Melting Kinetics of Nascent Poly(tetrafluoroethylene) Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
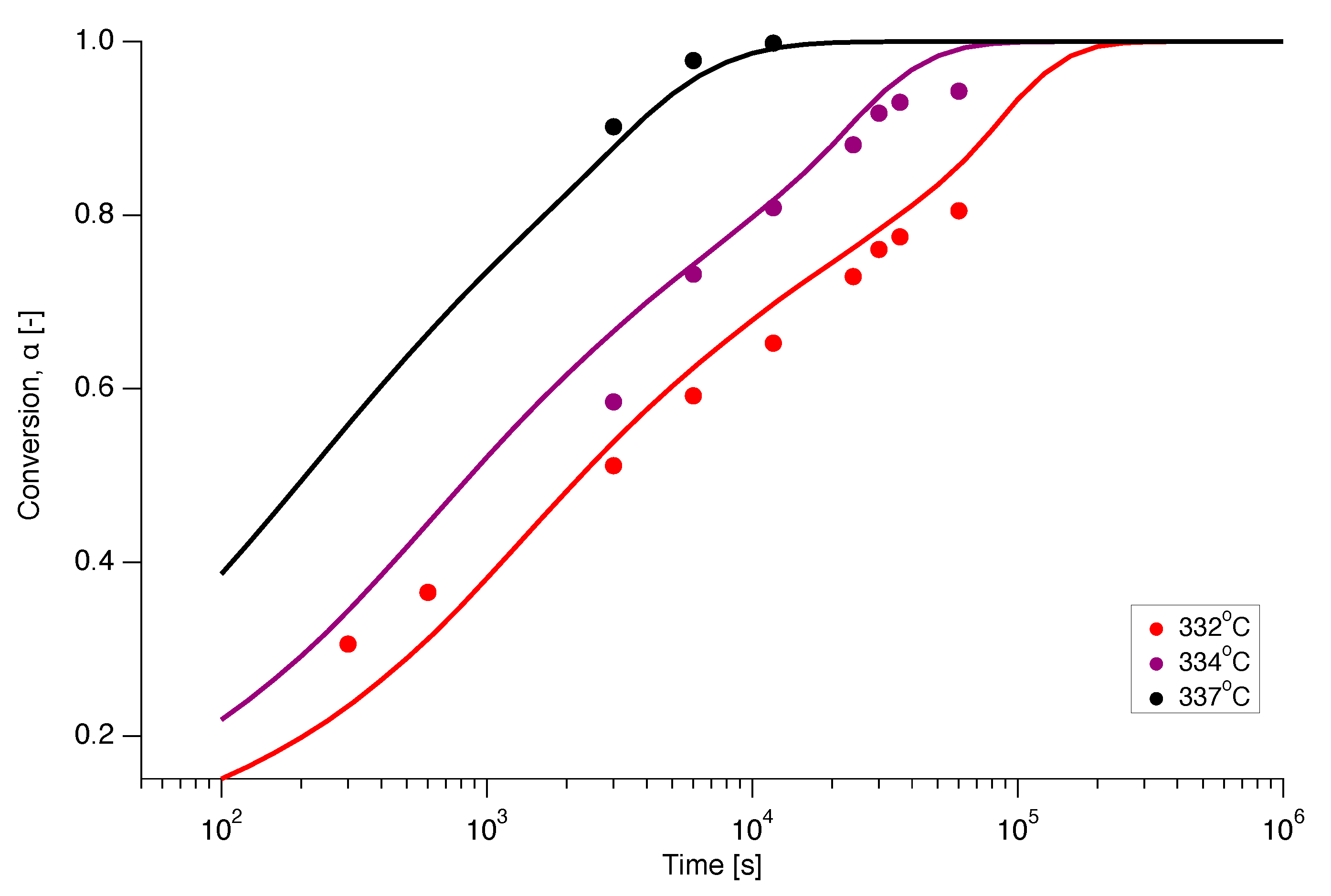
2.2. Kinetic Analysis
3. Results and Discussion
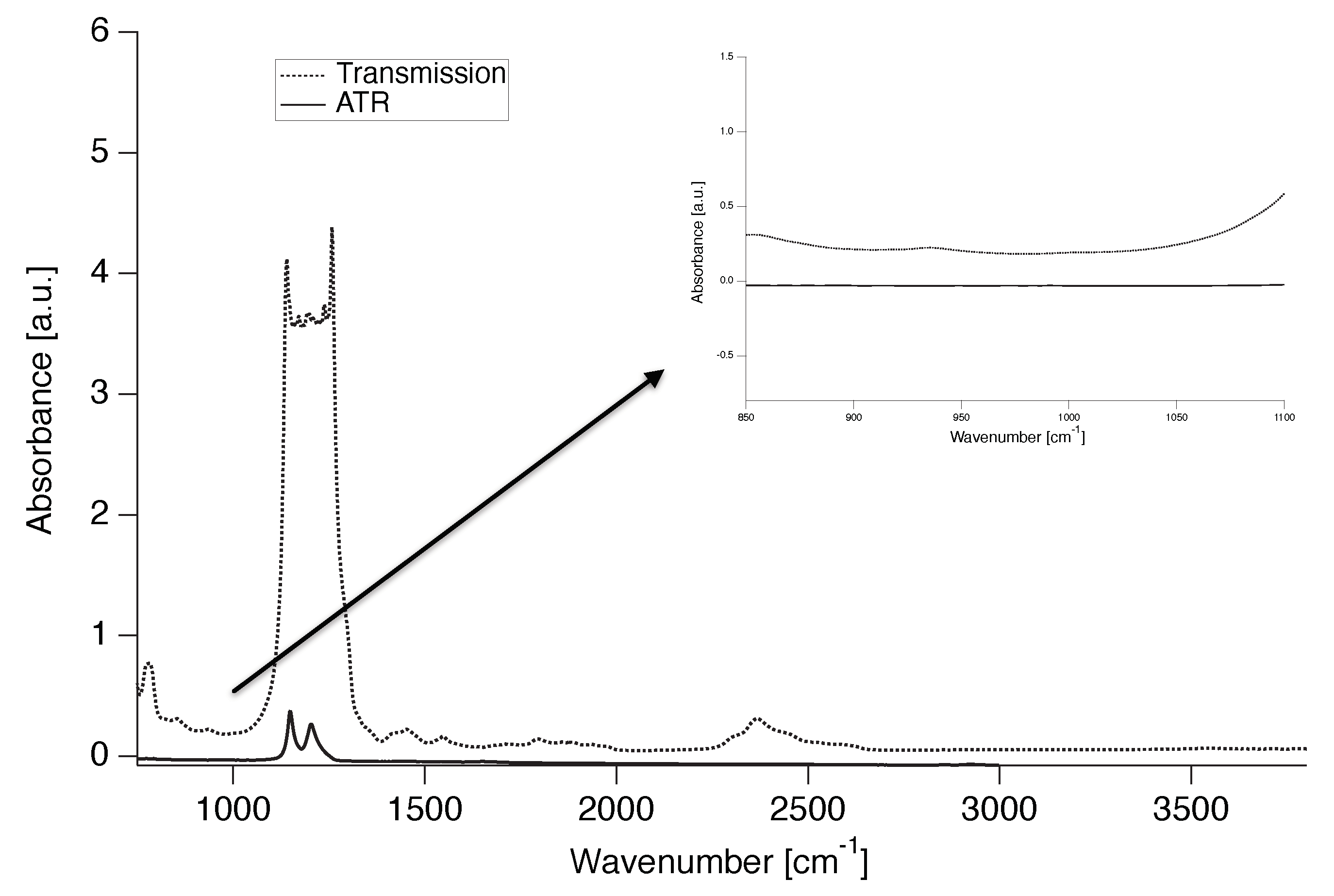
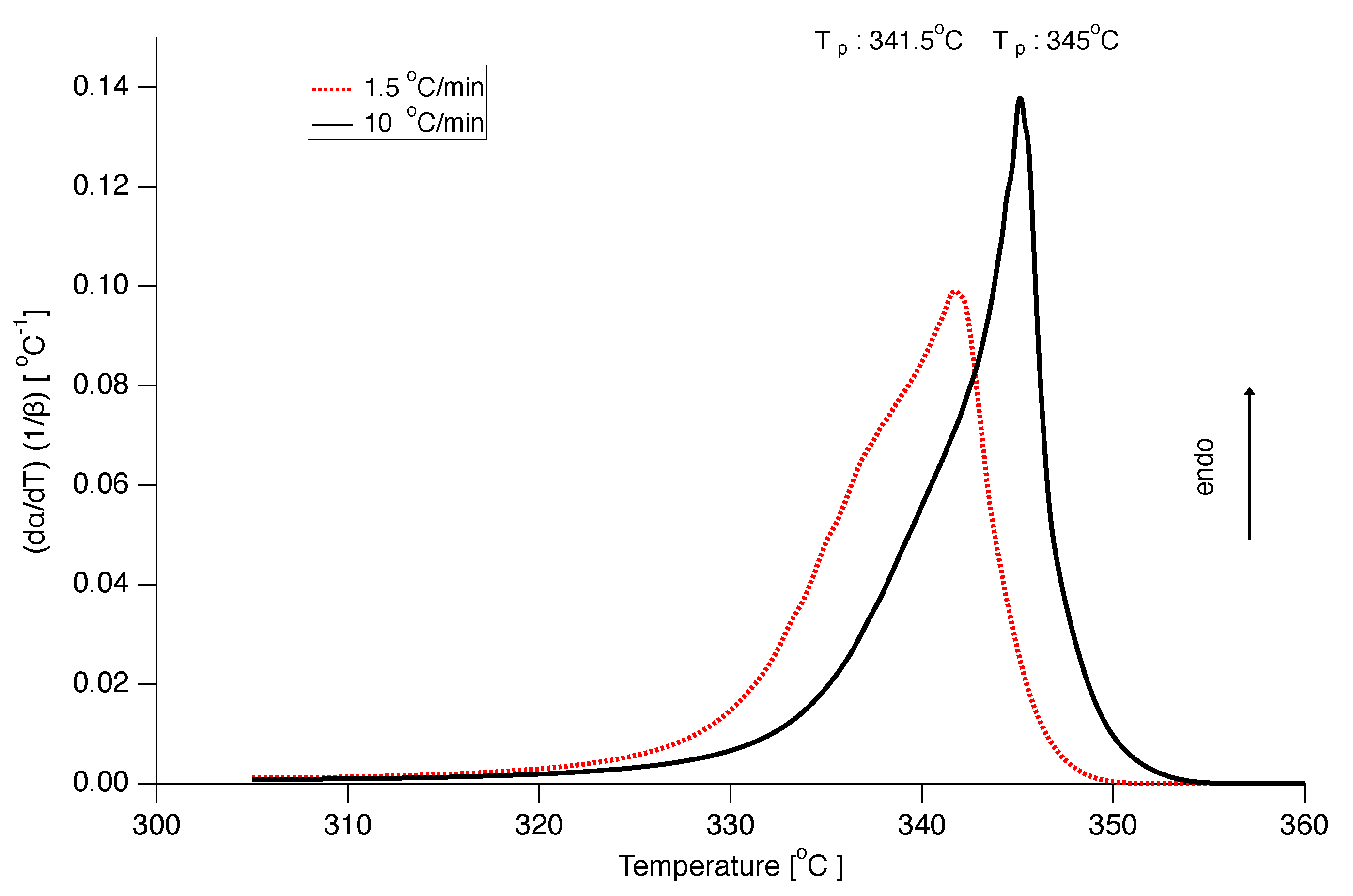
3.1. Material Characterization
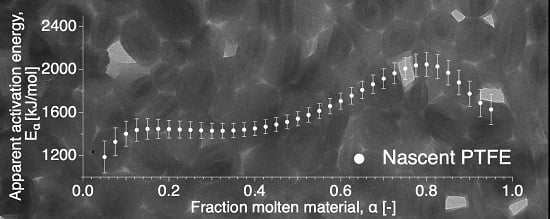
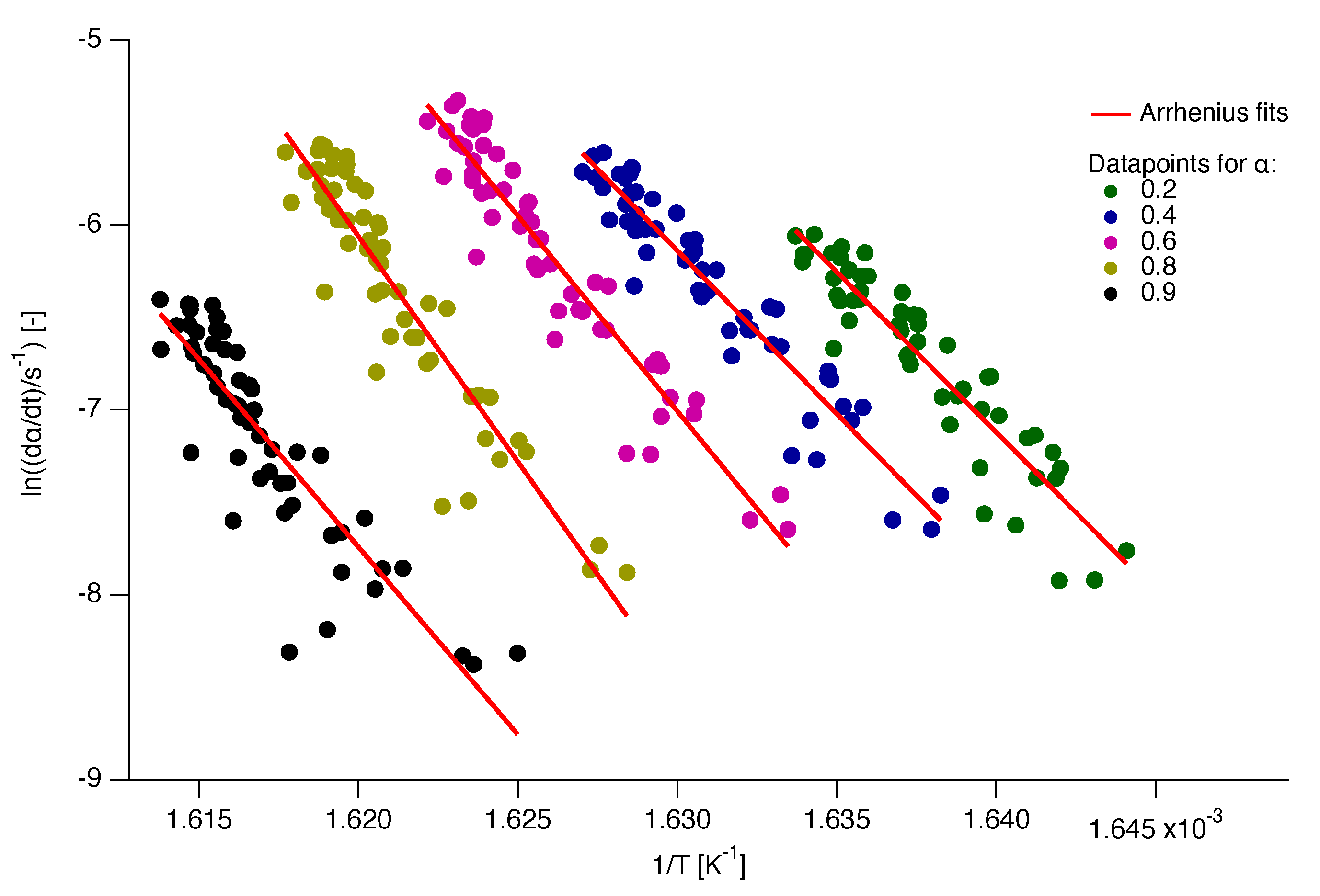
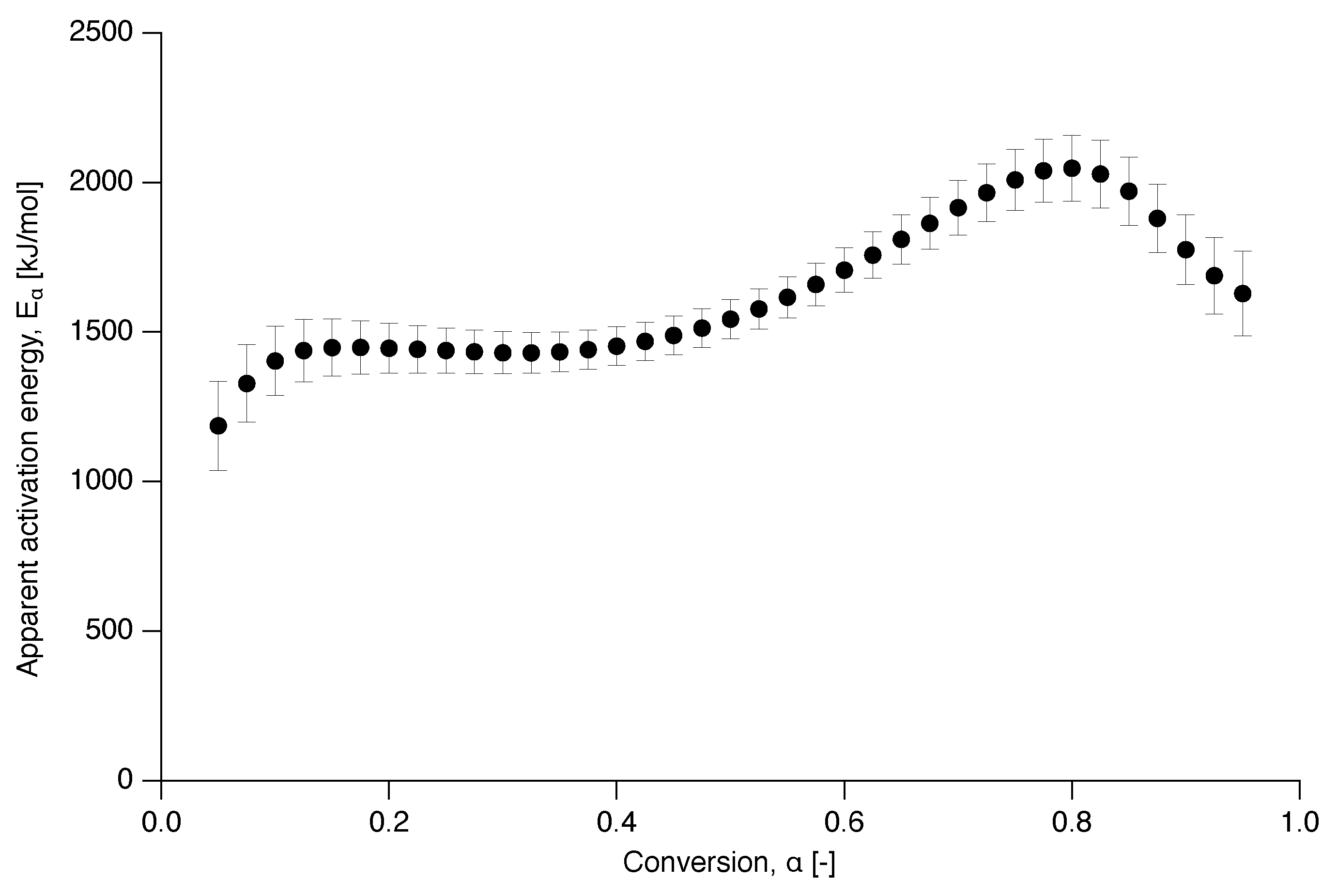
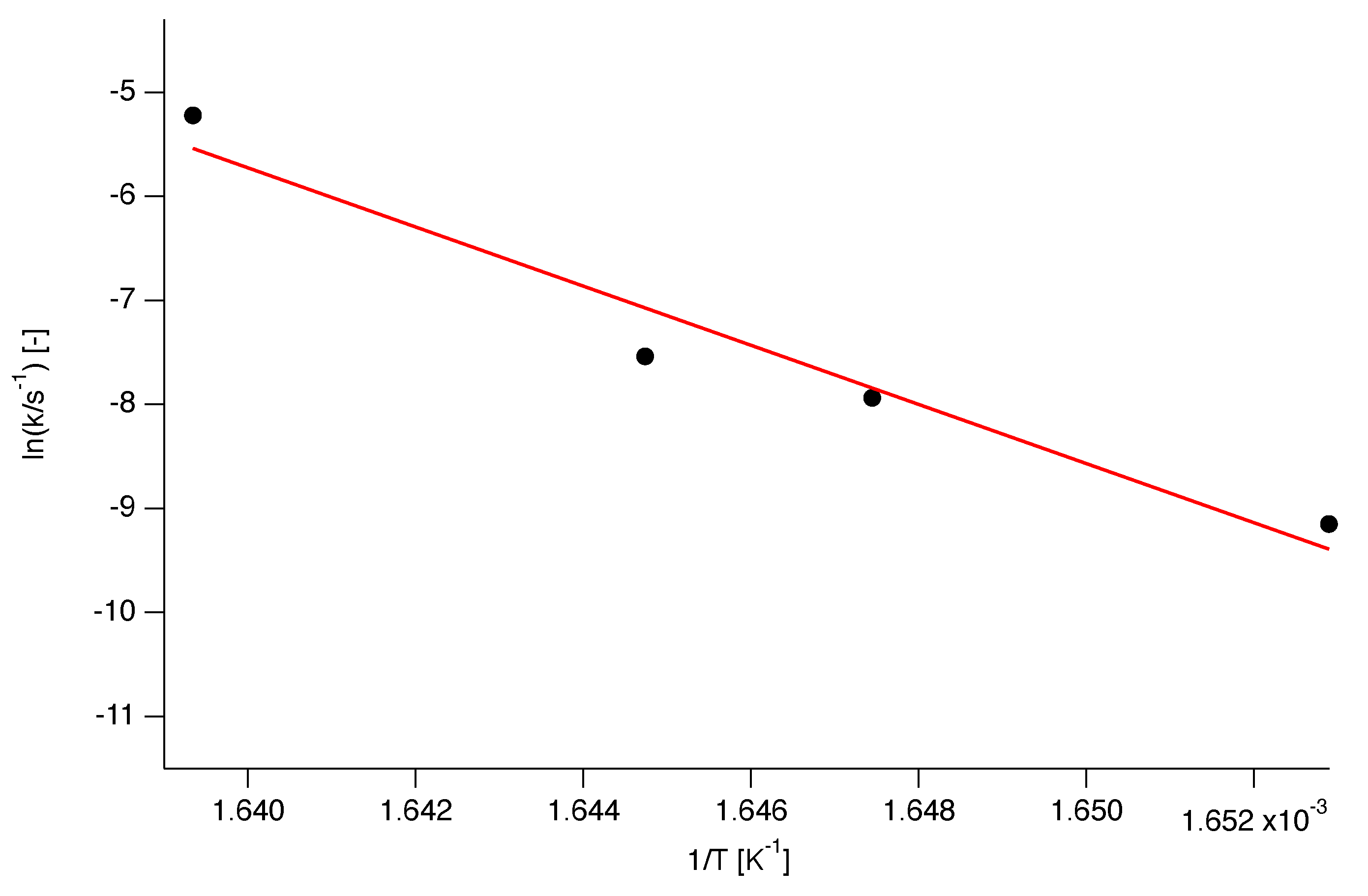
3.2. Apparent Activation Energy Calculation
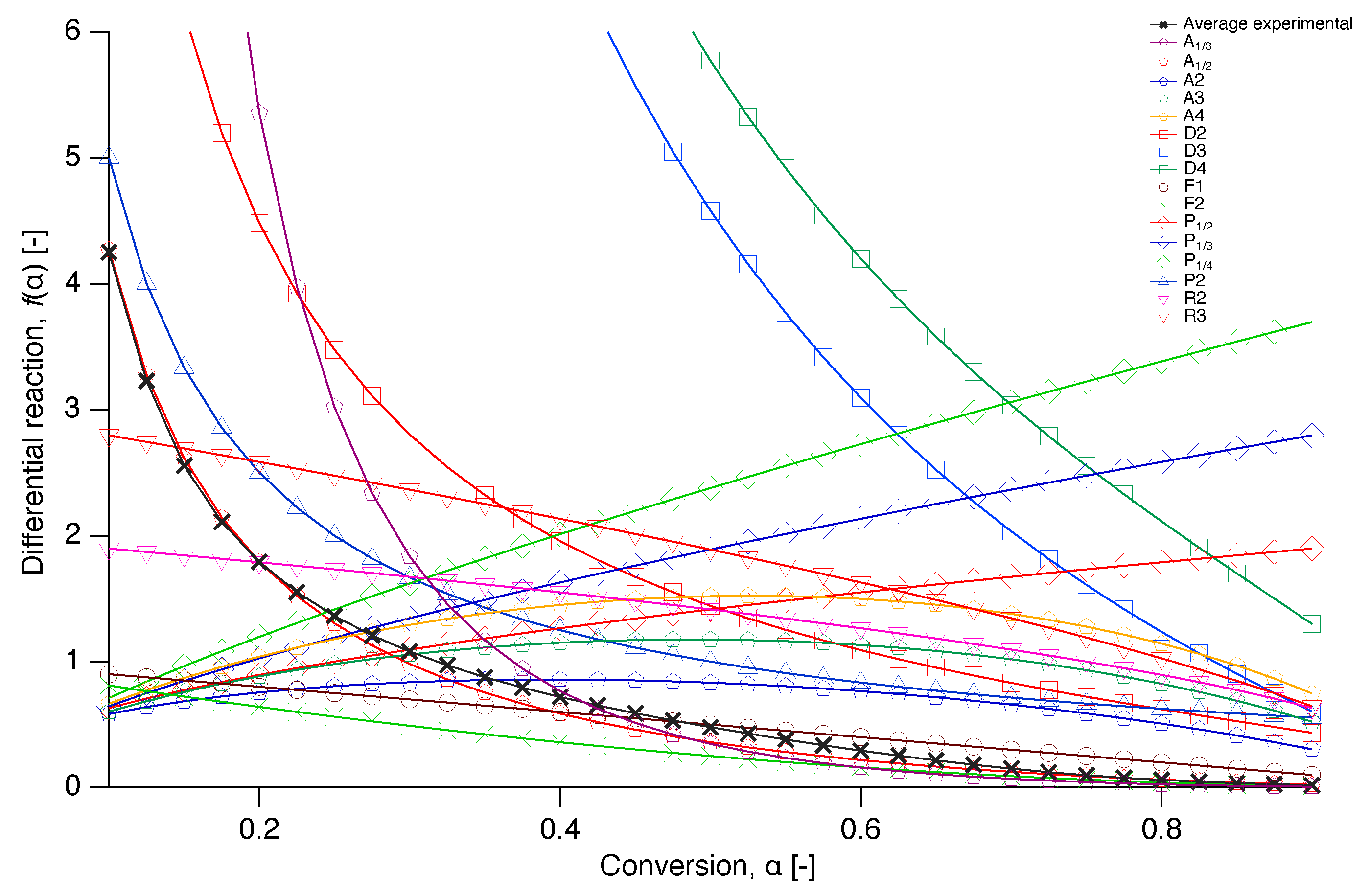
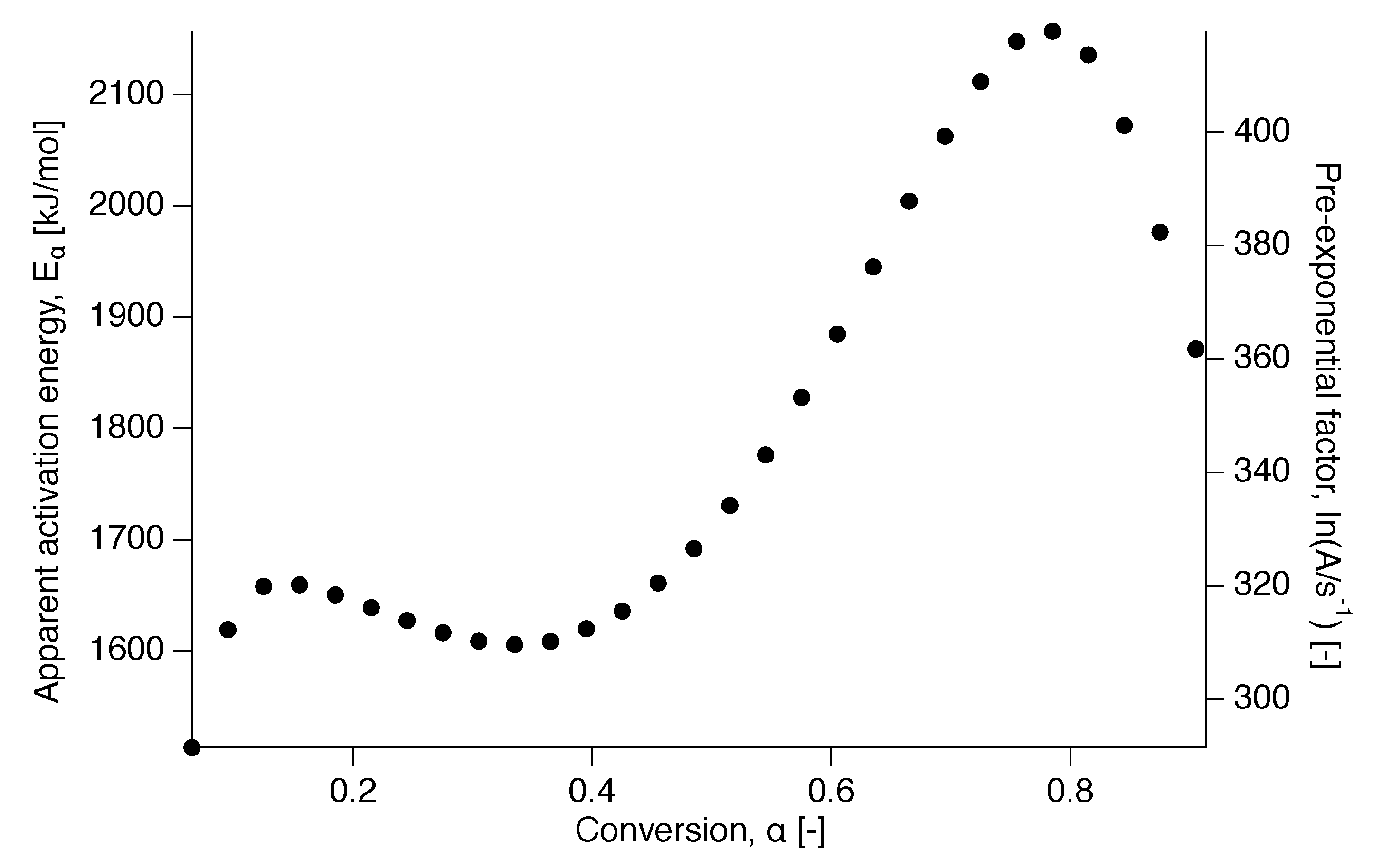
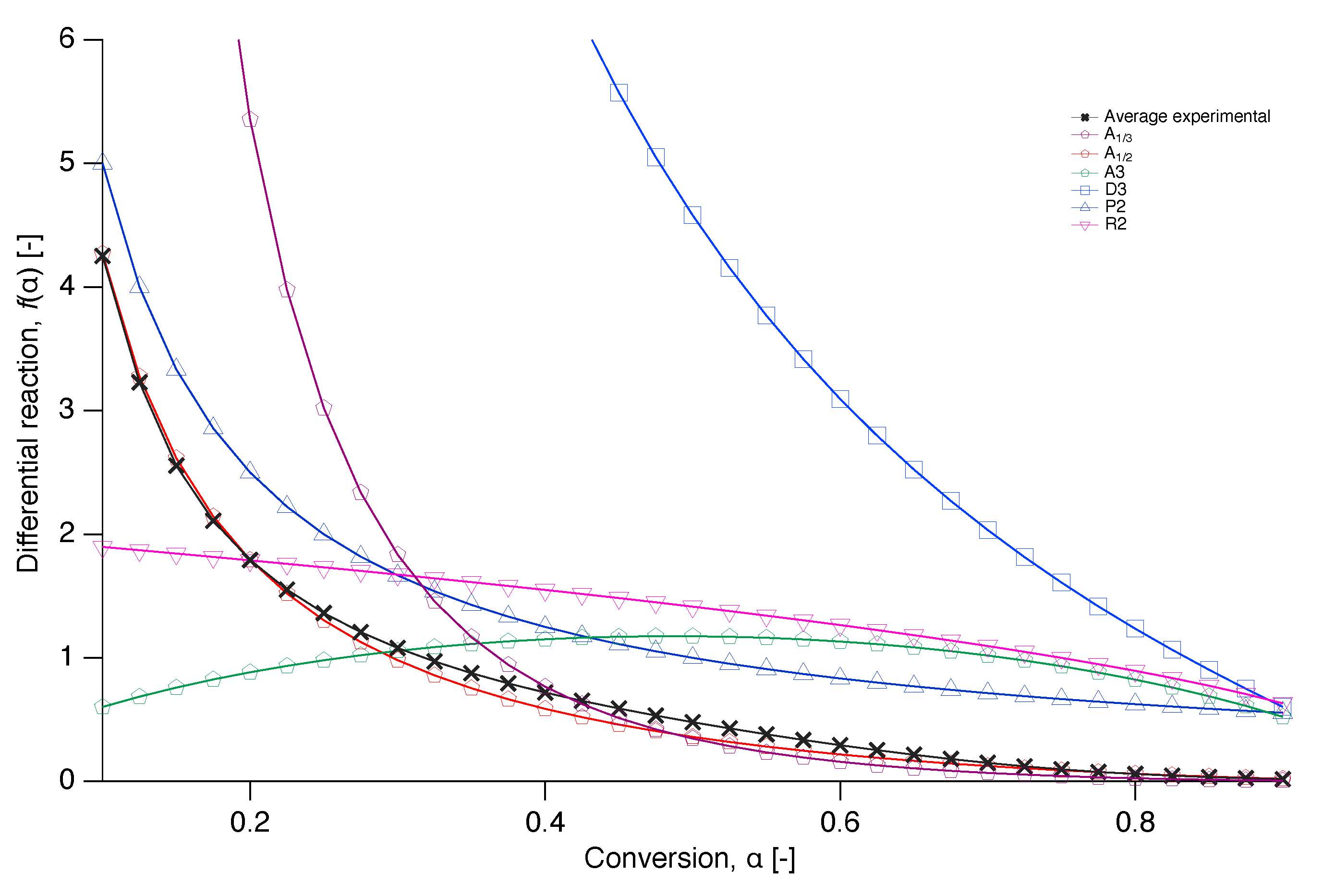
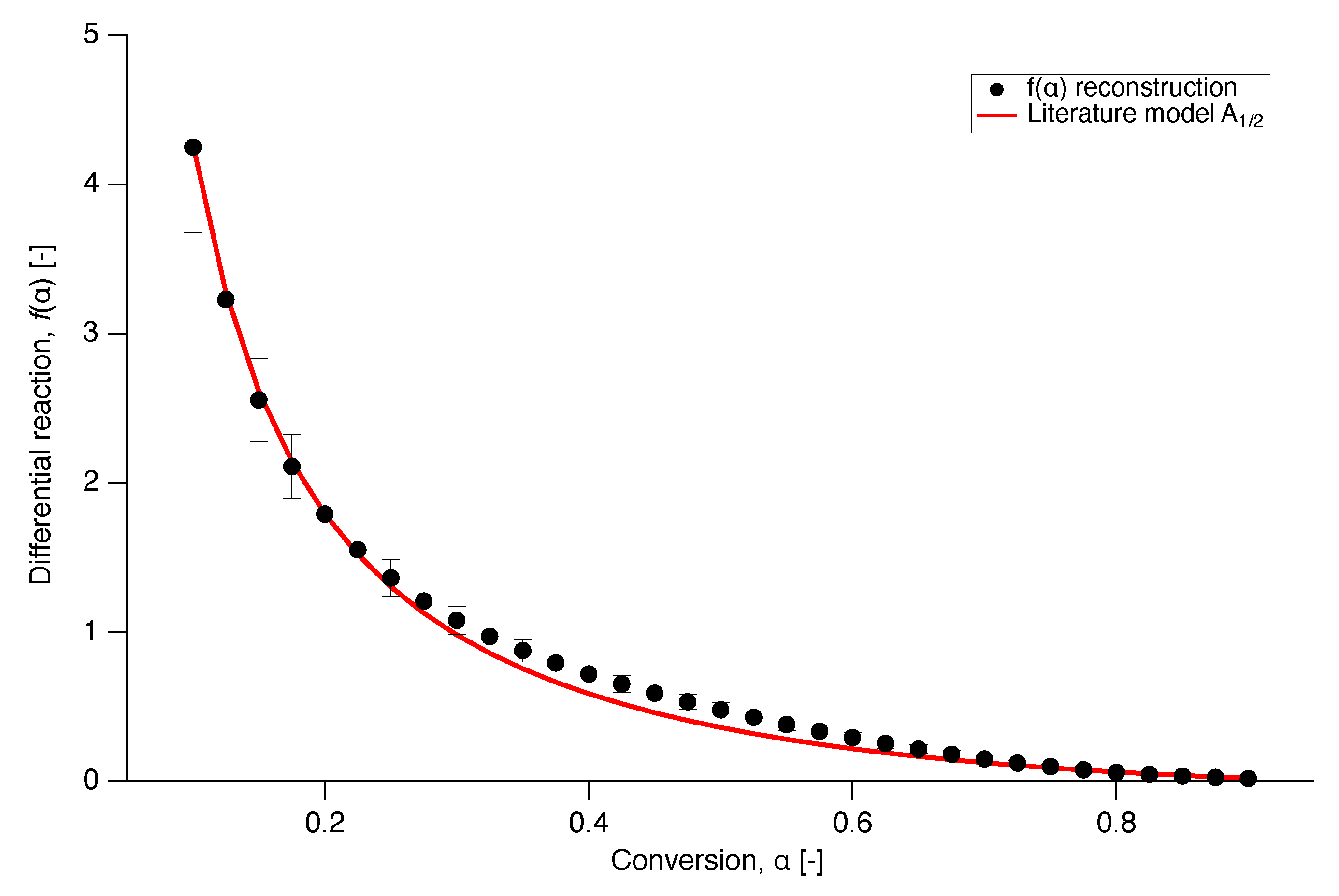
3.3. Pre-Exponential Factor and Reaction Model Calculation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Mandelkern, L. Crystallization of Polymers Volume 2. Kinetics and Mechanisms; Cambridge University Press: Cambridge, UK, 2004; p. 478. [Google Scholar]
- Chuah Hoe, H. Crystallization kinetics of Poly(Trimethylene Terephthalate). Polym. Eng. Sci. 2001, 41, 308–313. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Lauritzen, J.I. Crystallization of bulk polymers with chain folding: Theory of growth of lamellar spherulites. