Unidirectional All-Cellulose Composites from Flax via Controlled Impregnation with Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Chemical Composition of Fibers
2.2.2. Optical Microscope Observation of Fiber Evolution in Ionic Liquid
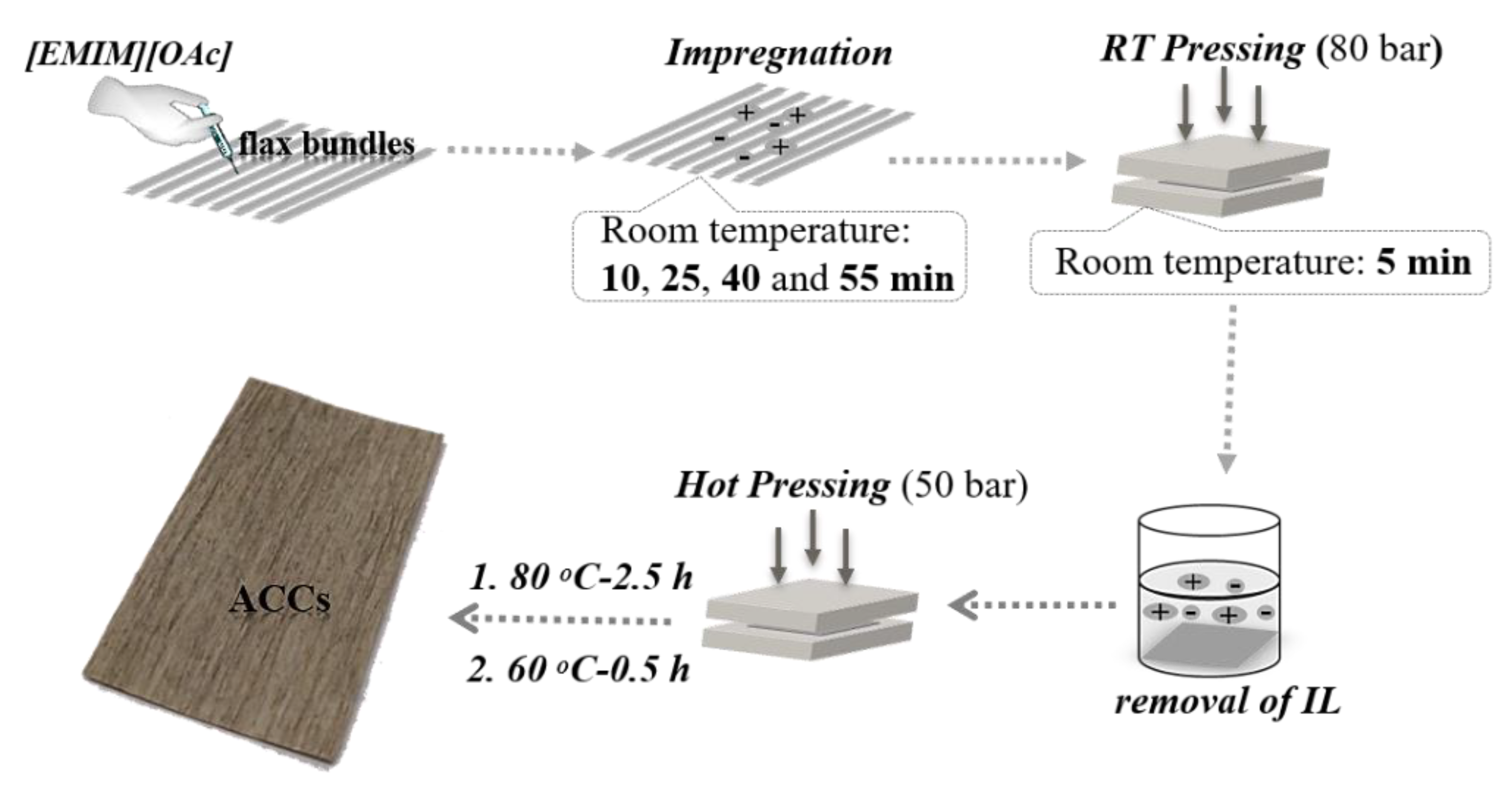
2.2.3. ACC Preparation
2.2.4. Calculation of Composite Apparent Density
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. X-ray Diffraction (XRD)
2.2.7. Tensile Testing
3. Results and Discussion
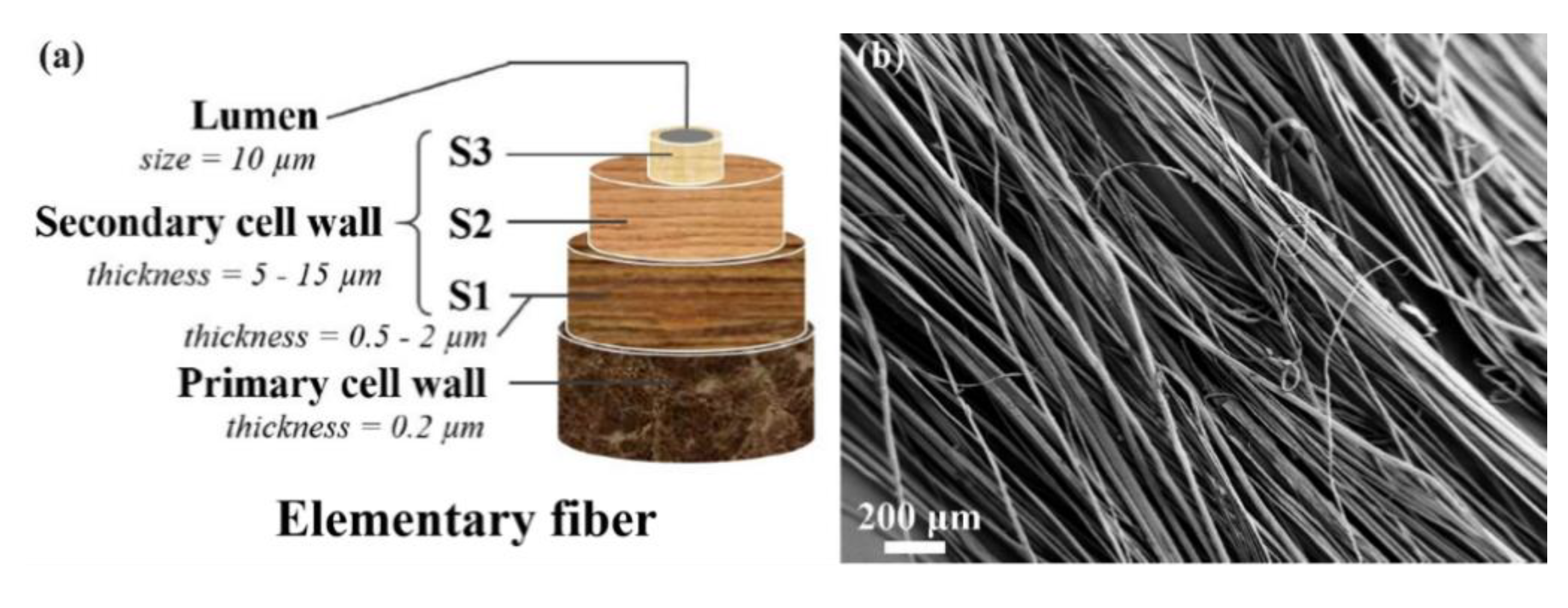
3.1. Analysis of Flax Fibers
3.2. Fiber Size Evolution in [EMIM][OAc]
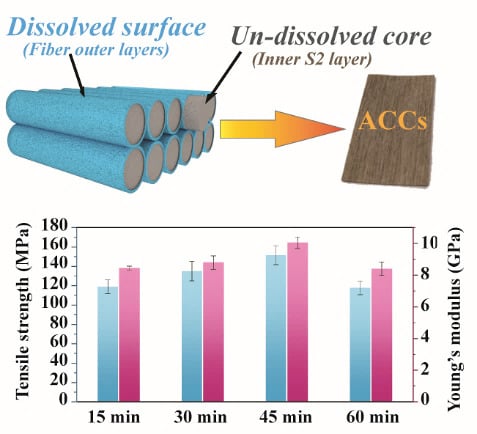
3.3. Morphology of the ACCs
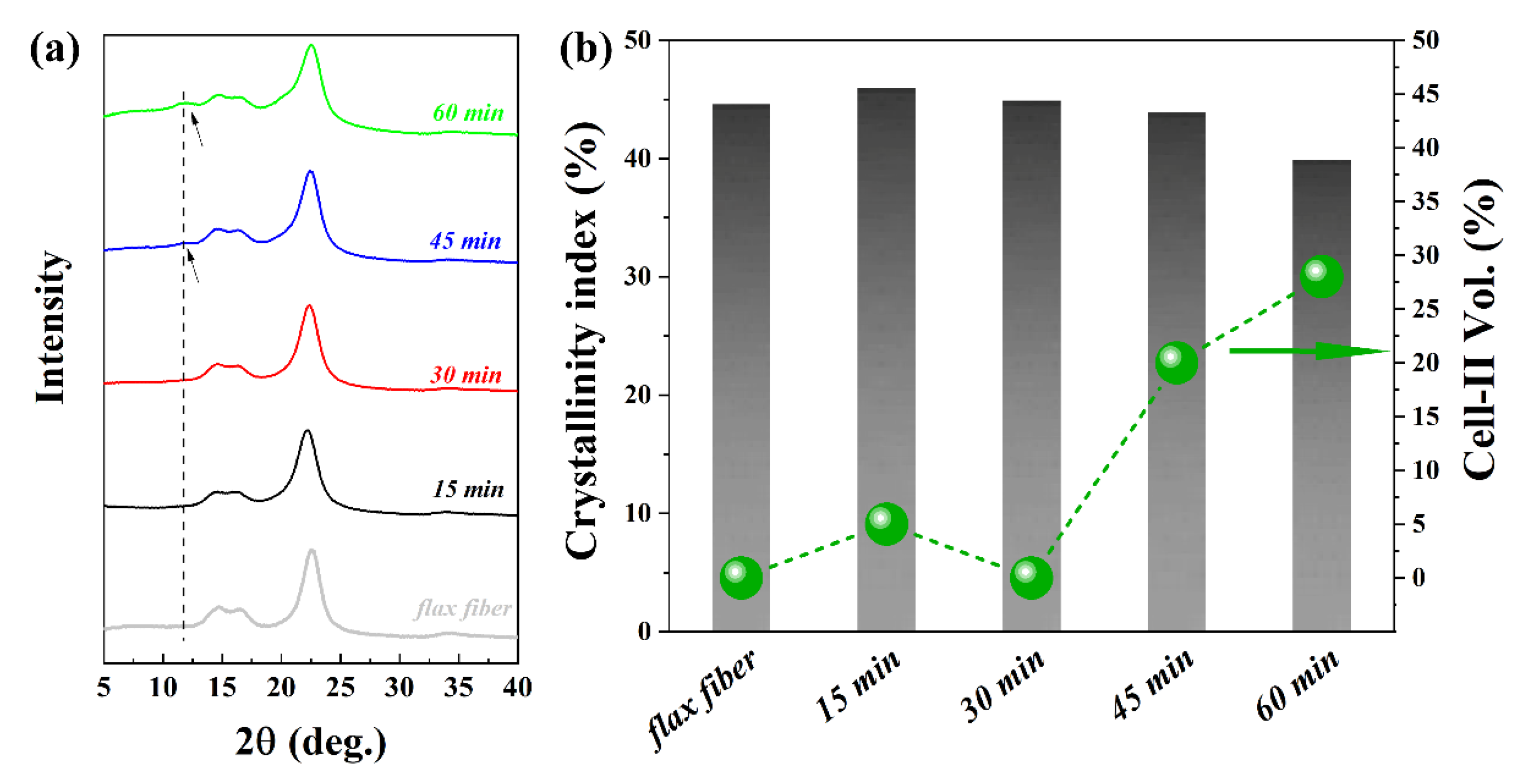
3.4. Crystallinity of the ACCs
3.5. Mechanical Performance of the ACCs
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nishino, T.; Matsuda, I.; Hirao, K. All-cellulose composite prepared by selective dissolving of fiber surface. Macromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Manjusri, M.; Lawrence, T. Surface modifications of natural fibers and performance of the resulting biocomposites: An overview. Compos. Interfaces 2012, 8, 313–343. [Google Scholar] [CrossRef]
- Huber, T.; Müssig, J.; Curnow, O.; Pang, S.; Bickerton, S.; Staiger, M.P. A critical review of all-cellulose composites. J. Mater. Sci. 2011, 47, 1171–1186. [Google Scholar] [CrossRef]
- Spörl, J.M.; Batti, F.; Vocht, M.P.; Raab, R.; Müller, A.; Hermanutz, F.; Buchmeiser, M.R. Ionic Liquid Approach Toward Manufacture and Full Recycling of All-Cellulose Composites. Macromol. Mater. Eng. 2017, 303, 1700335. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Kan, Z.; Hua, S.; Liu, Z.Y.; Yang, M.B. A new understanding concerning the influence of structural changes on the thermal behavior of cellulose. J. Polym. Res. 2015, 22, 225. [Google Scholar] [CrossRef]
- Gindl, W.; Keckes, J. All-cellulose nanocomposite. Polymer 2005, 46, 10221–10225. [Google Scholar] [CrossRef]
