Bovine Decellularized Amniotic Membrane: Extracellular Matrix as Scaffold for Mammalian Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Decellularization of the BAM
2.2. Determination of DNA Content
2.2.1. Extraction of DNA
2.2.2. DNA Quantification
2.3. Cell Culture
Cell Seeding on DM and 3-(4,5-d imethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) Assay
2.4. Histological Analysis with Hematoxylin-Eosin
2.5. Scanning Electron Microscopy (SEM)
2.6. FTIR-ATR Spectroscopy
2.7. Differential Scanning Calorimetry (DSC)
2.8. Statistic Analysis
3. Results and Discussion
3.1. Decellularization of BAM and DNA Content
3.2. MTT Assay
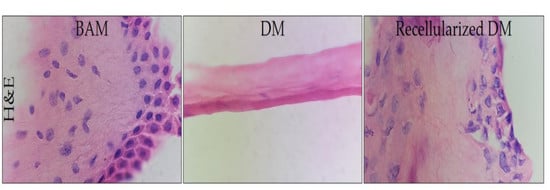
3.3. Histological Analysis
3.4. Scanning Electron Microscopy
3.5. FTIR-ATR Spectroscopy
3.6. Differential Scanning Calorimetry (DSC)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Parmaksiz, M.; Elcin, A.E.; Elcin, Y.M. Decellularization of bovine small intestinal submucosa and its use for the healing of a critical-sized full-thickness skin defect, alone and in combination with stem cells, in a small rodent model. J. Tissue Eng. Regen. Med. 2017, 11, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.E. Hybrid Biomaterials for Skin Tissue Engineering. In Skin Tissue Engineering and Regenerative Medicine; Elsevier: Winston-Salem, NC, USA, 2016; pp. 185–210. [Google Scholar]
- Downes, S.; Mishra, A.A. Tissue-biomaterial interactions. In Advanced Wound Repair Therapies; Elsevier Inc.: Cornwall, UK, 2011; pp. 174–185. ISBN 9781845697006. [Google Scholar]
- Naasani, L.S.; Damo Souza, A.F.; Rodrigues, C.; Vedovatto, S.; Azevedo, J.G.; Santin Bertoni, A.P.; Da Cruz Fernandes, M.; Buchner, S.; Wink, M.R. Decellularized human amniotic membrane associated with adipose derived mesenchymal stromal cells as a bioscaffold: Physical, histological and molecular analysis. Biochem. Eng. J. 2019, 107366. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Skin tissue regeneration. In Electrospinning for Tissue Regeneration; Elsevier: Cornwall, UK, 2011; pp. 298–316. [Google Scholar]
- Somuncu, Ö.S.; ßak Ballica, B.; Furkan Temiz, A.; Somuncu, D. Experimental study In vitro artificial skin engineering by decellularized placental scaffold for secondary skin problems of meningomyelocele. J. Clin. Neurosci. 2019, 59, 291–297. [Google Scholar] [CrossRef]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Park, S.M.; Yang, S.; Rye, S.-M.; Choi, S.W. Effect of pulsatile flow perfusion on decellularization. Biomed. Eng. Online 2018, 17, 15. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Singh, D.; Han, S. 3D Printing of Scaffold for Cells Delivery: Advances in Skin Tissue Engineering. Polymers 2016, 8, 19. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef]
- Catalano, E.; Cochis, A.; Varoni, E.; Rimondini, L.; Azzimonti, B. Tissue-engineered skin substitutes: An overview. J. Artif. Organs 2013, 16, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2018, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Nyström, A.; Bernasconi, R.; Bornert, O. Therapies for genetic extracellular matrix diseases of the skin. Matrix Biol. 2018, 71–72, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Gubbiotti, M.A. Extracellular matrix: The driving force of mammalian diseases. 2017, 71, 1–9. Matrix Biol. 2017, 71, 1–9. [Google Scholar]
