Thermal and Calorimetric Evaluations of Some Chemically Modified Carbohydrate-Based Substrates with Phosphorus-Containing Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthetic Procedures
Chemical Modification of the Base Substrates
2.2.2. Characterization Techniques
Spectroscopic Analyses (Solid-State NMR)
Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
Thermogravimetric Analysis (TGA)
Pyrolysis Combustion Flow Calorimetry (PCFC)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xuan, S.; Xing, W.; Bai, Z.; Lu, H. Flame retardancy and thermal degradation of intumescent flame retardant poly (lactic acid)/starch biocomposites. Ind. Eng. Chem. Res. 2010, 50, 713–720. [Google Scholar] [CrossRef]
- Demirgöz, D.; Elvira, C.; Mano, J.F.; Cunha, A.M.; Piskin, E.; Reis, R.L. Chemical modification of starch based biodegradable polymeric blends: Effects on water uptake, degradation behaviour and mechanical properties. Polym. Degrad. Stab. 2000, 70, 161–170. [Google Scholar] [CrossRef]
- Kishore, K.; Mohandas, K. Action of phosphorus compounds on fire-retardancy of cellulosic materials: A review. Fire Mater. 1982, 6, 54–58. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsiue, G.H.; Chiu, Y.S.; Jeng, R.J.; Ma, C. Synthesis and flame-retardant properties of phosphorus-containing polymers based on poly (4-hydroxystyrene). J. Appl. Polym. Sci. 1996, 59, 1619–1625. [Google Scholar] [CrossRef]
- Matko, S.; Toldy, A.; Keszei, S.; Anna, P.; Bertalan, G.; Marosi, G. Flame retardancy of biodegradable polymers and biocomposites. Polym. Degrad. Stab. 2005, 88, 138–145. [Google Scholar] [CrossRef]
- Keglevich, G.; Bagi, P.; Rapi, Z.; Bako, P.; Drahos, L.; Szolnoki, B.; Marosi, G. The Synthesis of Bio-Based Flame-Retarded Epoxy-Precursors. Macromol. Symp. 2015, 352, 46–50. [Google Scholar] [CrossRef]
- Blennow, A.; Nielsen, T.H.; Baunsgaard, L.; Mikkelsen, R.; Engelsen, S.B. Starch phosphorylation: A new front line in starch research. Trends Plant Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef]
- Passauer, L.; Liebner, F.; Fischer, K. Starch phosphate hydrogels. Part I: Synthesis by mono-phosphorylation and cross-linking of starch. Starch Stärke 2009, 61, 621–627. [Google Scholar] [CrossRef]
- Muhammad, K.; Hussin, F.; Man, Y.C.; Ghazali, H.M.; Kennedy, J.F. Effect of pH on phosphorylation of sago starch. Carbohydr. Polym. 2000, 42, 85–90. [Google Scholar] [CrossRef]
- Blennow, A.; Engelsen, S.B.; Munck, L.; Moller, B.L. Starch molecular structure and phosphorylation investigated by a combined chromatographic and chemometric approach. Carbohydr. Polym. 2000, 41, 163–174. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Green, J. Mechanisms for flame retardancy and smoke suppression-a review. J. Fire Sci. 1996, 14, 426–442. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Kandola, B.K.; Davies, P.J.; Zhang, S.; Padbury, S. Developments in flame retardant textiles—A review. Polym. Degrad. Stab. 2005, 88, 3–12. [Google Scholar] [CrossRef]
- Horrocks, A.R. Developments in flame retardants for heat and fire resistant textiles—The role of char formation and intumescence. Polym. Degrad. Stab. 1996, 54, 143–154. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N. Pyrolysis combustion flow calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27–46. [Google Scholar] [CrossRef]
- Joseph, P.; Tretsiakova-Mcnally, S. Reactive modifications of some chain-and step-growth polymers with phosphorus-containing compounds: Effects on flame retardance—A review. Polym. Adv. Technol. 2011, 22, 395–406. [Google Scholar] [CrossRef]
