Polyacrylamide-Metilcellulose Hydrogels Containing Aloe barbadensis Extract as Dressing for Treatment of Chronic Cutaneous Skin Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Aloe barbadensis Plant Material
2.3. Preparation of Aloe barbadensis Extract
2.4. Physical–Chemical Characterization Assays of Aloe barbadensis
2.4.1. pH Analysis of Aloe barbadensis
2.4.2. Relative Density Analysis
2.4.3. Fiber Content and Moisture Analysis
2.4.4. Analysis of the Extract of Aloe barbadensis by Liquid Chromatography of Ultra Efficiency Coupled to Mass Spectrometry (HPLC-DAD-EM)
2.5. Preparation of Hydrogel Containing Extract of Aloe barbadensis
2.6. Physical–Chemical Characteristics of Hydrogel containing Aloe barbadensis Extract
2.6.1. Determination of Degree of Swelling
2.6.2. Morphology of Hydrogels through the Scanning Electron Microscopy (SEM)
2.6.3. Analysis of Formulation Excipient Profiles
2.6.4. Thermogravimetric Profile
2.7. In Vitro Activity Test of Antibacterials and Antifungals
2.7.1. Preparation of Media and Biological Material
2.7.2. Determination of Minimum Inhibitory Concentration (MIC) of Aloe barbadensis Extract
3. Results and Discussions
3.1. Aloe barbadensis Extract
3.2. Physical–Chemical Characterization Cssays of Aloe barbadensis
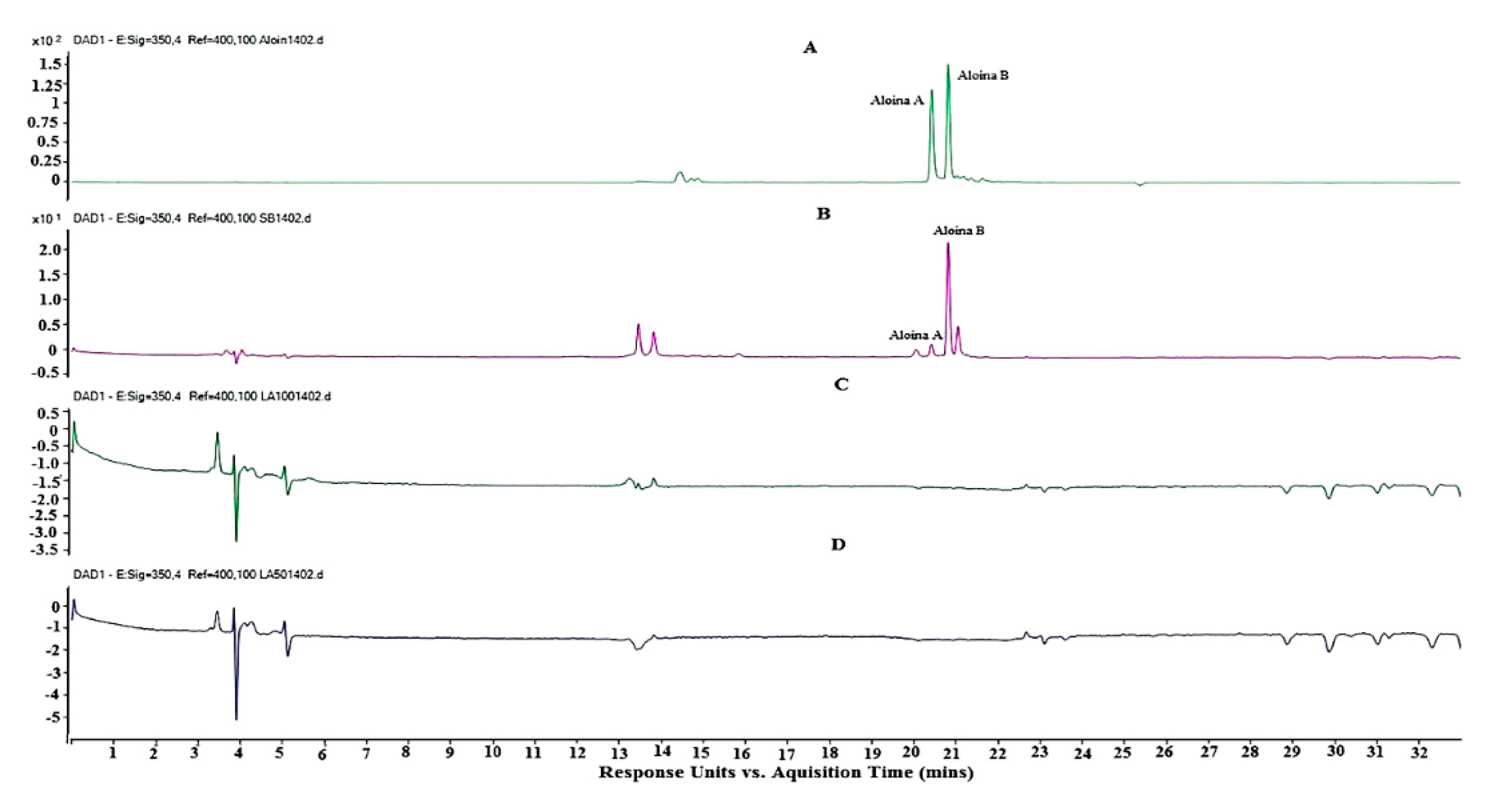
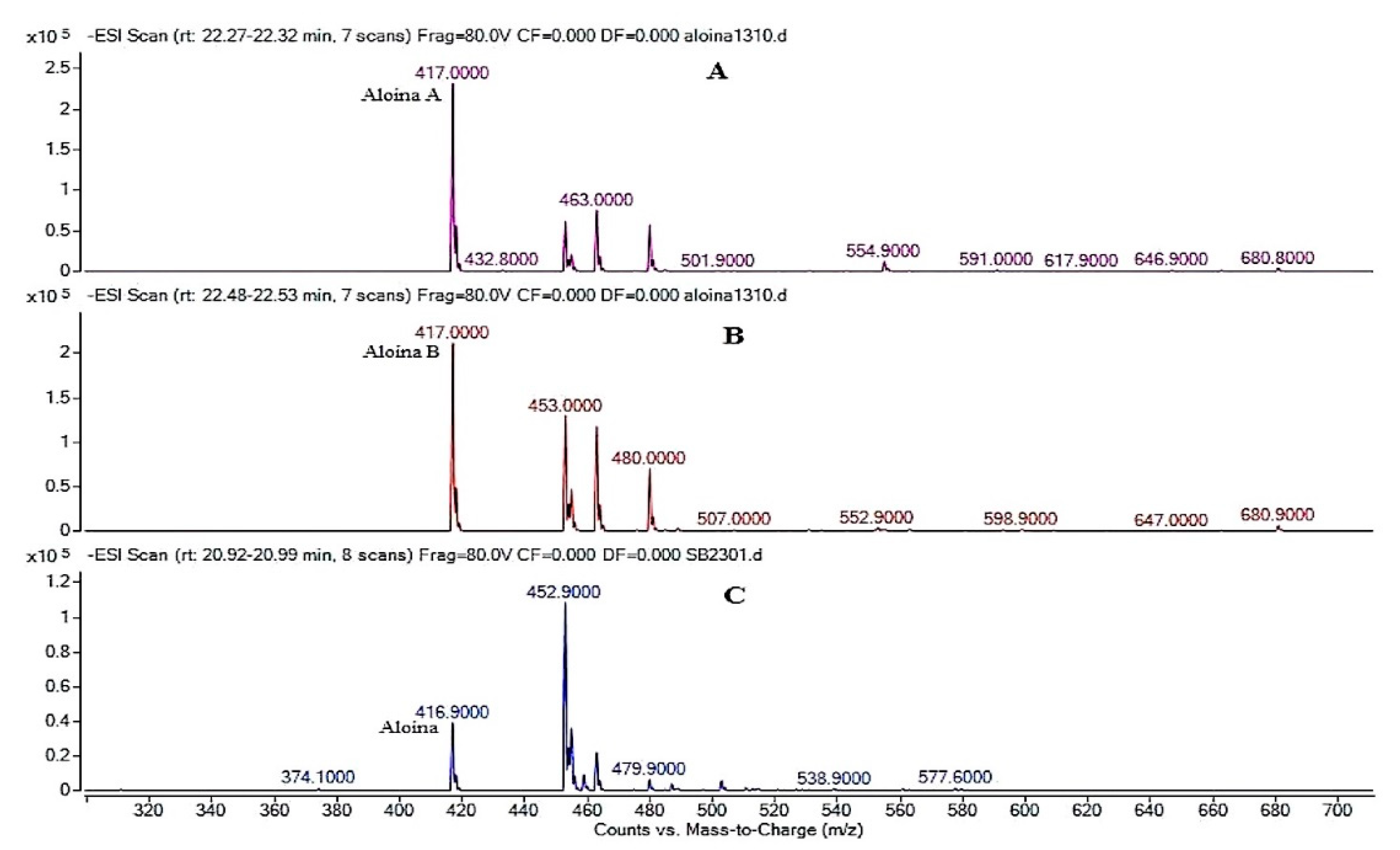
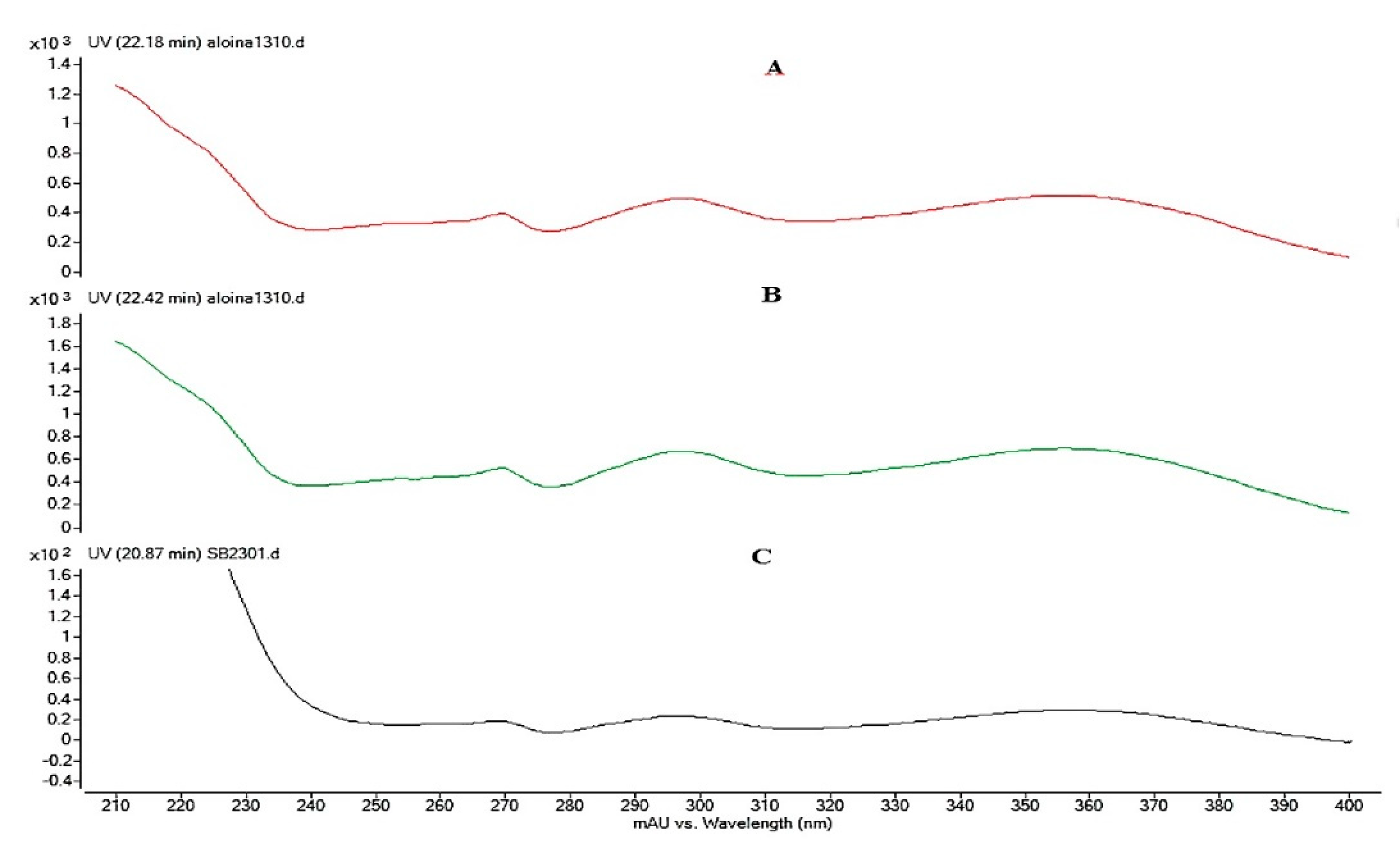
3.3. Analysis of the Extract of Aloe Barbadensis by Ultra High Performance Liquid Chromatography Coupled to Mass Spectrometry (HPLC-DAD-EM)
3.4. The Aloe barbadensis Hydrogel Synthesis
3.5. Physical–Chemical Characterization of Hydrogels
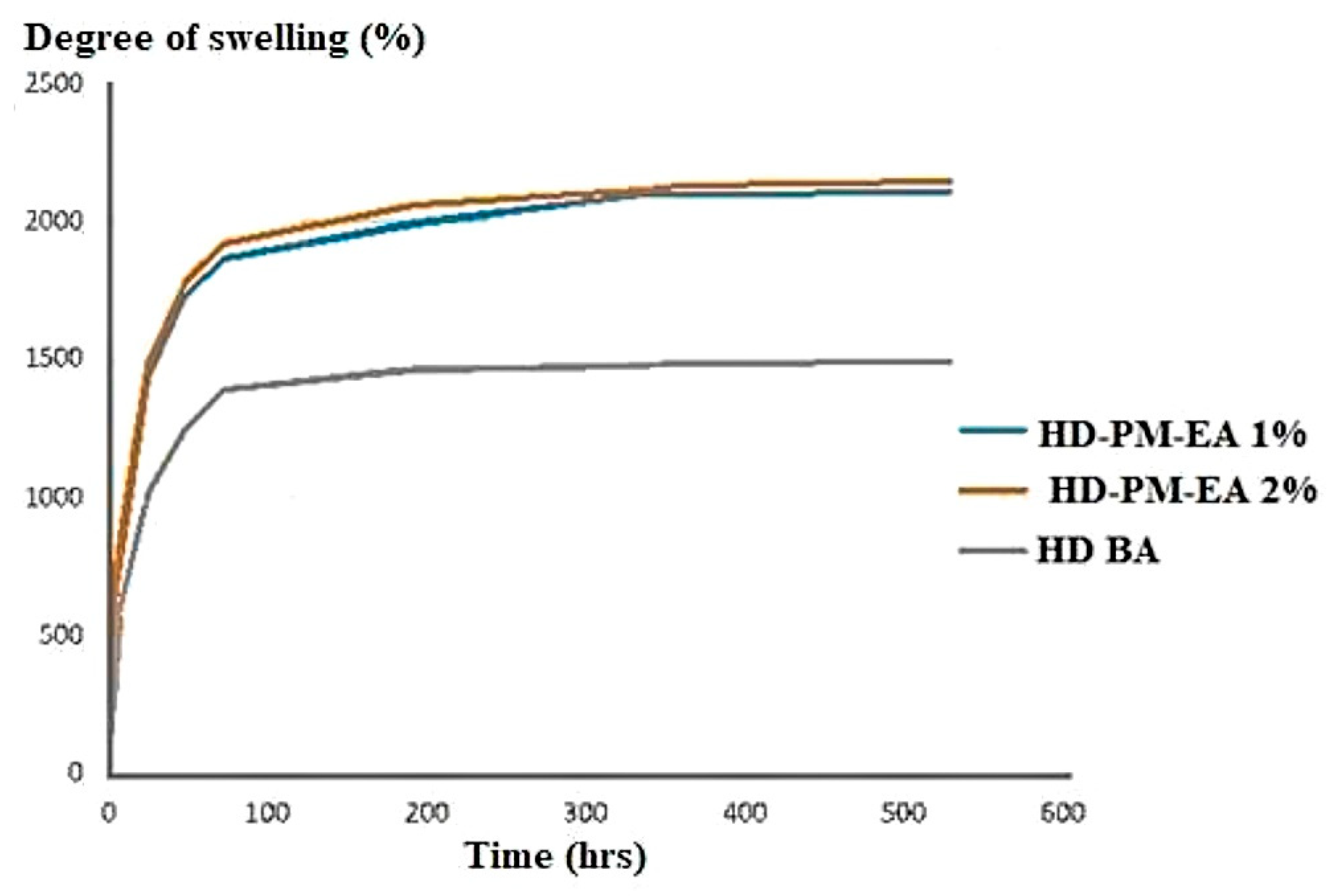
3.5.1. The Swelling Analysis
3.5.2. Scanning Electron Microscopy (SEM)
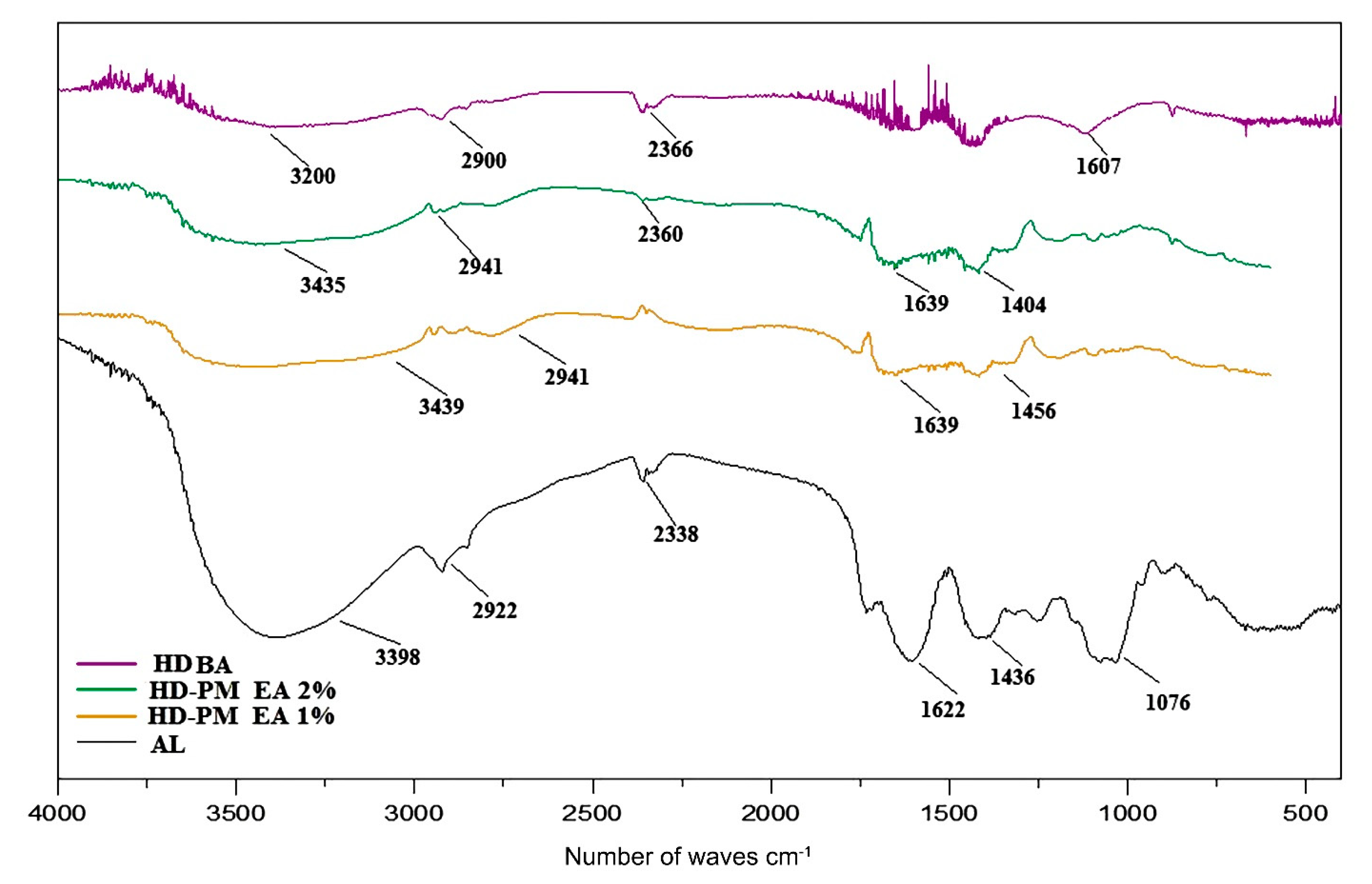
3.5.3. Spectroscopic Profile By FTIR
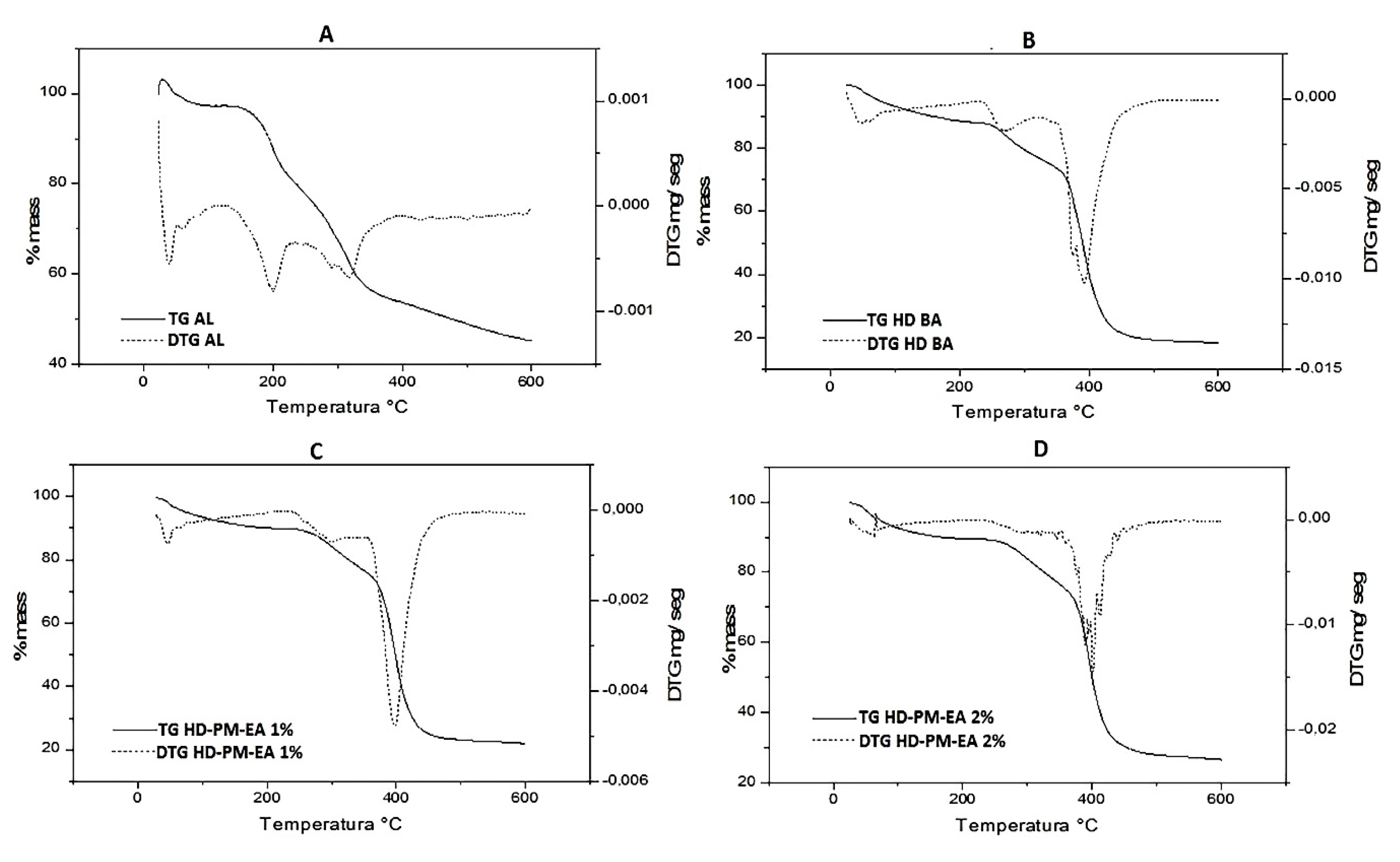
3.5.4. Thermogravimetric Profile
3.5.5. Evaluation of In Vitro Antibacterial and Antifungal Properties: Minimal Inhibitory Concentration (MIC) of Aloe barbadensis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ribeiro, D.M.L.; Carvalho, A.R., Jr.; de Macedo, V.; Rodrigues, G.H.; Chagas, V.L.; Silva, L.d.S.; Cutrim, B.d.S.; Santos , D.M.; Soares, B.L.L.; da Albuquerque, P.B.S.; et al. Polysaccharide-Based