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1961, 65A, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Yancey, B.; Walker, K. Nucleation-Driven Kinetics of Poly (ethylene terephthalate) Melting. Macromol. Chem. Phys. 2013, 214, 2562–2566. [Google Scholar] [CrossRef]
- Toda, A.; Hikosaka, M.; Yamada, K. Superheating of the melting kinetics in polymer crystals: A possible nucleation mechanism. Polymer 2002, 43, 1667–1679. [Google Scholar] [CrossRef]
- Toda, A.; Kojima, I.; Hikosaka, M. Melting kinetics of polymer crystals with an entropic barrier. Macromolecules 2008, 41, 120–127. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Berlin, Germany, 2015. [Google Scholar] [CrossRef] [Green Version]
- Vyazovkin, S. Isoconversional Kinetics of Polymers: The Decade Past. Macromol. Rapid Commun. 2017, 38, 1–21. [Google Scholar] [CrossRef]
- Toda, A.; Taguchi, K.; Nozaki, K.; Fukushima, T.; Kaji, H. Superheated Melting Kinetics of Metastable Chain-Folded Polymer Crystals. Cryst. Growth Des. 2018, 18, 3637–3643. [Google Scholar] [CrossRef]
- Starkweather, H.W. Effect of Heating Rate on the Melting of Polytetrafluoroethylene. J. Polym. Sci. Part A-2 Polym. Phys. 1985, 23, 1177–1185. [Google Scholar] [CrossRef]