- Tretsiakova-McNally, S.; Joseph, P. Thermal and calorimetric evaluations of polyacrylonitrile containing covalently-bound phosphonate groups. Polymers 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tretsiakova-McNally, S.; Joseph, P. Pyrolysis combustion flow calorimetry studies on some reactively modified polymers. Polymers 2015, 7, 453–467. [Google Scholar] [CrossRef] [Green Version]
- Joseph, P.; Tretsiakova-McNally, S. Combustion behaviours of chemically modified polyacrylonitrile polymers containing phosphorylamino groups. Polym. Degrad. Stab. 2012, 97, 2531–2535. [Google Scholar] [CrossRef]

















| Sl. No. | Substrate | Sample Weight (g) | DECP (g) | DCM (cm3) | TEA (cm3) | Recovered Yield (wt %) | P (wt %) |
|---|---|---|---|---|---|---|---|
| 1 | β-cyclodextrin | 2.5 | 2.63 | 40 | 5 | 49.90 | 1.7 |
| 2 | Dextran | 2.4 | 2.63 | 40 | 5 | 79.15 | 1.0 |
| 3 | Potato starch | 2.3 | 2.63 | 40 | 5 | 55.17 | 3.2 |
| 4 | Agar agar | 2.7 | 2.63 | 40 | 5 | 66.45 | 5.55 |
| 5 | Tamarind | 2.8 | 2.63 | 40 | 5 | 68.23 | 6.68 |
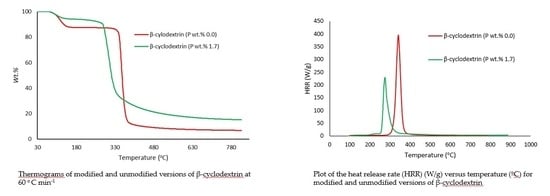
| Sample | P (wt %) | Induction Temperature (°C) | Temperature at 50 wt % (°C) | Residue at 400 °C (wt %) | Final Residue at 800 °C (wt %) |
|---|---|---|---|---|---|
| β-cyclodextrin | 0.0 | 94.0 | 356 | 11.4 | 6.00 |
| β-cyclodextrin | 1.7 | 96.0 | 334 | 39.7 | 22.9 |
| Dextran | 0.0 | 85.0 | 340 | 15.2 | 9.80 |
| Dextran | 1.0 | 93.0 | 273 | 29.3 | 18.7 |
| Potato Starch | 0.0 | 93.0 | 335 | 18.3 | 11.9 |
| Potato Starch | 3.2 | 129 | 366 | 45.6 | 25.4 |
| Agar agar | 0.0 | 77.0 | 333 | 36.7 | 14.8 |
| Agar agar | 5.5 | 90.0 | 280 | 36.9 | 23.7 |
| Tamarind | 0.0 | 86.0 | 357 | 38.5 | 21.3 |
| Tamarind | 6.6 | 105 | 338 | 39.6 | 24.0 |
| Sample | P (wt %) | Temp to pHRR (°C) | pHRR (W/g) | THR (kJ/g) | Heat Release Capacity (J/g K) | Char Yield (wt %) |
|---|---|---|---|---|---|---|
| β-cyclodextrin | 0.0 | 342 | 452.7 | 11.6 | 459 | 11.11 |
| β-cyclodextrin | 1.7 | 290 | 66.02 | 4.20 | 67.0 | 25.54 |
| Dextran | 0.0 | 319 | 289.0 | 10.4 | 288 | <1.0 |
| Dextran | 1.0 | 252 | 199.9 | 7.50 | 217 | 23.52 |
| Potato Starch | 0.0 | 310 | 362.8 | 10.4 | 368 | 12.50 |
| Potato Starch | 3.2 | 260 | 57.18 | 3.90 | 60.0 | 31.85 |
| Agar agar | 0.0 | 272 | 256.0 | 12.3 | 250 | 3.680 |
| Agar agar | 5.5 | 234 | 219.4 | 8.30 | 234 | 23.40 |
| Tamarind | 0.0 | 326 | 158.0 | 10.0 | 155 | 25.12 |
| Tamarind | 6.6 | 291 | 126.0 | 8.10 | 128 | 29.36 |
| Sample | P (wt %) | Pyrolysis Residue (g/g) | hc (kJ/g) |
|---|---|---|---|
| β-cyclodextrin | 0.0 | 0.11 | 13.03 |
| β-cyclodextrin | 1.7 | 0.25 | 5.600 |
| Dextran | 0.0 | — * | — |
| Dextran | 1.0 | 0.27 | 6.980 |
| Potato Starch | 0.0 | 0.23 | 11.81 |
| Potato Starch | 3.2 | 0.31 | 5.650 |
| Agar agar | 0.0 | 0.036 | 12.75 |
| Agar agar | 5.5 | 0.23 | 10.78 |
| Tamarind | 0.0 | 0.25 | 13.30 |
| Tamarind | 6.6 | 0.27 | 11.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, A.; Joseph, P.; Moinuddin, K.; Zhu, H.; Tretsiakova-McNally, S. Thermal and Calorimetric Evaluations of Some Chemically Modified Carbohydrate-Based Substrates with Phosphorus-Containing Groups. Polymers 2020, 12, 588. https://doi.org/10.3390/polym12030588
Thomas A, Joseph P, Moinuddin K, Zhu H, Tretsiakova-McNally S. Thermal and Calorimetric Evaluations of Some Chemically Modified Carbohydrate-Based Substrates with Phosphorus-Containing Groups. Polymers. 2020; 12(3):588. https://doi.org/10.3390/polym12030588
Chicago/Turabian StyleThomas, Ananya, Paul Joseph, Khalid Moinuddin, Haijin Zhu, and Svetlana Tretsiakova-McNally. 2020. "Thermal and Calorimetric Evaluations of Some Chemically Modified Carbohydrate-Based Substrates with Phosphorus-Containing Groups" Polymers 12, no. 3: 588. https://doi.org/10.3390/polym12030588