Formulations for Healing of Skin-Related Wound Infections: Lessons from Animal Models and Clinical Trials. Biomolecules 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werdin, F.; Tennenhaus, M.; Schaller, H.E.; Rennekampff, H.O. Evidence-based management strategies for treatment of chronic wounds. Eplasty 2009, 9, e19. [Google Scholar] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Gottrup, F. A specialized wound-healing center concept: Importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am. J. Surg. 2004, 187, 38S–43S. [Google Scholar] [CrossRef]
- Kloth, L. The Roles of Physical Therapists in Wound Management, Part II: Patient and Wound Evaluation. J. Am. Col. Certif. Wound Spec. 2009, 1, 49–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, J. Global initiative for wound and lymphoedema care (GIWLC). J. Lymphoedema 2009, 4, 92–95. [Google Scholar]
- Heyer, K.; Augustin, M.; Protz, K.; Herberger, K.; Spehr, C.; Rustenbach, S.J. Effectiveness of advanced versus conventional wound dressings on healing of chronic wounds: Systematic review and meta-analysis. Dermatology 2013, 226, 172–184. [Google Scholar] [CrossRef]
- Denny, K.; Lawand, C.; Perry, S.D. Compromised wounds in Canada. Health Q. 2014, 17, 7–10. [Google Scholar] [CrossRef]
- Posnett, J.; Franks, P.J. The burden of chronic wounds in the UK. Nurs. Times 2008, 104, 44–45. [Google Scholar]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health/Anvisa/Fiocruz. Protocol for the Prevention of Pressure Ulcer—Annex 02; Registered Nurses’ Association of Ontario: Toronto, ON, Canada, Revised; 2005. [Google Scholar]
- Kusuma, S.; Shen, Y.; Hanjaya-Putra, D.; Mali, P.; Cheng, L.; Gerecht, S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl. Acad. Sci. USA 2013, 110. [Google Scholar] [CrossRef] [Green Version]
- Gavini, E.; Mariani, A.; Rassu, G.; Bidali, S.; Spada, G.; Bonferoni, M.C.; Giunched, P. Frontal polymerization as a new method for developing drug controlled release systems (DCRS) based on polyacrylamide. Eur. Polym. J. 2009, 45, 690–699. [Google Scholar] [CrossRef]
- Gulrez, S.; Al-Assaf, S.; Phillips, G. Hydrogels: Methods of Preparation. Charact. Appl. 2011. [Google Scholar] [CrossRef] [Green Version]