- Rastogi, S.; Lippits, D.R.; Peters, G.W.M.; Graf, R.; Yao, Y.; Spiess, H.W. Heterogeneity in polymer melts from melting of polymer crystals. Nat. Mater. 2005, 4, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, E.; Wunderlich, B. Superheating of linear high-polymer polyethylene crystals. J. Appl. Phys. 1965, 36, 3039–3044. [Google Scholar] [CrossRef]
- Androsch, R.; Wunderlich, B.; Radusch, H.J. Analysis of reversible melting in polytetrafluoroethylene. J. Therm. Anal. Calorimet. 2005, 79, 615–622. [Google Scholar] [CrossRef]
- Wunderlich, B. One hundred years research on supercooling and superheating. Thermochim. Acta 2007, 461, 4–13. [Google Scholar] [CrossRef]
- Smith, P.; Chanzy, H.D.; Rotzinger, B.P. Drawing of virgin ultrahigh molecular weight polyethylene: An alternative route to high strength/high modulus materials—Part 2 Influence of polymerization temperature. J. Mater. Sci. 1987, 22, 523–531. [Google Scholar] [CrossRef]
- Rotzinger, B.P.; Chanzy, H.D.; Smith, P. High strength/high modulus polyethylene: Synthesis and processing of ultra-high molecular weight virgin powders. Polymer 1989, 30, 1814–1819. [Google Scholar] [CrossRef]
