Reduced Coefficients of Linear Thermal Expansion of Colorless and Transparent Semi-Alicyclic Polyimide Films via Incorporation of Rigid-Rod Amide Moiety: Preparation and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.3. PI Synthesis and Film Preparation
3. Results and Discussion
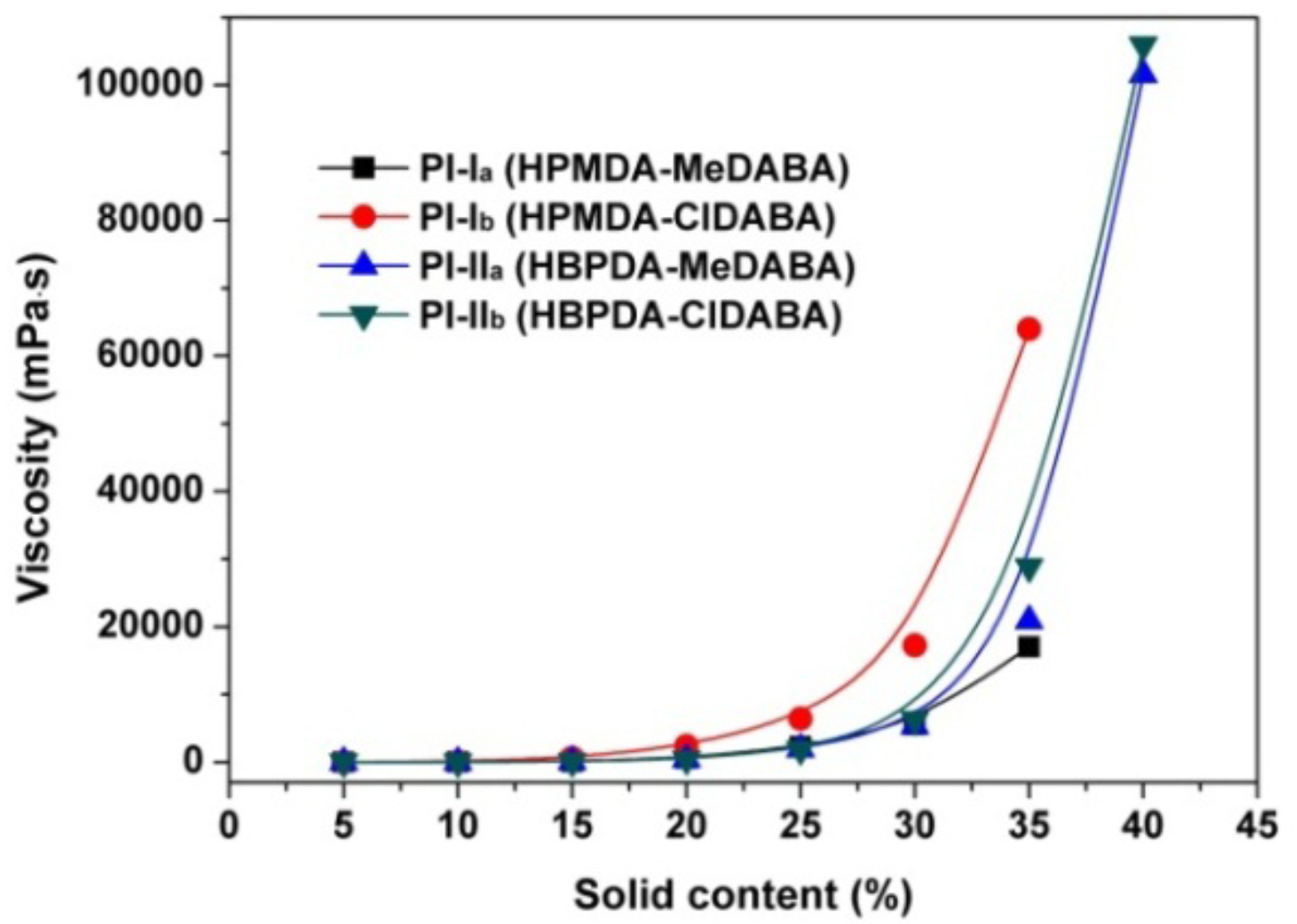
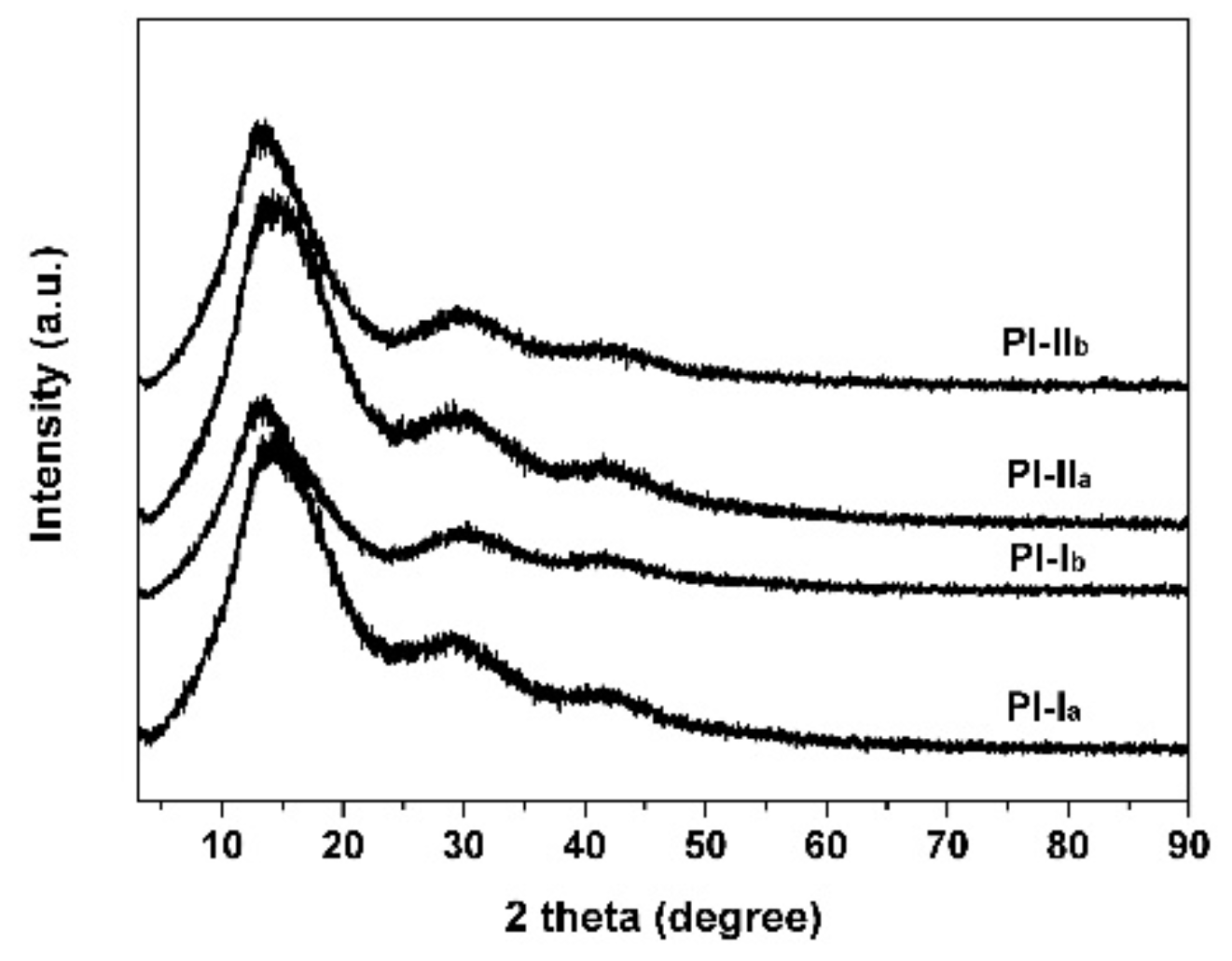
3.1. PI Synthesis and Film Preparation
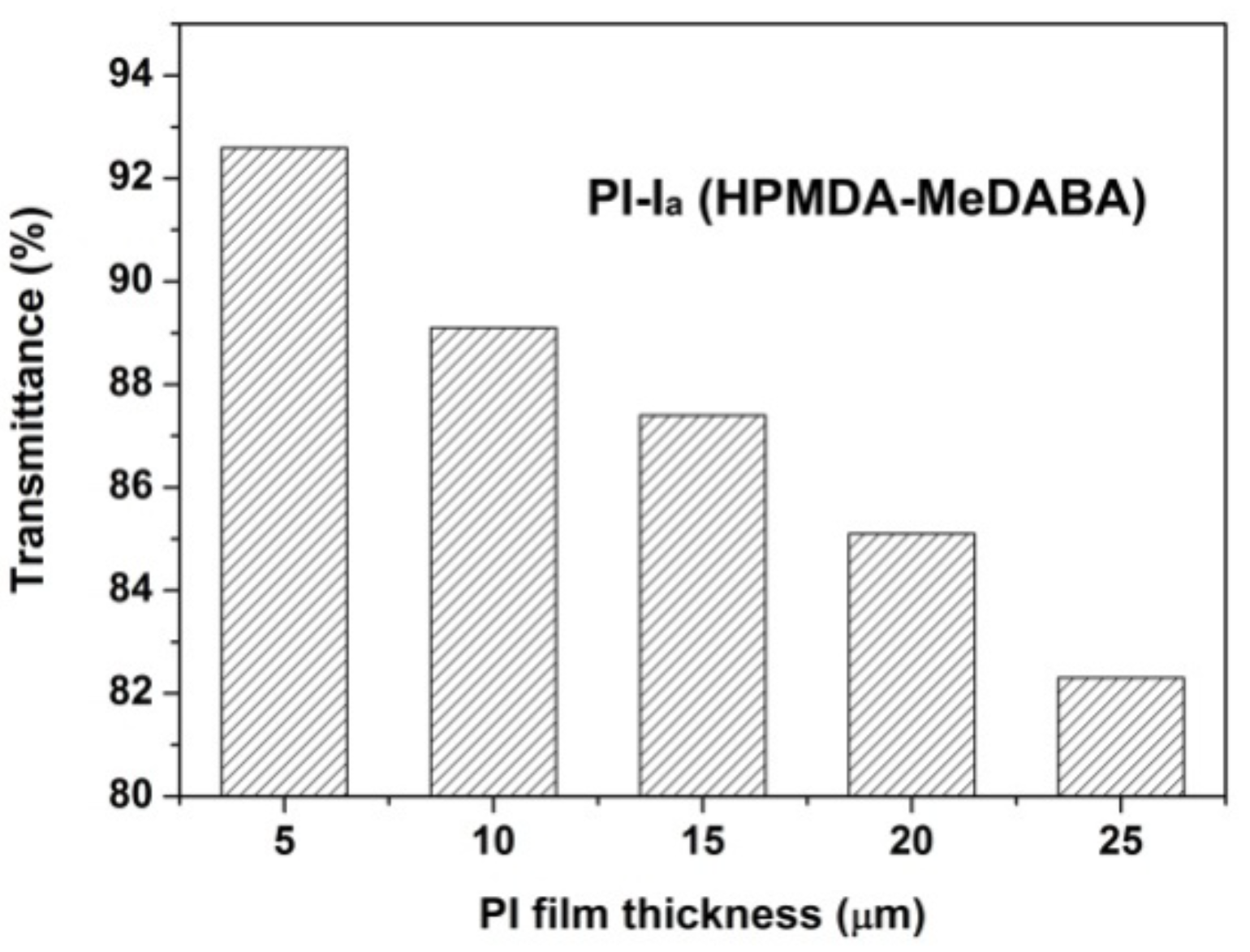
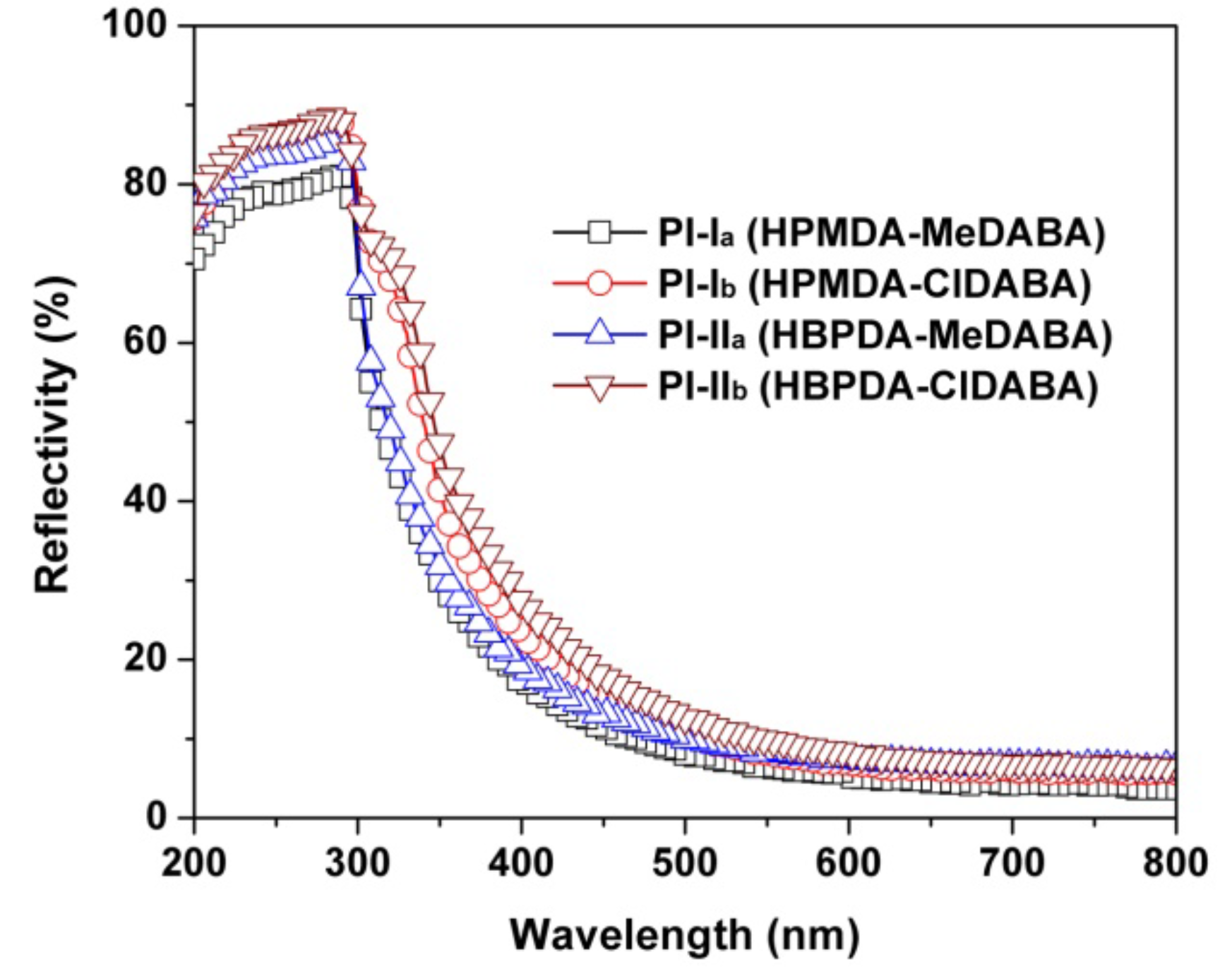
3.2. Optical Properties
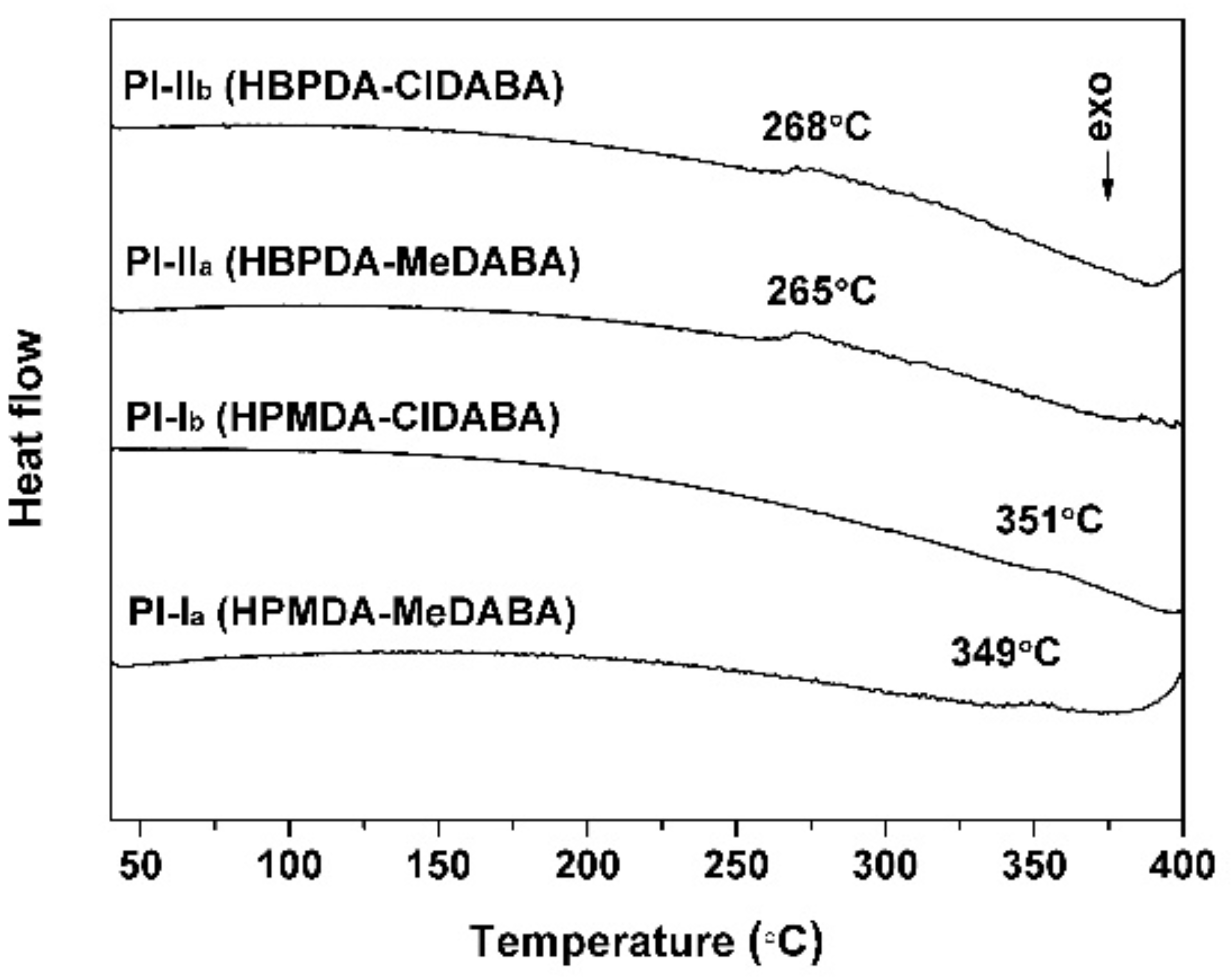
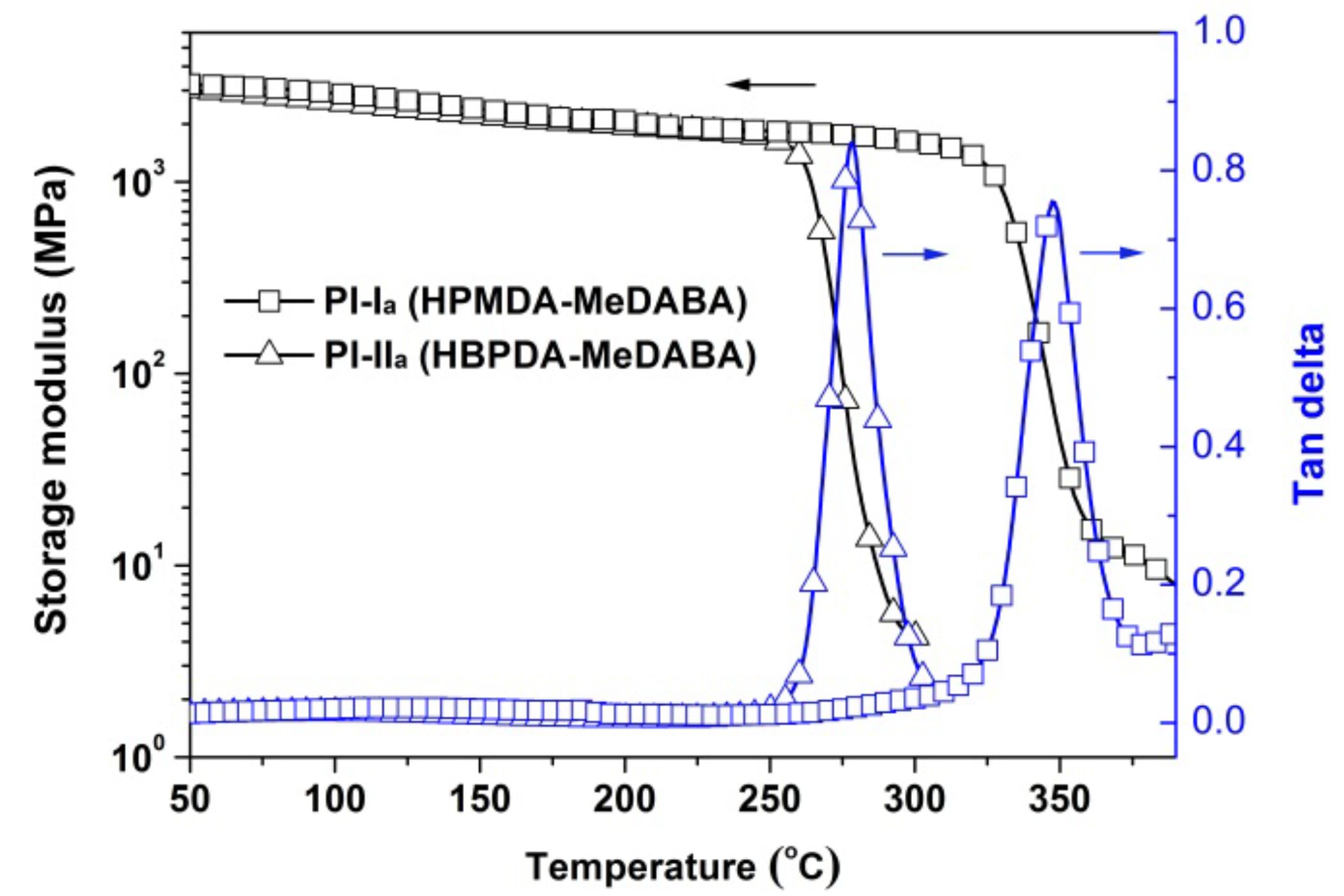