- Nilimanka, D. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Pharm. Sci. 2013, 5. [Google Scholar]
- Kumar, P.T.; Lakshmanan, V.-K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and Microporous Chitosan Hydrogel/Nano Zno Composite Bandages for Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef]
- Aouada, F.; Moura, M.; Menezes, E.; Nogueira, A.; Mattoso, L. Synthesis of hydrogels and release kinetics of ammonium and potassium. Soil. Sci. 2008, 1643–1649. [Google Scholar]
- Onoda-Yamamuro, N.; Yamamuro, O.; Inamura, Y.; Nomura, H. QENS study on thermal gelation in aqueous solution of methylcellulose. Phys. B Condens. Matter 2007, 393, 158–160. [Google Scholar] [CrossRef]
- Irani, P.S.; Varaie, S. Comparison of the Effect of Aloe Vera Gel And Nitrofurazone 2% On Epithelialization And Granulation Tissue Formation Regarding Superficial Second-Degree Burns. Iran. J. Med Sci. 2016, 41, S3. [Google Scholar]
- Kim, J.H.; Cho, C.W.; Lee, J.I.; Vinh, L.B.; Kim, K.T.; Cho, I.S. An investigation of the inhibitory mechanism of α-glucosidase by chysalodin from Aloe vera. Int. J. Biol. Macromol. 2018, 147, 314–318. [Google Scholar] [CrossRef]
- Juneby, H.B. Aloe barbadensis—A Legendary Medicinal Plant. Pharmacognosy 2009, 15, 1–14. [Google Scholar]
- Surjushe, A.; Resham, V.; Saple, D.G. Aloe Vera: A Short Review. Indian J. Dermatology Pmc. Web. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and Applications of Aloe Vera Leaf Gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [Green Version]
- Grundmann, O. Aloe Vera Gel Research Review: An Overview of Its Clinical Uses and Proposed Mechanisms of Action. Nat. Med. J. 2012, 4, 1–12. [Google Scholar]
- Hashemi, S.A.; Madani, S.A.; Abediankenari, S. The Review on Properties of Aloe Vera in Healing of Cutaneous Wounds. Hindawi Publishing Corporation. Biol. Med. Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Pandey, D.K.; Malik, T.; Banik, R.M. Quantitative Estimation of Barbaloin in Aloe Vera and Its Commercial Formulations by Using HPLC. Int. J. Med. Aromat. Plants 2012, 2, 420–427. [Google Scholar]
- Wang, Y.; Strong, K.J. A two year study monitoring some chemical and physical characteristics of field grown Aloe barbadensis Leaves. Subtrop. Plant Sci. 1995, 47, 34–38. [Google Scholar]