- Pandey, A.; Toda, A.; Rastogi, S. Influence of amorphous component on melting of semicrystalline polymers. Macromolecules 2011, 44, 8042–8055. [Google Scholar] [CrossRef]
- Romano, D.; Tops, N.; Andablo-Reyes, E.; Ronca, S.; Rastogi, S. Influence of polymerization conditions on melting kinetics of low entangled uhmwpe and its implications on mechanical properties. Macromolecules 2014, 47, 4750–4760. [Google Scholar] [CrossRef]
- Keller, A.; Willmouth, F.M. On the morphology and orgin of the fibres observed in Nascent Ziegler polyethylene. Die Makromolekulare Chemie 1969, 121, 42–50. [Google Scholar] [CrossRef]
- Tervoort-Engelen, Y.M.T.; Lemstra, P.J. Morphology of nascent ultra-high molecular weight polyethylene reactor powder: Chain-extended versus chain-folded crystals. Polym. Commun. 1991, 32, 343–345. [Google Scholar]
- Phillips, R.A. Morphology and melting behavior of nascent ultra-high molecular weight polyethylene. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 495–517. [Google Scholar] [CrossRef]
- Starkweather, H.W.; Zoller, P.; Jones, G.A.; Vega, A.J. The heat of fusion of polytetrafluoroethylene. J. Polym. Sci. Polym. Phys. Ed. 1982, 20, 751–761. [Google Scholar] [CrossRef]
