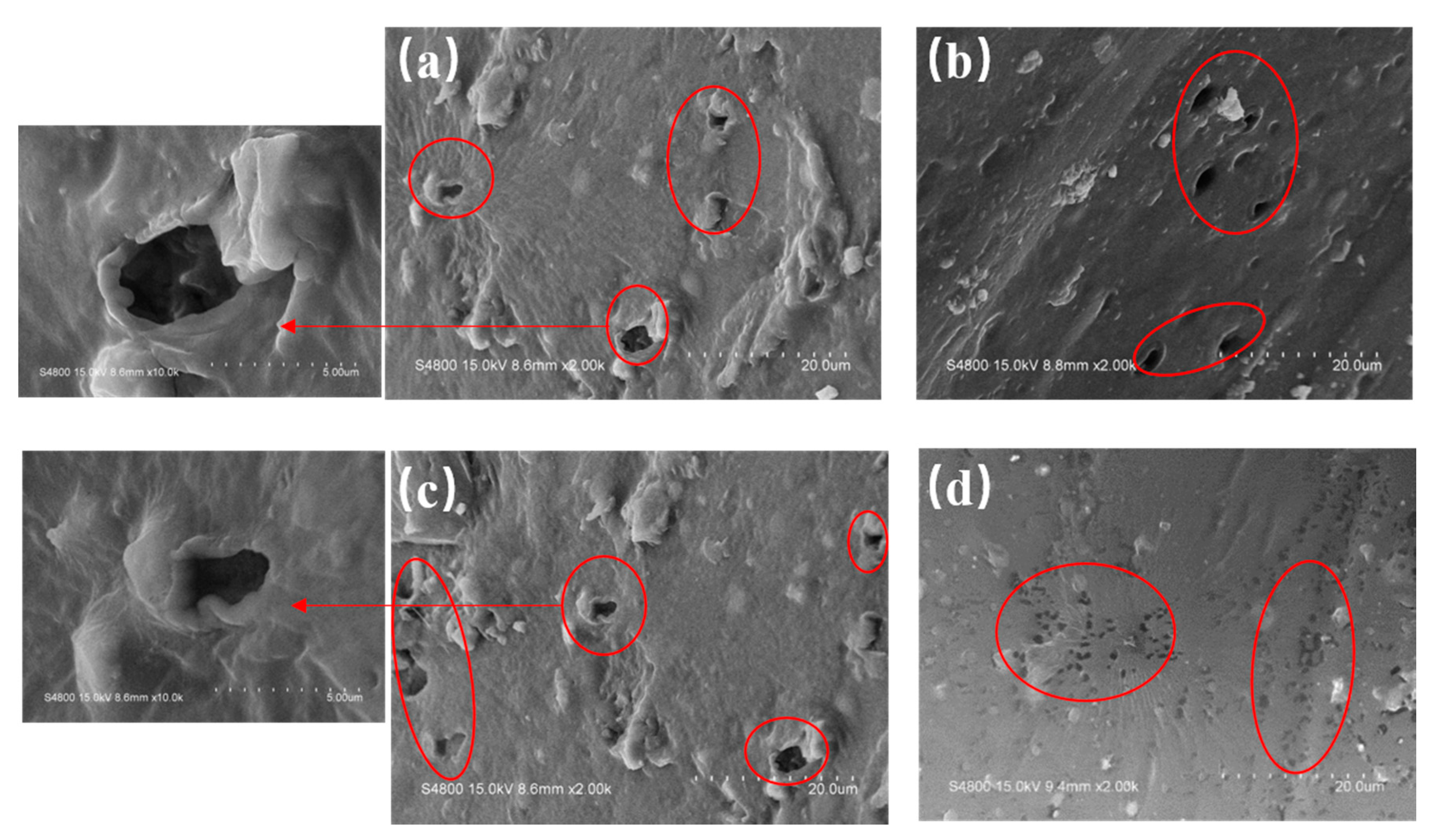
3.4. Surface Morphology of PVC Biofilm Carrier
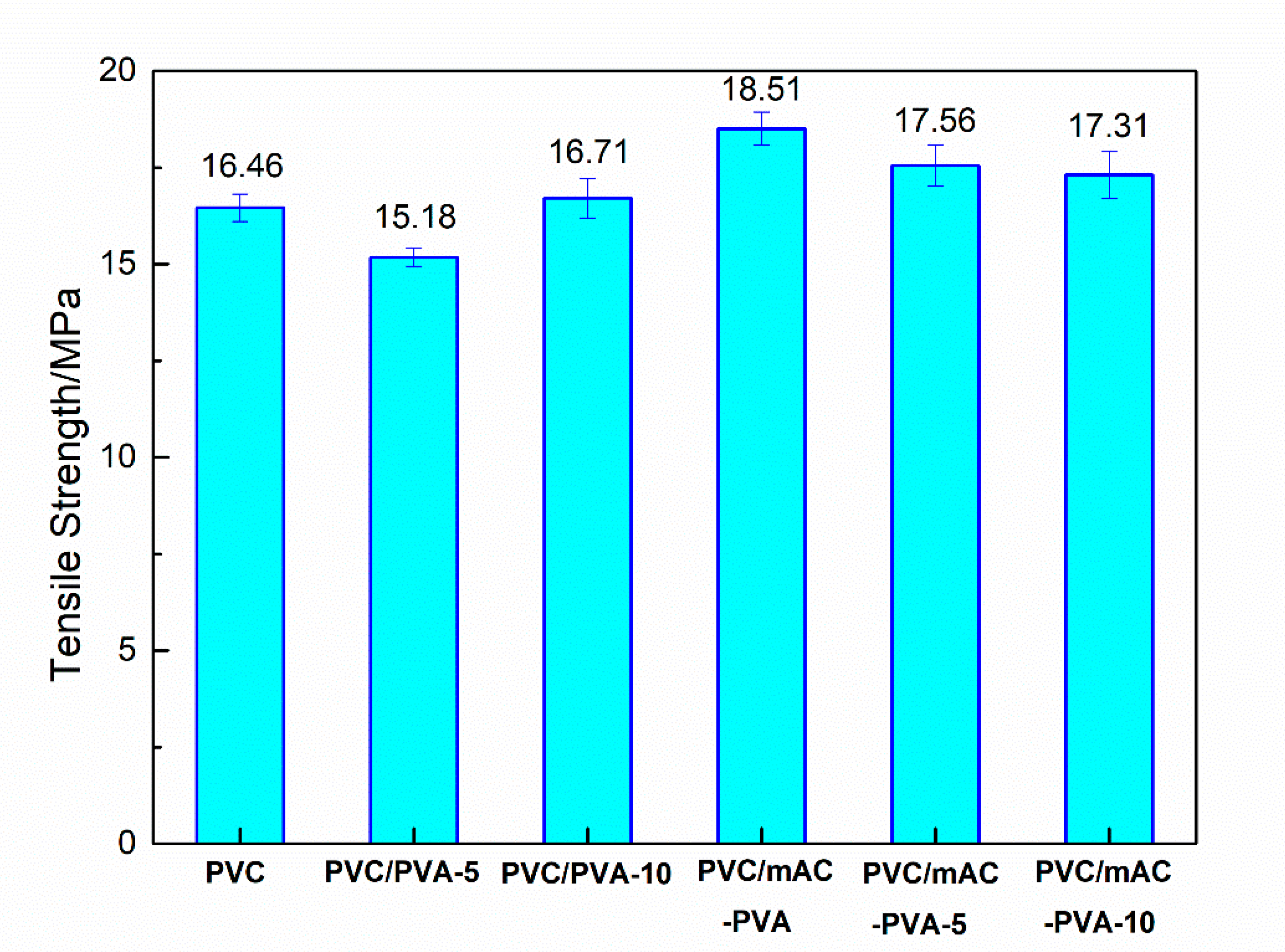
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Guo, W.; Ngo, H.; Nghiem, L.; Hai, F.; Zhang, J.; Liang, S.; Wang, X. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Ngo, H.; Urase, T.; Gin, K. A critical review on characterization strategies of organic matter for wastewater and water treatment processes. Bioresour. Technol. 2015, 193, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Tanaka, S. Biological removal and recovery of toxic heavy metals in water environment. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1007–1057. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Zhang, X.; Peng, S. A review of different drinking water treatments for natural organic matter removal. Water Sci. Technol. Water Supply 2015, 15, 442–455. [Google Scholar] [CrossRef]
- Xu, H.; Yang, B.; Liu, Y.; Li, F.; Shen, C.; Ma, C. Recent advances in anaerobic biological processes for textile printing and dyeing wastewater treatment: A mini-review. World J. Microbiol. Biotechnol. 2018, 34, 165–169. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Huang, W.; Yang, Y.; Ji, M.; Nghiem, L.; Trinh, Q.; Tran, N. Insights into biofilm carriers for biological wastewater treatment processes: Current state-of-the-art, challenges, and opportunities. Bioresour. Technol. 2019, 288, 1–15. [Google Scholar] [CrossRef]
- Feng, L.; Chen, K.; Han, D.; Zhao, J.; Lu, Y.; Yang, G.; Mu, J.; Zhao, X. Comparison of nitrogen removal and microbial properties in solid-phase denitrification systems for water purification with various pretreated lignocellulosic carriers. Bioresour. Technol. 2016, 224, 236–245. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.; Dharmawan, F.; Palmer, C. Roles of polyurethane foam in aerobic moving and fixed bed bioreactors. Bioresour. Technol. 2010, 101, 1435–1439. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Xu, J.; Cai, D. Efficiency of active barriers attaching biofilm as sediment capping to eliminate the internal nitrogen in eutrophic lake and canal. J. Environ. Sci. 2011, 23, 38–43. [Google Scholar] [CrossRef]