- Azaroual, L.; Liazid, A.; Barbero, G.F.; Brigul, J.; Palma, M.; Barroso, C.G. Improved Chromatographic Methods For determination of bioactive compounds from Aloe Vera Leaves. Int. Sch. Res. Netw. 2012. [Google Scholar] [CrossRef] [Green Version]
- Farmacopeia Brasileira, 5th ed.; Atheneu Publisher: São Paulo, Brazil, 2010.
- Pourjavadi, A.; Jahromi, P.E.; Seidi, F.; Salimi, H. Synthesis and swelling behavior of acrylatedstarch-g-poly (acrylic acid) and acrylatedstarch-g-poly (acrylamide) hydrogels. Carbohydrate Polym. 2010, 79, 933–940. [Google Scholar] [CrossRef]
- Aouada, F.A.; Pan, Z.; Orts, W.J.; Mattoso LH, C. Removal of paraquat pesticide from aqueous solutions using a novel adsorbent material based on polyacrylamide and methylcellulose hydrogels. J. Appl. Polym. Sci. 2009, 114, 2139–2148. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kapoor, S.; Kundu, S.C. Silk fibroin/Polyacrylamide Semi-Interpenetrating Network Hydrogels for Controlled Drug Release. Biomaterials 2009, 30, 2826–2836. [Google Scholar] [CrossRef]
- Aouada, F.; Guilherme, M.; Campese, G.M.; Girotto, E.M.; Rubira, A.; Muniz, E. Electrochemical And Mechanical Properties Of Hydrogels Based On Conductive Poly(3,4-Ethylene Dioxythiophene)/Poly(Styrenesulfonate) And PAAm. Polym. Test. 2006, 25, 158. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N.; Chauhan, N. Synthesis, Characterization and Swelling Studies of Ph Responsive Psyllium And Methacrylamide Based Hydrogels For The Use In Colon Specific Drug Delivery. Carbohydr. Polym. 2007, 69, 631. [Google Scholar] [CrossRef]
- Tang, Y.; Du, Y.; Hu, X.; Shi, X.; Kennedy, J.F. Rheological characterization of a novel thermosensitive chitosan/Poly(Vinyl Alcohol) Blend Hydrogel. Carbohydr. Polym. 2007, 67, 491. [Google Scholar] [CrossRef]
- Oliveira, D.N.; De Menezes, M.; Catharino, R.R. Thermal degradation of sucralose: A combination of analytical methods to determine stability and chlorinated by products. Sci. Rep. 2015, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Pellizzoni, M.; Růžičková, G.; Kalhotka, L.; Lucini, L. Antimicrobial activity of different Aloe barbadensis Mill. and Aloe arborescens Mill. leaf fractions. J. Med. Plants Res. 2012, 6, 1975–1981. [Google Scholar]