- Menczel, J.D.; Prime, R.B. Thermal Analysis of Polymers: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–688. [Google Scholar] [CrossRef] [Green Version]
- Vyazovkin, S. A Unified Approach to Nonisothermal Data. Unified Kinet. Process. 1995, 28, 95–101. [Google Scholar]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys.Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Zeitschrift für Physikalische Chemie 1889, 4. [Google Scholar] [CrossRef] [Green Version]
- Sbirrazzuoli, N. Determination of pre-exponential factors and of the mathematical functions f(α) or G(α) that describe the reaction mechanism in a model-free way. Thermochim. Acta 2013, 564, 59–69. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Gang Linert, W. Thermally induced reactions of solids: Isokinetic relationships of non-isothermal systems. Int. Rev. Phys. Chem. 1995, 14, 355–369. [Google Scholar] [CrossRef]
- Trache, D.; Abdelaziz, A.; Siouani, B. A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J. Therm. Anal. Calorim. 2017, 128, 335–348. [Google Scholar] [CrossRef]
- Lau, S.F.; Suzuki, H.; Wunderlich, B. Thermodynamic Properties of Polytetrafluoroethylene. J. Polym. Sci. Part A-2 Polym. Phys. 1984, 22, 379–405. [Google Scholar] [CrossRef]