- Korhonen, O.; Sawada, D.; Budtova, T. All-cellulose composites via short-fiber dispersion approach using NaOH–water solvent. Cellulose 2019, 26, 4881–4893. [Google Scholar] [CrossRef]
- Labidi, K.; Korhonen, O.; Zrida, M.; Hamzaoui, A.H.; Budtova, T. All-cellulose composites from alfa and wood fibers. Ind. Crop. Prod. 2019, 127, 135–141. [Google Scholar] [CrossRef]
- Adak, B.; Mukhopadhyay, S. Effect of pressure on structure and properties of lyocell fabric-based all-cellulose composite laminates. J. Text. Inst. 2016, 108, 1010–1017. [Google Scholar] [CrossRef]
- Mat, S.M.; Magniez, K.; Pang, S.; Dormanns, J.W.; Staiger, M.P. Parametric optimization of the processing of all-cellulose composite laminae. Adv. Manuf. Polym. Compos. Sci. 2017, 3, 73–79. [Google Scholar]
- Adak, B.; Mukhopadhyay, S. All-cellulose composite laminates with low moisture and water sensitivity. Polymer 2018, 141, 79–85. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Arimoto, N.; Nishino, T.; Peijs, T. All-cellulose composites by surface selective dissolution of aligned ligno-cellulosic fibres. Compos. Sci. Technol. 2008, 68, 2201–2207. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.N. Ramie fibre: Part I. Chemical composition and chemical properties. A critical review of recent developments. Text. Prog. 2007, 39, 1–66. [Google Scholar] [CrossRef]
- Pandey, S.N. Ramie fibre: Part II. Physical fibre properties. A critical appreciation of recent developments. Text. Prog. 2007, 39, 189–268. [Google Scholar] [CrossRef]
- Duchemin, B.J.C.; Mathew, A.P.; Oksman, K. All-cellulose composites by partial dissolution in the ionic liquid 1-butyl-3-methylimidazolium chloride. Part A-Appl. Sci. 2009, 40, 2031–2037. [Google Scholar] [CrossRef]
- Gindl, A.W.; Keckes, J.; Plackner, J.; Liebner, F.; Englund, K.; Laborie, M.P. All-cellulose composites prepared from flax and lyocell fibres compared to epoxy–matrix composites. Compos. Sci. Technol. 2012, 72, 1304–1309. [Google Scholar] [CrossRef]