- Waldeck, H.M.; Guerra, A.D.; Kao, W.J. Extracellular Matrix: Inspired Biomaterials. Compr. Biomater. II 2017, 2, 132–146. [Google Scholar] [CrossRef]
- Chalikias, G.K.; Tziakas, D.N. Biomarkers of the extracellular matrix and of collagen fragments. Clin. Chim. Acta 2015, 443, 39–47. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Reprint of: Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2015, 23, S17–S26. [Google Scholar] [CrossRef]
- De Castro Brás, L.E.; Ramirez, T.A.; Deleon-Pennell, K.Y.; Chiao, Y.A.; Ma, Y.; Dai, Q.; Halade, G.V.; Hakala, K.; Weintraub, S.T.; Lindsey, M.L. Texas 3-Step decellularization protocol: Looking at the cardiac extracellular matrix. J. Proteomics 2013, 86, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Vyas, K.; Vasconez, H.; Vyas, K.S.; Vasconez, H.C. Wound Healing: Biologics, Skin Substitutes, Biomembranes and Scaffolds. Healthcare 2014, 2, 356–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Wei, Y.; Hung, C.; Vunjak-Novakovic, G. Chondrogenic properties of collagen type XI, a component of cartilage extracellular matrix. Biomaterials 2018, 173, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, M.; Łabuś, W.; Klama-Baryla, A.; Kitala, D.; Kraut, M.; Glik, J.; Misiuga, M.; Nowak, M.; Bielecki, T.; Kasperczyk, A. A review of decellurization methods caused by an urgent need for quality control of cell-free extracellular matrix’ scaffolds and their role in regenerative medicine. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K. Innovations and Advances in Wound Healing; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-46586-8. [Google Scholar]
- Milan, P.B.; Kargozar, S.; Joghataie, M.T.; Samadikuchaksaraei, A. Nanoengineered biomaterials for skin regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, Netherlands, 2019; pp. 265–283. [Google Scholar]
- Hany Hussein, K.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng.: C 2016, 67, 766–788. [Google Scholar] [CrossRef] [PubMed]
- Dunckel, A.P. Acellular bovine-derived matrix used on a traumatic crush injury of the hand: A case study. Ostomy Wound Manag. 2009, 55, 44–49. [Google Scholar]
- Wurzer, P.; Keil, H.; Branski, L.K.; Parvizi, D.; Clayton, R.P.; Finnerty, C.C.; Herndon, D.N.; Kamolz, L.P. The use of skin substitutes and burn careda survey. J. Surg. Res. 2016, 201, 293–298. [Google Scholar] [CrossRef]
- Scarritt, M.; Murdock, M.; Badylak, S.F. Biologic Scaffolds Composed of Extracellular Matrix for Regenerative Medicine. Princ. Regen. Med. 2019, 613–626. [Google Scholar] [CrossRef]
- Barreto, R.d.S.N.; Romagnolli, P.; Mess, A.M.; Miglino, M.A. Decellularized bovine cotyledons may serve as biological scaffolds with preserved vascular arrangement. J. Tissue Eng. Regen. Med. 2018, 12, e1880–e1888. [Google Scholar] [CrossRef]
- Nyame, T.T.; Chiang, H.A.; Orgill, D.P. Clinical Applications of Skin Substitutes. Surg. Clin. NA 2014, 94, 839–850. [Google Scholar] [CrossRef]
- Yamamoto, T.; Iwase, H.; King, T.W.; Hara, H.; Cooper, D.K.C. Skin xenotransplantation: Historical review and clinical potential. Burns 2018, 44, 1738–1749. [Google Scholar] [CrossRef]