- Illers, K.H. Die ermittlung des schmelzpunktes von kristallinen polymeren mittels wärmeflusskalorimetrie (DSC). Eur. Polym. J. 1974, 10, 911–916. [Google Scholar] [CrossRef]
- Liavitskaya, T.; Birx, L.; Vyazovkin, S. Melting kinetics of superheated crystals of glucose and fructose. Phys. Chem. Chem. Phys. 2017, 19, 26056–26064. [Google Scholar] [CrossRef]
- De Bruijn, T.J.W.; De Jong, W.A.; Van Den Berg, P.J. Kinetic parameters in Avrami-Erofeev type reactions from isothermal and non-isothermal experiments. Thermochim. Acta 1981, 45, 315–325. [Google Scholar] [CrossRef]
- Vyazovkin, S. A time to search: Finding the meaning of variable activation energy. Phys. Chem. Chem. Phys. 2016, 18, 18643–18656. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Bos, C.; Mittemeijer, E.J. Analysis of solid state phase transformation kinetics: Models and recipes. Int. Mater. Rev. 2007, 52, 193–212. [Google Scholar] [CrossRef]
- Grapes, M.D.; Santala, M.K.; Campbell, G.H.; LaVan, D.A.; Weihs, T.P. A detailed study of the Al3Ni formation reaction using nanocalorimetry. Thermochim. Acta 2017, 658, 72–83. [Google Scholar] [CrossRef]