- Müller-Renno, C.; Buhl, S.; Davoudi, N.; Aurich, J.; Ripperger, S.; Ulber, R. Novel Materials for Biofilm Reactors and their Characterization. Adv. Biochem. Eng. Biotechnol. 2013, 146, 207–233. [Google Scholar] [CrossRef]
- Tarjányi-Szikora, S.; Oláh, J.; Makó, M.; Palkó, G.; Barkács, K.; Záray, G. Comparison of different granular solids as biofilm carriers. Microchem. J. 2013, 107, 101–107. [Google Scholar] [CrossRef]
- Ahmad, M.; Liu, S.; Mahmood, N.; Mahmood, A.; Ali, M.; Zheng, M.; Ni, J. Effects of porous carrier size on biofilm development, microbial distribution and nitrogen removal in microaerobic bioreactors. Bioresour. Technol. 2017, 234, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.; Wang, J.; Quan, F.; Xing, X.; Tang, L.; Zhang, C. Modification of polyurethane foam carriers and application in a moving bed biofilm reactor. Process. Biochem. 2014, 49, 1979–1982. [Google Scholar] [CrossRef]
- Deng, L.; Guo, W.; Ngo, H.; Zhang, X.; Wang, X.; Zhang, Q. New functional biocarriers for enhancing the performance of a hybrid moving bed biofilm reactor-membrane bioreactor system. Bioresour. Technol. 2016, 208, 87–93. [Google Scholar] [CrossRef]
- Mao, Y.; Quan, X.; Zhao, H.; Zhang, Y.; Chen, S.; Liu, T.; Quan, W. Accelerated startup of moving bed biofilm process with novel electrophilic suspended biofilm carriers. Chem. Eng. J. 2017, 315, 364–372. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys. Chem. 2005, 117, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, X.; Ni, H. Surface Modification of Basalt Fiber, with Organic/Inorganic Composites for Biofilm Carrier Used in Wastewater Treatment. ACS Sustain. Chem. Eng. 2018, 6, 2596–2602. [Google Scholar] [CrossRef]
- Renner, L.; Weibel, D. Physicochemical regulation of biofilm formation. Mrs Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Choi, H. Effect of surface hydrophobicity on the adhesion of S. cerevisiae onto modified surfaces by poly(styrene-ran-sulfonic acid) random copolymers. Colloids Surf. B Biointerfaces 2005, 46, 70–77. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, Y.; Wang, S.; Borca-Tasciuc, D.; Worobo, R.; Moraru, C. Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: How small is small enough? Npj Biofilms Microbiomes 2015, 1, 15022. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.; Hardwidge, P.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef] [Green Version]