- Macleod, T.M.; Sarathchandra, P.; Williams, G.; Sanders, R.; Green, C.J. Evaluation of a porcine origin acellular dermal matrix and small intestinal submucosa as dermal replacements in preventing secondary skin graft contraction. Burns 2004, 30, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Schallberger, S.P.; Stanley, B.J.; Hauptman, J.G.; Steficek, B.A. Effect of Porcine Small Intestinal Submucosa on Acute Full-Thickness Wounds in Dogs. Vet. Surg. 2008, 37, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Brown-Etris, M.; Milne, C.T.; Hodde, J.P. An extracellular matrix graft (Oasis ® wound matrix) for treating full-thickness pressure ulcers: A randomized clinical trial. J. Tissue Viability 2018, 28, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Middelkoop, E.; Sheridan, R.L. Skin Substitutes and ‘the next level’. Total Burn Care 2018, 167–173. [Google Scholar] [CrossRef]
- Chawla, R.; Seifalian, A.; Moiemen, N.S.; Butler, P.E.; Seifalian, A.M. The Use of Skin Substitutes in the Treatment of Burns. Regen. Med. Appl. Organ Transplant. 2014, 771–782. [Google Scholar] [CrossRef]
- Haddad, A.G.; Giatsidis, G.; Orgill, D.P.; Halvorson, E.G. Skin Substitutes and Bioscaffolds Temporary and Permanent Coverage. Clin. Plast. Surg. 2017, 44, 627–634. [Google Scholar] [CrossRef]
- Halim, A.; Khoo, T.; Shah, J.Y. Biologic and synthetic skin substitutes: An overview. Indian J. Plast. Surg. 2010, 43, 23. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Saffle, J.R. Closure of the Excised Burn Wound: Temporary Skin Substitutes. Clin. Plast. Surg. 2009, 36, 627–641. [Google Scholar] [CrossRef]
- Park, M.; Kim, S.; Kim, I.S.; Son, D. Healing of a porcine burn wound dressed with human and bovine amniotic membranes. Wound Repair Regen. 2008, 16, 520–528. [Google Scholar] [CrossRef]
- Valladares, M. Estudio de Tres Diferentes Métodos de Preparación y Conservación de la Membrana Amniótica Para Usos Oftalmológicos; Universidade de Santiago de Compostela: Sandriago de Compostela, Spain, 2008. [Google Scholar]
- Francisco, J.C.; Correa Cunha, R.; Cardoso, M.A.; Baggio Simeoni, R.; Mogharbel, B.F.; Picharski, G.L.; Silva Moreira Dziedzic, D.; Guarita-Souza, L.C.; Carvalho, K.A.T. Decellularized Amniotic Membrane Scaffold as a Pericardial Substitute: An In Vivo Study. Transplant. Proc. 2016, 48, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Long, D.; Hsu, C.-C.; Liu, H.; Chen, L.; Slavin, B.; Lin, H.; Li, X.; Tang, J.; Yiu, S.; et al. Nanofiber-reinforced decellularized amniotic membrane improves limbal stem cell transplantation in a rabbit model of corneal epithelial defect. Acta Biomater. 2019, 97, 310–320. [Google Scholar] [CrossRef] [PubMed]
- da Anunciação, A.; Mess, A.; Orechio, D.; Aguiar, B.; Favaron, P.; Miglino, M. Extracellular matrix in epitheliochorial, endotheliochorial and haemochorial placentation and its potential application for regenerative medicine. Reprod. Domest. Anim. 2017, 52, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Yoon, J.Y.; Park, S.Y.; Kwon, H.H.; Suh, D.H. Clinical effect of bovine amniotic membrane and hydrocolloid on wound by laser treatment: Prospective comparative randomized clinical trial. Wound Repair Regen. 2014, 22, 212–219. [Google Scholar] [CrossRef]
- Leonel, L.; Miranda, C.; Coelho, T.; Ferreira, G.; Cañada, R.; Miglino, M.; Lobo, S. Decellularization of placentas: Establishing a protocol. Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Kang, M.; Choi, S.; Cho Lee, A.-R. Effect of freeze dried bovine amniotic membrane extract on full thickness wound healing. Arch. Pharm. Res. 2013, 36, 472–478. [Google Scholar] [CrossRef]