- Torrens-Serra, J.; Venkataraman, S.; Stoica, M.; Kuehn, U.; Roth, S.; Eckert, J. Non-isothermal kinetic analysis of the crystallization of metallic glasses using the master curve method. Materials 2011, 4, 2231–2243. [Google Scholar] [CrossRef] [Green Version]
- Ebnesajjad, S. FLUOROPLASTICS Volume 1: Non-Melt Processible Fluoropolymers—The Definitive User’s Guide and Data Book; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]




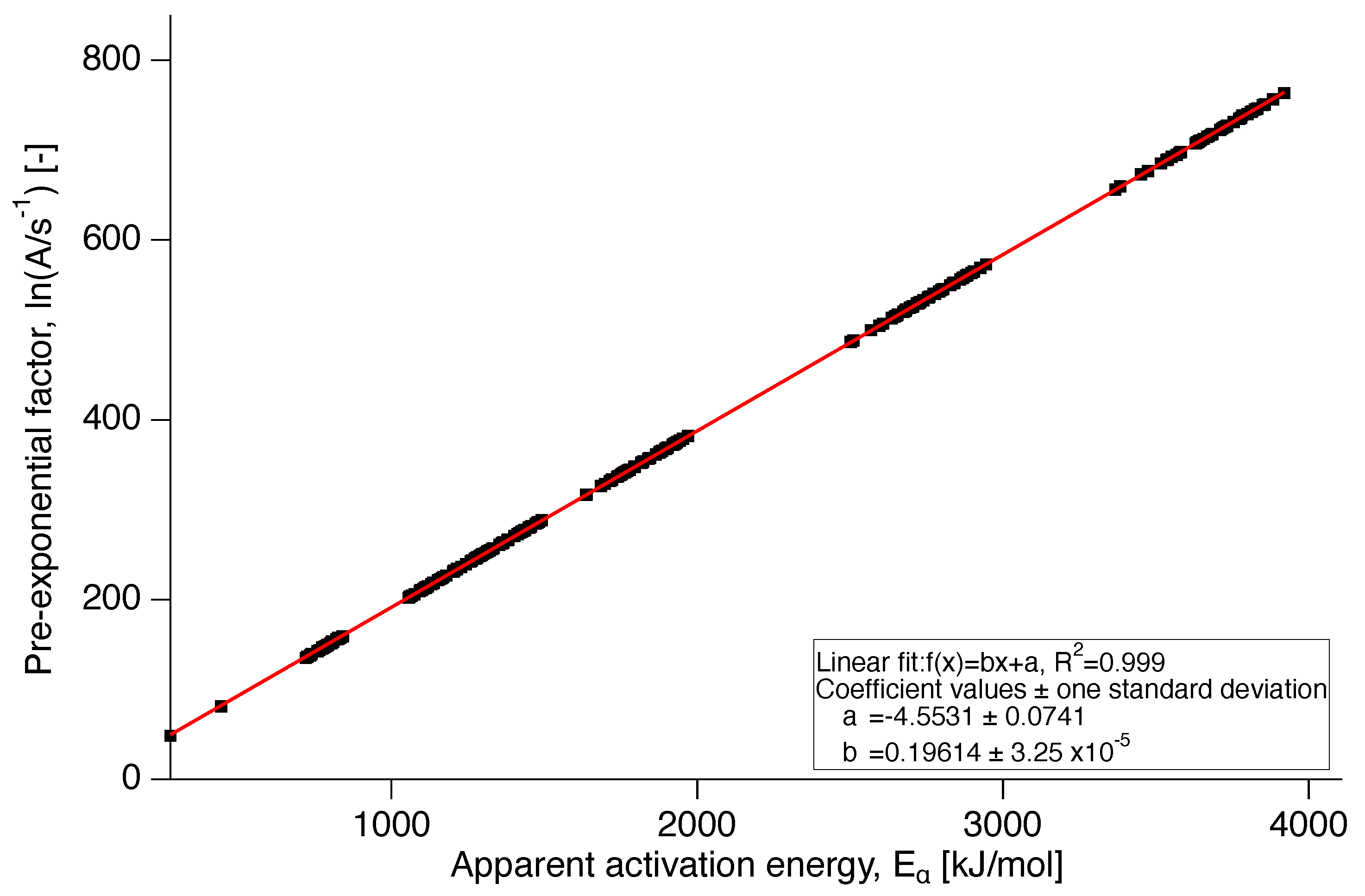


| Number | Model | Rate-Determining Mechanism | |
|---|---|---|---|
| 1 | Chemical reaction | ||
| 2 | Chemical reaction | ||
| 3 | Chemical reaction | ||
| 4 | Chemical reaction | ||
| 5 | Chemical reaction | ||
| 6 | Chemical reaction | ||
| 7 | Chemical reaction | ||
| 8 | Chemical reaction | ||
| 9 | Chemical reaction | ||
| 10 | Nucleation (power law) | ||
| 11 | Nucleation (power law) | ||
| 12 | Nucleation (power law) | ||
| 13 | Nucleation (power law) | ||
| 14 | Nucleation (parabolic law) | ||
| 15 | Nucleation (exponential law) | ||
| 16 | Nucleation (exponential law) | ||
| 17 | Random nucleation/ first order (Mampel) | ||
| 18 | Random nucleation (Avrami-Erofeev) | ||
| 19 | Random nucleation (Avrami-Erofeev) | ||
| 20 | Random nucleation (Avrami-Erofeev) | ||