- Terada, A.; Okuyama, K.; Nishikawa, M.; Tsuneda, S.; Hosomi, M. The effect of surface charge property on Escherichia coli initial adhesion and subsequent biofilm formation. Biotechnol. Bioeng. 2012, 109, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cheng, X.; Zhang, X.; Sun, D. Influence of surface modification of polyethylene biocarriers on biofilm properties and wastewater treatment efficiency in moving-bed biofilm reactors. Water Sci. Technol. 2012, 65, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Van Merode, A.; Van Der Mei, H.; Busscher, H.; Krom, B. Influence of Culture Heterogeneity in Cell Surface Charge on Adhesion and Biofilm Formation by Enterococcus faecalis. J. Bacteriol. 2006, 188, 2421–2426. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, Y.; Jia, H.; Yong, X.; Zhang, L.; Zhou, J.; Cao, Z.; Kruse, A.; Wei, P. Effects of different biofilm carriers on biogas production during anaerobic digestion of corn straw. Bioresour. Technol. 2017, 48, 445–451. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, Q. Surface modification of carbon fiber support by ferrous oxalate for biofilm wastewater treatment system. J. Clean. Prod. 2018, 194, 416–424. [Google Scholar] [CrossRef]
- Nguyen, V.; Karunakaran, E.; Collins, G.; Biggs, C. Physicochemical analysis of initial adhesion and biofilm formation of Methanosarcina barkeri on polymer support material. Colloids Surf. B Biointerfaces 2016, 143, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wu, C.; Xu, S. Mechanical, thermal and flame retardant properties of magnesium hydroxide filled poly(vinyl chloride) composites: The effect of filler shape. Compos. Part. A 2018, 113, 1–11. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends. Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Liu, T.; Jia, G.; Quan, X. Accelerated start-up and microbial community structures of simultaneous nitrification and denitrification by using novel suspended carriers. J. Chem. Technol. Biotechnol. 2018, 93, 577–584. [Google Scholar] [CrossRef]