- Le Moigne, N.; Bikard, J.; Navard, P. Rotation and contraction of native and regenerated cellulose fibers upon swelling and dissolution: The role of morphological and stress unbalances. Cellulose 2010, 17, 507–519. [Google Scholar] [CrossRef] [Green Version]
- Dormanns, J.W.; Schuermann, J.; Müssig, J.; Duchemin, B.J.C.; Staiger, M.P. Solvent infusion processing of all-cellulose composite laminates using an aqueous NaOH/urea solvent system. Compos. Part A-Appl Sci. 2016, 82, 130–140. [Google Scholar] [CrossRef]
- Hauru, L.K.; Hummel, M.; King, A.W.; Kilpelainen, I.; Sixta, H. Role of solvent parameters in the regeneration of cellulose from ionic liquid solutions. Biomacromolecules 2012, 13, 2896–2905. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A Review on the Partial and Complete Dissolution and Fractionation of Wood and Lignocelluloses Using Imidazolium Ionic Liquids. Polymers 2020, 12, 195. [Google Scholar] [CrossRef] [Green Version]
- Zweckmair, T.; Hettegger, H.; Abushammala, H.; Bacher, M.; Potthast, A.; Laborie, M.P.; Rosenau, T. On the mechanism of the unwanted acetylation of polysaccharides by 1, 3-dialkylimidazolium acetate ionic liquids: Part 1—Analysis, acetylating agent, influence of water, and mechanistic considerations. Cellulose 2015, 22, 3583–3596. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Nakamura, T.; Saitoh, Y. All-cellulose and all-wood composites by partial dissolution of cotton fabric and wood in ionic liquid. Carbohyd. Polym. 2013, 98, 1532–1539. [Google Scholar]
- Bapan, A.; Samrat, M. Jute based all-cellulose composite laminates. Indian J. Fibre Text. 2016, 41, 380–384. [Google Scholar]
- Huber, T.; Bickerton, S.; Mussig, J.; Pang, S.; Staiger, M.P. Solvent infusion processing of all-cellulose composite materials. Carbohyd. Polym. 2012, 90, 730–733. [Google Scholar] [CrossRef]
- Huber, T.; Pang, S.; Staiger, M.P. All-cellulose composite laminates. Compos. Part A-Appl Sci. 2012, 43, 1738–1745. [Google Scholar] [CrossRef]