- Pandey, R.; Mishra, A. Antibacterial activities of crude extract of Aloe barbadensis to clinically isolated bacterial pathogens. Appl. Biochem. Biotechnol. 2010, 160, 1356–1361. [Google Scholar] [CrossRef]
- Hombach, M.; Wolfensberger, A.; Kuster, S.P.; Böttger, E.C. Influence of Clinical Breakpoint Changes From CLSI 2009 To EUCAST 2011 Antimicrobial Susceptibility Testing Guidelines On Multidrug Resistance Rates Of Gram-Negative Rods. J. Clin. Microbiol. 2013, 51, 2385–2387. [Google Scholar] [CrossRef] [Green Version]
- Özcan, K. Antibacterial, antioxidant and enzyme inhibition activity capacities of Doronicum macrolepis (FREYN&SINT): An endemic plant from Turkey. Saudi Pharmaceutical Journal. 2019, 28, 95–100. [Google Scholar] [CrossRef]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Filho, B.P.D. Screening of Some Plants Used In The Brazilian Folk Medicine For The Treatment Of Infectious Diseases. Mem Inst Oswaldo Cruz Rio De Jan. 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Yan, C.; Fuwei, P.; Yuliang, C.; Yahui, G.; He, Q. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [Green Version]
- Dal’Belo, S.E.; Gaspar, L.R.; Berardo Gonçalves Maia Campos, P.M. Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Res. Technol. 2006, 12, 241–246. [Google Scholar] [CrossRef]
- Priyanka, S.; Amit, K.; Harsha, K.; Ajit, V. A Review on Pharmacological Properties of Aloe vera International. J. Pharm. Sci. Rev. Res. 2014, 29, 31–37. [Google Scholar]
- Attah, M.O.; Jacks, T.W.; Jacob, A.; Eduitem, O.; John, B. The Effect of Aloe vera (Linn) On Cutaneous Wound Healing and Wound Contraction Rate in Adult Rabbits. Nova J. Med Biol. Sci. 2016, 5, 1–5. [Google Scholar]
- Nagaraju, M.; Ramulla, S.; Murthy, N. Extraction and preliminary analysis of Aloin obtained from Aloe barbadensis Miller. Asian J. Chem. 2011, 23, 2421–2423. [Google Scholar]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Mariscal-Domínguez, M.F.; Cruz-Flores, P.; Campas-Baypoli, O.N.; Cantú-Soto, E.U.; Sanches-Silva, A. An HPLC Procedure for the Quantification of Aloin in Latex and Gel from Aloe barbadensis Leaves. J. Chromatogr. Sci 2016, 55, 251–257. [Google Scholar] [CrossRef]