- Zhu, Y. Preparation and Characterization of a New Hydrophilic and Biocompatible Magnetic Polypropylene Carrier used in Wastewater Treatment. Environ. Technol. 2017, 39, 1–29. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, N.; Li, X. Effect of inorganic–organic surface modification of calcium sulfate whiskers on mechanical and thermal properties of calcium sulfate whisker/poly(vinyl chloride) composites. RSC Adv. 2017, 7, 46486–46498. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Cui, J.; Cai, Y.; Xu, S. A novel surface modification for calcium sulfate whisker used for reinforcement of poly(vinyl chloride). J. Polym. Res. 2015, 22, 173. [Google Scholar] [CrossRef]
- Abbasnezhad, H.; Gray, M.; Foght, J. Two different mechanisms for adhesion of Gram-negative bacterium, Pseudomonas fluorescens LP6a, to an oil–water interface. Colloids Surf. B Biointerfaces 2008, 62, 36–41. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S. Influences of particle sizes and contents of chemical blowing agents on foaming wood plastic composites prepared from poly(vinyl chloride) and rice hull. Mater. Des. 2011, 32, 2844–2850. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, Y.; Wang, T.; Zheng, H.; Chu, L.; Zhang, C.; Chen, H.; Kong, X.; Xing, X. Effects of packing rates of cubic-shaped polyurethane foam carriers on the microbial community and the removal of organics and nitrogen in moving bed biofilm reactors. Bioresour. Technol. 2012, 117, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Gin, K. Occurrence and removal of pharmaceuticals, hormones, personal care products, and endocrine disrupters in a full-scale water reclamation plant. Sci. Total Environ. 2017, 599–600, 1503–1506. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Zhang, M.; He, S. Simultaneous improving nitrogen removal and decreasing greenhouse gas emission with biofilm carriers addition in ecological floating bed. Bioresour. Technol. 2019, 292, 1–8. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, H.; Tian, Y.; Liu, X.; Yang, Y.; Zhu, L.; Yan, S.; Liu, G. Treatment of polluted surface water with nylon silk carrier-aerated biofilm reactor (CABR). Bioresour. Technol. 2019, 289, 1–9. [Google Scholar] [CrossRef]
- Mulinari, J.; Andrade, C.; Brandao, H.; Silva, A.; Souza, S.; Souza, A. Enhanced textile wastewater treatment by a novel biofilm carrier with adsorbed nutrients. Biocatal. Agric. Biotechnol. 2020, 24, 1–8. [Google Scholar] [CrossRef]