- Kröling, H.; Duchemin, B.; Dormanns, J.; Schabel, S.; Staiger, M.P. Mechanical anisotropy of paper-based all-cellulose composites. Compos. Part A-Appl Sci. 2018, 113, 150–157. [Google Scholar] [CrossRef]
- Khakalo, A.; Tanaka, A.; Korpela, A.; Hauru, L.K.J.; Orelma, H. All-Wood Composite Material by Partial Fiber Surface Dissolution with an Ionic Liquid. ACS Sustain. Chem. Eng. 2019, 7, 3195–3202. [Google Scholar] [CrossRef]
- Haverhals, L.M.; Reichert, W.M.; De Long, H.C.; Trulove, P.C. Natural Fiber Welding. Macromol. Mater. Eng. 2010, 295, 425–430. [Google Scholar] [CrossRef]
- Charlet, K.; Eve, S.; Jernot, J.P.; Gomina, M.; Breard, J. Tensile deformation of a flax fiber. Procedia Eng. 2009, 1, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Charlet, K.; Jernot, J.P.; Gomina, M.; Bizet, L.; Bréard, J. Mechanical properties of flax fibers and of the derived unidirectional composites. J. Compos. Mater. 2010, 44, 2887–2896. [Google Scholar] [CrossRef]
- Janson, J. Calculation of the polysaccharide composition of wood and pulp. PAP PUU-PAP TIM 1970, 52, 323–329. [Google Scholar]
- Brückner, S. Estimation of the background in powder diffraction patterns through a robust smoothing procedure. J. Appl. Crystallogr. 2000, 33, 977–979. [Google Scholar] [CrossRef]
- Frost, K.; Kaminski, D.; Kirwan, G.; Lascaris, E.; Shanks, R. Crystallinity and structure of starch using wide angle X-ray scattering. Carbohyd. Polym. 2009, 78, 543–548. [Google Scholar]
- Savitzky, A.; Golay, M.J. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Newville, M.; Stensitzki, T.; Allen, D.B. LMFIT: Non-linear Least-square Minimization and Curve-fitting for Python; Astrophysics Source Code Library; Michigan Technological University: Houghton, MI, USA, 2016. [Google Scholar]