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, X.; Liu, L.; Liu, Y.; Huang, X. LTPS TFT process on polyimide substrate for flexible AMOLED. J. Disp. Technol. 2015, 11, 666–669. [Google Scholar] [CrossRef]
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A review on high temperature resistant polyimide films: Heterocyclic structures and nanocomposites. Compo. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Spechler, J.A.; Koh, T.W.; Herb, J.T.; Rand, B.P.; Arnold, C.B. A transparent, smooth, thermally robust, conductive polyimide for flexible electronics. Adv. Funct. Mater. 2015, 48, 7428–7434. [Google Scholar] [CrossRef]
- Zhang, Q.; Tsai, C.Y.; Li, L.J.; Liaw, D.J. Colorless-to-colorful switching electrochromic polyimides with high contrast ratio. Nat. Commun. 2019, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Tapaswi, P.K.; Ha, C.S. Recent trends on transparent colorless polyimides with balanced thermal and optical properties: Design and synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Yang, Y.; Park, J.H.; Jung, Y.; Lee, S.G.; Park, S.K.; Kwon, S. Effect of fluorination on haze reduction in transparent polyimide films for flexible substrates. J. Appl. Polym. Sci. 2017, 134, 44375. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Solar Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Zhang, X.M.; Song, Y.Z.; Liu, J.G.; Yang, S.Y. Synthesis and properties of cost-effective light-color and highly transparent polyimide films from fluorine-containing tetralin dianhydride and aromatic diamines. J. Photopolym. Sci. Technol. 2016, 29, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.A.A.; Song, J.Y.; Lee, J.H.; Kim, S.M.; Kim, Y. Experimental