- Sanluis-Verdes, A.; Yebra-Pimentel Vilar, M.T.; García-Barreiro, J.J.; García-Camba, M.; Ibáñez, J.S.; Doménech, N.; Rendal-Vázquez, M.E. Production of an acellular matrix from amniotic membrane for the synthesis of a human skin equivalent. Cell Tissue Bank. 2015, 16, 411–423. [Google Scholar] [CrossRef]
- Favaron, P.; Carvalho, R.; Borghesi, J.; Anunciação, A.; Miglino, M. The Amniotic Membrane: Development and Potential Applications - A Review. Reprod. Domest. Anim. 2015, 50, 881–892. [Google Scholar] [CrossRef]
- Milan, P.B.; Amini, N.; Joghataei, M.T.; Ebrahimi, L.; Amoupour, M.; Sarveazad, A.; Kargozar, S.; Mozafari, M. Decellularized human amniotic membrane: From animal models to clinical trials. Methods 2019. [Google Scholar] [CrossRef]
- Bruyneel, A.A.N.; Carr, C.A. Ambiguity in the Presentation of Decellularized Tissue Composition: The Need for Standardized Approaches. Artif. Organs 2017, 41, 778–784. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Freund, J.; Badylak, S.F. Quantification of DNA in Biologic Scaffold Materials. J. Surg. Res. 2009, 152, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Valencia, M.; Patiño-Vargas, M.; Correa-Londoño, L.; Restrepo-Múnera, L. Evaluación del método químico-enzimático de descelularización para la obtención de matrices extracelulares de tráquea en el modelo porcino. Iatreia 2016, 29, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.-C.; Kuo, Y.-J.; Sun, F.-W.; Chen, C.-H.; Chiang, C.-J.; Weng, P.-W.; Tsuang, Y.-H.; Huang, Y.-Y. Optimized decellularization protocol including α-Gal epitope reduction for fabrication of an acellular porcine annulus fibrosus scaffold. Cell Tissue Bank. 2017, 18, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed. Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Olgierd, B.; Sklarek, A.; Siwek, P.; Waluga, E. Methods of Biomaterial-Aided Cell or Drug Delivery: Extracellular Matrix Proteins as Biomaterials. Stem Cells Biomater. Regen. Med. 2019, 163–189. [Google Scholar] [CrossRef]
- Vargas, N.; González, C. Técnicas de Cultivos Celulares e Ingeniería de Tejidos; México, 2016; ISBN 978-607-28-0688-7. [Google Scholar]
- Ganjibakhsh, M.; Mehraein, F.; Koruji, M.; Aflatoonian, R.; Farzaneh, P. Three-dimensional decellularized amnion membrane scaffold as a novel tool for cancer research; cell behavior, drug resistance and cancer stem cell content. Mater. Sci. Eng. C 2019, 100, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Zvarova, B.; Uhl, F.E.; Uriarte, J.J.; Borg, Z.D.; Coffey, A.L.; Bonenfant, N.R.; Weiss, D.J.; Wagner, D.E. Residual Detergent Detection Method for Nondestructive Cytocompatibility Evaluation of Decellularized Whole Lung Scaffolds. Tissue Eng. Part C Methods 2016, 22, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.-S.; Vaas, A.P.J.P.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, W.; Ahn, G.; Lee, D.-S.; et al. A keratinocyte and integrated fibroblast culture model for studying particulate matter-induced skin lesions and therapeutic intervention of fucosterol. Life Sci. 2019, 233, 116714. [Google Scholar] [CrossRef]