- Logaranjan, K.; Devasena, T.; Pandian, K. Quantitative Detection of Aloin and Related Compounds Present in Herbal Products and Aloe Vera Plant Extract Using HPLC Method. Am. J. Anal. Chem. 2013, 4, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Garrett, R.H.; Grisham, C.M. Biochemistry, 3rd ed.; Thomson: Belmont, CA, USA, 2005; p. 205. ISBN 0-534-41020-0. [Google Scholar]
- Gabbay Alvez, T.V.; Tavares, E.J.M.; Aouada, F.A.; Negrão, C.A.B.; Oliveira, M.E.C.; Júnior, A.P.D.; Da Costa, C.E.F.; Júnior, J.O.C.S.; Costa, R.M.R. Thermal analysis characterization of PAAm-co-MC Hydrogels. J. Therm. Anal. Calorim. 2011, 106, 717–724. [Google Scholar]
- Sandeep, C.; Harikumar, S.L.; Kanupriy, A. Hydrogels: A Smart Drug Delivery System. Int. J. Res. Pharm. Chem. 2012, 2, 603–614. [Google Scholar]
- Aouada, F.; Moura, A.; Fernandez, P.; Rubira, A.; Muniz, E. Optical and Morphological Characterization of Polyacrylamide Hydrogel and Liquid Crystal Systems. Eur. Polym. J. 2005, 41, 2134–2141. [Google Scholar] [CrossRef]
- Silva Da Costa, R.; Brito Negrão, C.A.; Pereira Camelo, S.R.; Ribeiro-Costa, R.M.; Ramos Barbosa, W.L.; Da Costa, C.E.F.; Silva Júnior, J.O.C. Investigation of thermal behavior of Heliotropium indicum L. lyophilized extract by TG and DSC. J. Therm. Anal. Calorim. 2013, 111, 1959–1964. [Google Scholar] [CrossRef]
- Choi, S.; Chung, M. A review on the relationship of Aloe Vera components and their biologic effects. Semin. Integr. Med. 2003, 1, 53–62. [Google Scholar] [CrossRef]
- Palmieri, B.; Vadalà, M.; Laurino, C. Nutrition in wound healing: Investigation of the molecular mechanisms, a narrative review. J. Wound Care 2019, 28, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.G.; Kamble V., M. Quantitative Assessment of Mineral Composition of Aloe vera (L.) Burm.f. leaves by ICP-MS and CHNS Analyzer. Int. J. Sci. Res. 2015, 4. [Google Scholar]
- Miranda, M.; Vega-Gálvez, A.; García, P.; Di Scala, K.; Shi, J.; Xue, S.; Uribe, E. Effect of Temperature on structural properties of Aloe vera (Aloe barbadensis Miller) gel and Weibull distribution for modelling drying process. Food Bioprod. Process. 2010, 88, 138–144. [Google Scholar] [CrossRef]
- Mbese, J.E.; Ajibade, P. Preparation and Characterization of ZnS, CdS and HgS/Poly(methyl methacrylate) Nanocomposites. Polymers 2014, 6, 2332–2344. [Google Scholar] [CrossRef] [Green Version]