- Alexander, L.; Klug, H.P. Basic aspects of X-ray absorption in quantitative diffraction analysis of powder mixtures. Anal. Chem. 1948, 20, 886–889. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules 2001, 2, 410–416. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Liu, Y.; Yang, G.C.; Zeng, H.M. The effect of fiber treatment on the mechanical properties of unidirectional sisal-reinforced epoxy composites. Compos. Sci. Technol. 2001, 61, 1437–1447. [Google Scholar] [CrossRef]
- Charlet, K.; Baley, C.; Morvan, C.; Jernot, J.P.; Gomina, M.; Bréard, J. Characteristics of Hermès flax fibres as a function of their location in the stem and properties of the derived unidirectional composites. Compos. Part. A-Appl Sci. 2007, 38, 1912–1921. [Google Scholar] [CrossRef]
- Cuissinat, C.; Navard, P. Swelling and Dissolution of Cellulose Part 1: Free Floating Cotton and Wood Fibres in N-Methylmorpholine-N-oxide–Water Mixtures. Macromol. Symp. 2006, 244, 1–18. [Google Scholar] [CrossRef]
- Chen, W.; Lickfield, G.C.; Yang, C.Q. Molecular modeling of cellulose in amorphous state. Part I: Model building and plastic deformation study. Polymer 2004, 45, 1063–1071. [Google Scholar] [CrossRef]
- Chen, W.; Lickfield, G.C.; Yang, C.Q. Molecular modeling of cellulose in amorphous state part II: Effects of rigid and flexible crosslinks on cellulose. Polymer 2004, 45, 7357–7365. [Google Scholar] [CrossRef]
- Cottrell, A.H. Strong solids. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1964, 282, 2–9. [Google Scholar]
- Moryganov, A.P.; Zavadskii, A.E.; Stokozenko, V.G. Special Features of X-ray Analysis of Cellulose Crystallinity and Content in Flax Fibres. Fibre Chem. 2018, 49, 382–387. [Google Scholar] [CrossRef]
- Yan, L.; Chouw, N.; Jayaraman, K. Flax fibre and its composites—A review. Compos. Part. B-Eng. 2014, 56, 296–317. [Google Scholar] [CrossRef]
- Bos, H.L.; Donald, A.M. In situ ESEM study of the deformation of elementary flax fibres. J. Mater. Sci. 1999, 34, 3029–3034. [Google Scholar] [CrossRef]