- Celik, S.B.; Dominici, S.R.; Filby, B.W.; Das, A.A.; Madden, L.A.; Paunov, V.N. Fabrication of Human Keratinocyte Cell Clusters for Skin Graft Applications by Templating Water-in-Water Pickering Emulsions. Biomimetics 2019, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Sapru, S.; Das, S.; Mandal, M.; Ghosh, A.K.; Kundu, S.C. Nonmulberry silk protein sericin blend hydrogels for skin tissue regeneration - in vitro and in vivo. Int. J. Biol. Macromol. 2019, 137, 545–553. [Google Scholar] [CrossRef]
- Izadyari Aghmiuni, A.; Heidari Keshel, S.; Sefat, F.; Akbarzadeh Khiyavi, A. Quince seed mucilage-based scaffold as a smart biological substrate to mimic mechanobiological behavior of skin and promote fibroblasts proliferation and h-ASCs differentiation into keratinocytes. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Feng, B.; Guo, X.; Wang, J.; Zhao, L.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, W.J. Engineering of epidermis skin grafts using electrospun nanofibrous gelatin/polycaprolactone membranes. Int. J. Nanomed. 2013, 8, 2077–2084. [Google Scholar]
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.A.; D’Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef]
- Wood, F.M. Therapeutic Applications: Tissue Engineering of Skin. Princ. Regen. Med. 2019, 1281–1295. [Google Scholar] [CrossRef]
- Petreaca, M.; Martins-Green, M. Cell–Extracellular Matrix Interactions in Repair and Regeneration. In Principles of Regenerative Medicine; Elsevier: London, UK, 2019; pp. 15–35. [Google Scholar]
- Leonel, L.C.P.C.; Miranda, C.M.F.C.; Coelho, T.M.; Ferreira, G.A.S.; Caãada, R.R.; Miglino, M.A.; Lobo, S.E. Decellularization of placentas: Establishing a protocol. Braz. J. Med Biol. Res. 2017, 51, e6382. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Son, D.; Choi, T.H.; Jung, S.; Kwon, S.; Kim, J.; Han, K. Evaluation of an Amniotic Membrane-Collagen Dermal Substitute in the Management of Full-Thickness Skin Defects in a Pig. Arch. Plast. Surg. 2013, 40, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barraza-Garza, G.; de la Rosa, L.A.; Martínez-Martínez, A.; Castillo-Michel, H.; Cotte, M.; Alvarez-Parrilla, E. La microespectroscopia de infrarrojo con t ransformada de Fourier (FTIRM) en el estudio de sistemas biológicos. Revista Latinoamericana de Química RLQ. 2013, 41, 125–148. [Google Scholar]
- Bernal, A.; Balkova, R.; Kuritka, I.; Saha, P. Preparation and characterisation of a new double-sided bio-artificial material prepared by casting of poly(vinyl alcohol) on collagen. Polym. Bull. 2013, 70, 431–453. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Guo, Z.; Xie, S.; Hu, J.; Lu, H. SR-FTIR as a tool for quantitative mapping of the content and distribution of extracellular matrix in decellularized book-shape bioscaffolds. BMC Musculoskelet. Disord. 2018, 19, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi Ting Au-Yeung, G.; Sarig, U.; Sarig, H.; Bogireddi, H.; Bronshtein, T.; Baruch, L.; Spizzichino, A.; Bortman, J.; Freddy, B.Y.C.; Machluf, M.; et al. Restoring the biophysical properties of decellularized patches through recellularization. Biomater. Sci. 2017, 5, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Liu, F.; Yu, Z.; Chang, B.; Douglas Goff, H.; Zhong, F. Effect of aging treatment on the physicochemical properties of collagen films. Food Hydrocoll. 2018, 87, 436–447. [Google Scholar] [CrossRef]