| 21 | Random nucleation (Avrami-Erofeev) | ||
| 22 | Random nucleation (Avrami-Erofeev) | ||
| 23 | Random nucleation (Avrami-Erofeev) | ||
| 24 | Random nucleation (Avrami-Erofeev) | ||
| 25 | Random nucleation (Avrami-Erofeev) | ||
| 26 | Random nucleation (Avrami-Erofeev) | ||
| 27 | Random nucleation (Avrami-Erofeev) | ||
| 28 | 1 | Contracting disc | |
| 29 | Contracting cylinder | ||
| 30 | Contracting sphere | ||
| 31 | One-dimensional diffusion | ||
| 32 | Three-dimensional diffusion | ||
| 33 | Three-dimensional diffusion (Jander) | ||
| 34 | Three-dimensional diffusion (Ginstling-Brounshtein) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christakopoulos, F.; Troisi, E.; Tervoort, T.A. Melting Kinetics of Nascent Poly(tetrafluoroethylene) Powder. Polymers 2020, 12, 791. https://doi.org/10.3390/polym12040791
Christakopoulos F, Troisi E, Tervoort TA. Melting Kinetics of Nascent Poly(tetrafluoroethylene) Powder. Polymers. 2020; 12(4):791. https://doi.org/10.3390/polym12040791
Chicago/Turabian StyleChristakopoulos, Fotis, Enrico Troisi, and Theo A. Tervoort. 2020. "Melting Kinetics of Nascent Poly(tetrafluoroethylene) Powder" Polymers 12, no. 4: 791. https://doi.org/10.3390/polym12040791