- Romhány, G.; Karger-Kocsis, J.; Czigány, T. Tensile Fracture and Failure Behavior of Thermoplastic Starch with Unidirectional and Cross-Ply Flax Fiber Reinforcements. Macromol. Mater. Eng. 2003, 288, 699–707. [Google Scholar] [CrossRef]
- Bax, B.; Müssig, J. Impact and tensile properties of PLA/Cordenka and PLA/flax composites. Compos. Sci. Technol. 2008, 68, 1601–1607. [Google Scholar] [CrossRef] [Green Version]
- Van de Weyenberg, I.; Ivens, J.; De Coster, A.; Kino, B.; Baetens, E.; Verpoest, I. Influence of processing and chemical treatment of flax fibres on their composites. Compos. Sci. Technol. 2003, 63, 1241–1246. [Google Scholar] [CrossRef]
- Oksman, K. High quality flax fibre composites manufactured by the resin transfer moulding process. J. Reinf. Plast. Compos. 2001, 20, 621–627. [Google Scholar] [CrossRef]
- Miao, M.; Shan, M. Highly aligned flax/polypropylene nonwoven preforms for thermoplastic composites. Compos. Sci. Technol. 2001, 71, 1713–1718. [Google Scholar] [CrossRef]
- Bodros, E.; Pillin, I.; Montrelay, N.; Baley, C. Could biopolymers reinforced by randomly scattered flax fibre be used in structural applications? Compos. Sci. Technol. 2007, 67, 462–470. [Google Scholar] [CrossRef]
- Bjurhager, I. Effects of Cell Wall Structure on Tensile Properties of Hardwood. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, April 2011. [Google Scholar]
- Ashby, M.F. Materials Selection in Mechanical Design, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2010. [Google Scholar]





| IL | Raw Materials | Impregnation Condition | Method | Ref. |
|---|---|---|---|---|
| [BMIM]Cl | Cotton fabric | 100 °C (30 min) and 150 °C (hot press, 30 min) | One-step | [22] |
| Hinoki lumber | 100 °C (30 min) and 210 °C (hot press, 30 min) | |||
| [BMIM]Cl | Jute fabric | 110 °C (2–8 h) | [23] | |
| Filter paper | ||||
| [BMIM]Cl | Microfibrillated cellulose | 80 °C (20, 40, 80 and 160 min) | [15] | |
| [BMIM]Cl | Lyocell fabric | 110 °C (3 h) | [9] | |
| [BMIM]Cl | Lyocell fabric | 110 °C (hot press, 0.5–4 h) | [11] | |
