1. Introduction
Rubber can be used in many aspects of industry and beyond thanks to its excellent properties such elasticity, reversible deformation, insulation, and wear resistance after being processed. According to the International Rubber Group (IRSG), almost 60% of global consumption (about 30.12 million tons in 2019) is attributed to the world’s tire manufacturing industry, with nearly 18 million tons of waste tires produced each year [
1].
However, due to factors such as oxidation, wear, and mechanical damage, a significant number of tires are becoming waste items. Increasing ‘black pollution’ from disposed tires has caused tremendous pressure and damage to the world’s ecological environment. Therefore, the problem of finding a solution to treat the waste tires has attracted the attention of research groups around the world [
2,
3,
4,
5]. In the past, the primary ways of reusing or disposing waste tires were either retreading, incinerating, or disposing in a landfill. Nowadays, pyrolysis is considered to have the most potential for an environmentally friendly disposal. Due to the extremely difficult degradation of waste tires, disposing them in landfills will occupy a large amount of land resources and even lead to the breeding of bacteria. Incineration of waste tires is also not a viable answer, as doing so would release acidic gas and a large amount of small-particle-sized dust into the air, thus seriously polluting the environment. One advantage is that the pyrolysis of waste tires can overcome the shortcomings of landfilling and incineration. In addition, this method can also be used to recover high value-added recycled materials with high economic benefits [
6,
7]. Lastly, pyrolysis has good comprehensive environmental benefits and can save resources.
Thermogravimetric analysis and multi-kinetics research methods have been of recent focus for the treatment of waste plastics and tires [
8]. These methods can be used for other polymer complexes, as many other products manufactured from polymers do not degrade in nature in a short period of time and will bring great challenges to the environment. For example, the products of the pyrolysis of other types of waste plastics have been studied, such as plastic casings of television sets [
9,
10,
11,
12]. In most cases, pyrolysis is a new and useful waste treatment method because the products can often be recycled in other applications. For example, pyrolysis chars from coals can even be used as highly insulating building material [
13]. Meanwhile, bio-bitumen can be obtained from organic fractions of municipal solid waste [
14], while liquid hydrocarbon biofuels can be obtained from microalgae or waste cooking oil by catalytic pyrolysis [
15,
16], and the types and principles of catalysts are also of great significance for research [
17,
18]. Considering the value of the products of catalytic pyrolysis, carbon nanofibers obtained from the catalytic pyrolysis of acetylene have good development prospects [
19,
20]. It is even possible to recover graphite or electrode materials from spent lithium-ion batteries via pyrolysis [
21,
22]. In addition, natural macromolecule materials such as coconut copra and rice husk can also produce useful pyrolysis products such as biochar [
23,
24]. Not only is studying the products important, it is also important to consider the effects of various pyrolysis system parameters on the biomass pyrolysis process [
25,
26].
Previous studies have focused on the pyrolysis equipment and methods for waste compounds, or have concentrated on the pyrolysis process and products of other macromolecular substances [
27,
28,
29]. The pyrolysis kinetics and the corresponding mechanistic model for waste tires have an important guiding significance in the overall pyrolytic process, but they are yet to be fully revealed. Therefore, it is necessary to study the process used for tires so that the parameters of the general pyrolysis process can be improved and the pyrolysis product structure can be optimized. To investigate this, the three kinetic factors (activation energy
E, pre-exponential factor
A, and kinetic equation
f(α)) that describe the pyrolysis process need to be obtained.