| Material | Sales/Manufacturer | Material Properties |
|---|---|---|
| PVC (SG-5) | Yuyao Maiduo Plastic Chemical Co., Ltd. | GB/T 5761-2006 Injection Grade |
| PVA | Shanghai Lingfeng Chemical Reagent Co., Ltd. | A.R, Average degree of polymerization:1750 ± 50 |
| Stearic acid | Shanghai Lingfeng Chemical Reagent Co., Ltd. | A.R, MW:284.48 |
| cPAM | Zhengzhou Jintai Environmental Protection Technology Co., Ltd. | A.R, MW:1.2 × 107 |
| Azodicarbonamide | Shanghai Tengzhun Biological Technology Co., Ltd. | A.R, MW:116.08 |
| Calcium zinc stabilizer | Guangdong Winner New Material Technology Co., Ltd. | WWP-F02 A.R |
| Dioctyl terephthalate | Jining Baichuan Chemical Co., Ltd. | A.R |
| Zinc oxide | Sinopharm Chemical Reagent Co., Ltd. | A.R, MW:81.39 |
| Antioxidant 1010 | Shanghai Xian Ding Biological Technology Co., Ltd. | C.P, MW:1177.63 |
| Samples | PVC/g | PVA/g | mAC/g | cPAM/g |
|---|---|---|---|---|
| PVC | 100 | 0 | 0 | 0 |
| PVC/PVA-5 | 100 | 5 | 0 | 0 |
| PVC/PVA-10 | 100 | 10 | 0 | 0 |
| PVC/mAC-PVA | 100 | 0 | 1 | 0 |
| PVC/mAC-PVA-5 | 100 | 5 | 1 | 0 |
| PVC/mAC-PVA-10 | 100 | 10 | 1 | 0 |
| PVC/cPAM | 100 | 5 | 1 | 0 |
| PVC/cPAM-3 | 100 | 5 | 1 | 3 |
| PVC/cPAM-6 | 100 | 5 | 1 | 6 |
| PVC/cPAM-9 | 100 | 5 | 1 | 9 |
| PVC/cPAM-12 | 100 | 5 | 1 | 12 |
| PVC/cPAM-15 | 100 | 5 | 1 | 15 |
| Sample | PVC | PVC/PVA-5 | ||
|---|---|---|---|---|
| Weight (wt %) | Atom (at. %) | Weight (wt %) | Atom (at. %) | |
| C | 45.4 ± 2.4 | 67.4 ± 2.1 | 63.7 ± 4.2 | 76.6 ± 4.0 |
| O | 8.4 ± 0.5 | 9.3 ± 0.4 | 17.5 ± 1.4 | 15.8 ± 1.2 |
| Cl | 46.2 ± 0.6 | 23.3 ± 0.5 | 18.8 ± 0.3 | 7.6 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Wang, Y.; Wu, K.; Guo, W. Enhanced Hydrophilic and Electrophilic Properties of Polyvinyl Chloride (PVC) Biofilm Carrier. Polymers 2020, 12, 1240. https://doi.org/10.3390/polym12061240
Cai H, Wang Y, Wu K, Guo W. Enhanced Hydrophilic and Electrophilic Properties of Polyvinyl Chloride (PVC) Biofilm Carrier. Polymers. 2020; 12(6):1240. https://doi.org/10.3390/polym12061240
Chicago/Turabian StyleCai, Haifeng, Yang Wang, Kai Wu, and Weihong Guo. 2020. "Enhanced Hydrophilic and Electrophilic Properties of Polyvinyl Chloride (PVC) Biofilm Carrier" Polymers 12, no. 6: 1240. https://doi.org/10.3390/polym12061240