| [BMIM][OAc] | Cordenka textile | 95 °C (hot press, 60 min) | [24] | |
| [BMIM][OAc] | Linen textile | 110 °C (hot press, 80 min) | [25] | |
| Rayon textile | ||||
| [EMIM][OAc] | Paper | 95 °C (10 s) and 95 °C (hot press, 0.5–6.5 h) | [26] | |
| [EMIM][OAc] | Birch wood plies | 95 °C (30 min) | [27] | |
| [EMIM][OAc] | Silk/hemp/cotton thread | 60 °C (5 min) | [28] | |
| [BMIM]Cl | Lyocell nonwoven mats | 103 °C (1 min) | Two-step | [16] |
| [EMIM][OAc] | Cordenka fabric | 80 °C (hot press, 0.5–1 h) | [4] | |
| Flax nonwoven mats |
| Cellulose (%) | Hemicellulose (%) | Lignin (%) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Strain (%) |
|---|---|---|---|---|---|
| 83.3 | 11.3 | 2.3 | 1364 ± 283 | 59 ± 9 | 2.9 ± 0.5 |
| Matrix | Flax Content | Longitudinal Tensile Strength (MPa) | Young’s Modulus (GPa) | Ref. |
|---|---|---|---|---|
| Starch | 60 wt. %-UD | 79 | 9.3 | [48] |
| Polylactic acid | 30 wt. %-UD | 54 | 6.3 | [49] |
| Epoxy | 40 vol. %-UD | 133 | 28 | [50] |
| Epoxy | 37 wt. %-UD | 132 | 15 | [51] |
| Polypropylene | 30 wt. %-UD | 13 | 6.1 | [52] |
| Cellulose solution | 50 wt. %-ISO | 34 | 4.6 | [16] |
| Flax-based ACC | 100 wt. %-ISO | 46 | 0.86 | [25] |
| Flax-based ACC | 100 wt. %-UD | 151.3 | 10.1 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Sawada, D.; Hummel, M.; Sixta, H.; Budtova, T. Unidirectional All-Cellulose Composites from Flax via Controlled Impregnation with Ionic Liquid. Polymers 2020, 12, 1010. https://doi.org/10.3390/polym12051010
Chen F, Sawada D, Hummel M, Sixta H, Budtova T. Unidirectional All-Cellulose Composites from Flax via Controlled Impregnation with Ionic Liquid. Polymers. 2020; 12(5):1010. https://doi.org/10.3390/polym12051010
Chicago/Turabian StyleChen, Feng, Daisuke Sawada, Michael Hummel, Herbert Sixta, and Tatiana Budtova. 2020. "Unidirectional All-Cellulose Composites from Flax via Controlled Impregnation with Ionic Liquid" Polymers 12, no. 5: 1010. https://doi.org/10.3390/polym12051010