It should be noted that the production process of the tire is complicated but precise, and it usually requires several types of composite rubber. In particular, the composition of the tread comprises a significant proportion in the whole tire, which contains two or three kinds of rubber, as well as over 10 types of additives. Additionally, the total pyrolysis process of the tread rubber from waste tires cannot be characterized clearly and accurately by merely one method [
7,
30,
31]. Using the Kissinger–Akahira–Sunose (KAS) method to calculate the pre-exponential factor is not feasible when the reaction model/mechanism has not been determined [
32,
33,
34]. When some optimization algorithms such as the genetic algorithm (GA) and shuffled complex evolution (SCE) are used to calculate the kinetic parameters including the pre-exponential factor, the reaction model/mechanism still needs to be assumed in advance [
8]. Based on the peak differentiating analysis using a Gaussian function, the pyrolysis of waste rubber is divided into several sub-reactions. The pyrolysis kinetics can be obtained by analyzing the sub-reactions, but there is no reliable evidence for the establishment of an obtained mechanism [
35]. Gonzalez et al. focused their research on product distribution, but did not determine a kinetic model and did not establish a corresponding physical model [
36]. Complex reactions will overlap when the entire tire is selected as the experimental material, which is not conducive to the calculation of kinetic results. Unlike previous literature, this study first separated the compounds according to the different structures of the tires to obtain more accurate kinetic results. It should be emphasized that if the kinetic method requires the assumption of a kinetic model in advance, errors will be introduced [
37,
38]. Leung et al. used a single-rate scanning method to conclude that it is impossible to establish an accurate kinetic model for waste tire pyrolysis [
38], and Conesa et al. also reached a similar conclusion that a fractional model is not sufficient to explain the pyrolysis of waste tires, but a model was not given to explain the first reaction [
39]. At the beginning of the century, the International Thermal Analysis and Calorimetry Society (ICTAC) Kinetic Branch and multinational thermal analysts showed that using a single scan rate method to process thermal analysis kinetic data gives results that are not reliable and cannot reflect the complex nature of a solid-state reaction [
40,
41]. As a result, the international thermal analysis community has called for the use of multiple scan rate methods to determine thermal analysis data. In addition, as a way to determine the complex nature of the reaction, it is necessary to determine the change in activation energy with conversion using the iso-conversion method [
40,
41]. The multi-kinetic method integrated in this study can avoid pre-supposition models (assuming a kinetic model in advance) when studying the pyrolysis of a mixture such as waste tire tread rubber.
In summary, a more systematic and rigorous kinetics method must be adopted in dealing with the pyrolysis kinetics and the mechanistic model of a heterogeneous solid-phase material such as a tire. Therefore, a new research method must be defined to determine thermal analysis data and to reveal the complex nature of the reaction by determining the change in activation energy with conversion.
4. Conclusions
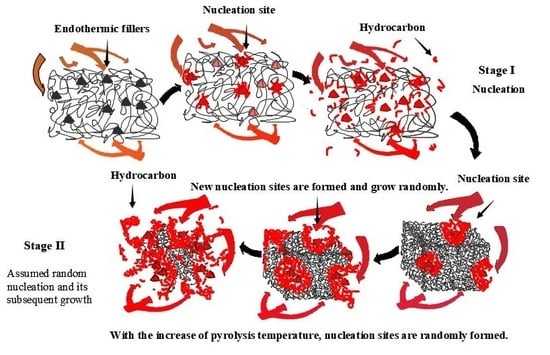
When considering the pyrolysis of a material with complex components such as the tread rubber of a silica-filled tire, a single kinetic method often cannot obtain an accurate kinetic reaction mechanism and its corresponding factors. A reasonable and effective use of multi-kinetic methods, especially methods with model detection and model revision, can obtain pyrolysis kinetic equations and reaction mechanisms of such materials from different angles. It should be emphasized that this method does not have to assume the reaction model in advance to avoid unnecessary errors. Moreover, the correction and detection component of the kinetic function included in the method improves the confidence of the result. The kinetic results of this compound are listed in
Table 6, and different reaction types can be controlled according to the activation energy and the temperature range of the reaction. The results show that the pyrolysis reaction process of this compound can be divided into Reaction I and Reaction II by the research methods proposed in this paper. In the case of insufficient thermal energy, the revised kinetic model,
, and the nucleation mechanism can accurately characterize Reaction I. During this stage, non-pyrolytic fillers such as SiO
2 and ZnO will take the lead in becoming nucleation sites for the pyrolysis reactions to occur. Moreover, thermal energy is transferred along the direction of the molecular chain, resulting in a one-dimensional fracture of the molecular chain. With the increase in thermal energy, the revised kinetic model,
and the reaction mechanism of random nucleation and its subsequent growth can accurately characterize Reaction II. In comparison to the previously fixed nucleation sites, the appearance of new nucleation sites is random but mainly appears around randomly distributed fillers and exposed molecular chain edges. Future research will focus on the establishment of a pyrolysis reaction simulation system based on the three kinetic factors obtained and the kinetic reaction mechanism.