- Li, M.; Han, M.; Sun, Y.; Hua, Y.; Chen, G.; Zhang, L. Oligoarginine mediated collagen/chitosan gel composite for cutaneous wound healing. Int. J. Biol. Macromol. 2018, 122, 1120–1127. [Google Scholar] [CrossRef]
- Zouhair, S.; Aguiari, P.; Iop, L.; Vásquez-Rivera, A.; Filippi, A.; Romanato, F.; Korossis, S.; Wolkers, W.F.; Gerosa, G. Preservation strategies for decellularized pericardial scaffolds for off-the-shelf availability. Acta Biomater. 2019, 84, 208–221. [Google Scholar] [CrossRef]
- Badea, E.; Della Gatta, G.; Usacheva, T. Effects of temperature and relative humidity on fibrillar collagen in parchment: A micro differential scanning calorimetry (micro DSC) study. Polym. Degrad. Stab. 2012, 97, 346–353. [Google Scholar] [CrossRef]
- Latorre, M.E.; Velázquez, D.E.; Purslow, P.P. Differences in the energetics of collagen denaturation in connective tissue from two muscles. Int. J. Biol. Macromol. 2018, 113, 1294–1301. [Google Scholar] [CrossRef]
- Schroepfer, M.; Meyer, M. DSC investigation of bovine hide collagen at varying degrees of crosslinking and humidities. Int. J. Biol. Macromol. 2017, 103, 120–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Snow, T.; Smith, A.J.; Holmes, G.; Prabakar, S. A guide to high-efficiency chromium (III)-collagen cross-linking: Synchrotron SAXS and DSC study. Int. J. Biol. Macromol. 2019, 126, 123–129. [Google Scholar] [CrossRef]
- Rochdi, A.; Foucat, L.; Renou, J.-P. NMR and DSC studies during thermal denaturation of collagen. Food Chem. 2000, 69, 295–299. [Google Scholar] [CrossRef]
- Bozec, L.; Odlyha, M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarti, B.; Scandola, M. Viscoelastic and thermal properties of collagen/poly(vinyl alcohol) blends. Biomaterials 1995, 16, 785–792. [Google Scholar] [CrossRef]









| No. | Protocols |
|---|---|
| I | SDS 0.1% for 4 h NaOH 0.1 M for 1 h PAA + ascorbic acid 0.1 for 12 h Ethanol 70% for 1 h PBS for 2 h |
| II | SDS 0.1% for 4 h NaOH 0.1 M for 1 h PAA 0.15% + EtOH for 12 h NaOH 0.1 M for 1 h PAA for 1 h Ethanol 70% for 1 h PBS for 2 h |
| III | Tween 80 for 4 h NaOH 0.1 M for 1 h, PAA + ascorbic acid 0.1 for 12 h Ethanol 70% for 1 h PBS for 2 h |
| IV | Tween 80 for 4 h NaOH 0.1 M for 1 h PAA 0.15% + EtOH for 12 h NaOH 0.1 M for 1 h PAA for 1 h Ethanol 70% for 1 h PBS for 2 h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villamil Ballesteros, A.C.; Segura Puello, H.R.; Lopez-Garcia, J.A.; Bernal-Ballen, A.; Nieto Mosquera, D.L.; Muñoz Forero, D.M.; Segura Charry, J.S.; Neira Bejarano, Y.A. Bovine Decellularized Amniotic Membrane: Extracellular Matrix as Scaffold for Mammalian Skin. Polymers 2020, 12, 590. https://doi.org/10.3390/polym12030590
Villamil Ballesteros AC, Segura Puello HR, Lopez-Garcia JA, Bernal-Ballen A, Nieto Mosquera DL, Muñoz Forero DM, Segura Charry JS, Neira Bejarano YA. Bovine Decellularized Amniotic Membrane: Extracellular Matrix as Scaffold for Mammalian Skin. Polymers. 2020; 12(3):590. https://doi.org/10.3390/polym12030590
Chicago/Turabian StyleVillamil Ballesteros, Andrea Catalina, Hugo Ramiro Segura Puello, Jorge Andres Lopez-Garcia, Andres Bernal-Ballen, Diana Lorena Nieto Mosquera, Diana Milena Muñoz Forero, Juan Sebastián Segura Charry, and Yuli Alexandra Neira Bejarano. 2020. "Bovine Decellularized Amniotic Membrane: Extracellular Matrix as Scaffold for Mammalian Skin" Polymers 12, no. 3: 590. https://doi.org/10.3390/polym12030590