- Dhakshnamoorthy, M.; Vikram, S.; Vasanthakumari, R. Development of Flexible Low Dielectric Constant Polyimide Films Based on Iso-Propylidene, Aryl-Ether Linked Dianhydride/Diamine. Int. J. Sci. Eng. Res. 2012, 3, 2229–2234. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating microbial methods: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Yadav, M.; Yadav, A.; Yadav, J.P. Comparative analysis of antimicrobial activity of methanolic extracts of Aloe vera and quantification of Aloe emodin collected from different climatic zones of India. Arch. Clin. Microbiol. 2015, 6, 1–10. [Google Scholar]
- Marzano, A.V.; Mercogliano, M.; Fachetti, M.; Caputo, R. Cutaneous Infection Caused by Salmonella typhi. J. Eur. Acad. Dematol. Venereol. 2003, 7, 575–577. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, And Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Pastar, I.; Nusbaum, A.G.; Gil, J.; Patel, S.B.; Chen, J.; Valdes, J.; Stojadinovic, O.; Plano, L.R.; Tomic-Canic, M.; Davis, S.C. Interactions of Methicillin Resistant Staphylococcus Aureus Usa300 And Pseudomonas Aeruginosa In Polymicrobial Wound Infection. PLoS ONE 2013, 8, E56846. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.D.; Beland, F.A. An evaluation of the biological and toxicological properties of Aloe barbadensis (Miller), Aloe vera. J. Environ. Sci. Health—Part. C Environ. Carcinog. Ecotoxicol. Rev. 2006, 25, 103–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banu, A.; Sathyanarayana, B.C.; Chattannavar, B. Efficacy of fresh Aloe Vera Gel against multi-drug resistant bacteria in infected leg ulcers. Australas. Med J. 2012, 5, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.; Lakshmanan, V.-K.; Biswas, R.; Nair, S.V.; Jayakumar, R. Synthesis and biological evaluation of chitin hydrogel/nano zno composite bandage as antibacterial wound dressing. J. Biomed Nanotechnol 2012, 8, 891–900. [Google Scholar] [CrossRef]
- National Institute of Meterology. Climate Data. INMET. 2019. Available online: http://www.inmet.gov.br/portal/index.php?r=home2/index (accessed on 5 March 2019).

| Tests | Aloe barbadensis in Natura | Aloe barbadensis Extract |
|---|---|---|
| pH | 4.19 ± 0.09 | 4.65 ± 0.25 |
| Density (g/mL) | 1.012 ± 0.001 | 1.002 ± 0.001 |
| Humidity (%) | 99.35 ± 0.162 | - |
| Fiber Content (%) | 0.6 ± 0.13 | - |
| Functional Groups | Aloe barbadensis Hydrogels | Basic Hydrogel | |
|---|---|---|---|
| O–H stretching | Alcohols and phenols | + | + |
| N–H stretching | Amines | + | + |
| C–H stretching | Alkanes, | + | + |
| C=O stretching | Aldehydes and ketones | + | + |
| C=C stretching | Alkenes | + | – |
| C=C aromatics | Alkenes | + | – |
| C=O curves | Aldehydes and ketones | + | – |
| C=C curves | Alkenes | + | – |
| C–C curves | Alkanes | + | + |
| C–N stretching | Nitriles e Amines | + | + |
| C–O stretching | Alcohols, Aldehydes and ketones | + | + |
| Microdilution of Aloe barbadensis (µg/mL) | Gram Negative Bacteria | Gram Positive Bacteria | Gram Positive Fungi | ||
|---|---|---|---|---|---|
| Enterobacter spp. | E. coli | Salmonella entérica | S. aureus | Candida albicans | |
| 1000 | N A | NA | A | A | NA |
| 500 | NA | NA | A | A | NA |
| 250 | NA | NA | A | A | NA |
| 125 | NA | NA | A | NA | NA |
| 62.5 | NA | NA | A | NA | NA |
| 31.25 | NA | NA | NA | NA | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alesa Gyles, D.; Pereira Júnior, A.D.; Diniz Castro, L.; Santa Brigida, A.; Nobre Lamarão, M.L.; Ramos Barbosa, W.L.; Carréra Silva Júnior, J.O.; Ribeiro-Costa, R.M. Polyacrylamide-Metilcellulose Hydrogels Containing Aloe barbadensis Extract as Dressing for Treatment of Chronic Cutaneous Skin Lesions. Polymers 2020, 12, 690. https://doi.org/10.3390/polym12030690
Alesa Gyles D, Pereira Júnior AD, Diniz Castro L, Santa Brigida A, Nobre Lamarão ML, Ramos Barbosa WL, Carréra Silva Júnior JO, Ribeiro-Costa RM. Polyacrylamide-Metilcellulose Hydrogels Containing Aloe barbadensis Extract as Dressing for Treatment of Chronic Cutaneous Skin Lesions. Polymers. 2020; 12(3):690. https://doi.org/10.3390/polym12030690
Chicago/Turabian StyleAlesa Gyles, Desireé, Anivaldo Duarte Pereira Júnior, Lorena Diniz Castro, Andressa Santa Brigida, Maria Louze Nobre Lamarão, Wagner Luiz Ramos Barbosa, José Otávio Carréra Silva Júnior, and Roseane Maria Ribeiro-Costa. 2020. "Polyacrylamide-Metilcellulose Hydrogels Containing Aloe barbadensis Extract as Dressing for Treatment of Chronic Cutaneous Skin Lesions" Polymers 12, no. 3: 690. https://doi.org/10.3390/polym12030690