investigation on micro-fabrication of wearable dry-patching flexible substrate using transparent superhydrophobic polyimide. Mater. Res. Exp. 2019, 6, 086434. [Google Scholar] [CrossRef]
- Yu, H.C.; Jung, J.W.; Choi, J.Y.; Chung, C.M. Kinetic study of low-temperature imidization of poly(amic acid)s and preparation of colorless, transparent polyimide films. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1593–1602. [Google Scholar] [CrossRef]
- Yang, Y.; Jung, Y.; Cho, M.D.; Lee, S.G.; Kwon, S. Transient color changes in oxidative-stable fluorinated polyimide film for flexible display substrates. RSC Adv. 2015, 5, 57339–57345. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.Y.; Liu, J.G.; Zhi, X.X.; Huangfu, M.G.; Jiang, G.L.; Wu, X.; Zhang, X.M. Molecular design, synthesis and characterization of intrinsically black polyimide films with high thermal stability and good electrical properties. J. Polym. Res. 2019, 26, 171. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Liu, S.; Chi, Z.; Chen, X.; Xu, J. Flexible and highly fluorescent aromatic polyimide: Design, synthesis, properties, and mechanism. J. Mater. Chem. C 2016, 4, 10509–10517. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.G.; Jiang, G.L.; Zhang, Y.; Guo, C.Y.; Zhang, Y.J.; Qi, L.; Zhang, X.M. Highly transparent preimidized semi-alicyclic polyimide varnishes with low curing temperatures and desirable processing viscosities at high solid contents: Preparation and applications for LED chip passivation. J. Mater. Sci. Mater. Electron. 2019, 30, 549–560. [Google Scholar] [CrossRef]
- Bong, S.; Yeo, H.; Goh, M.; Ku, B.C.; Kim, Y.Y.; Bong, P.H.; Park, B.; You, N.H. Synthesis and characterization of colorless polyimides derived from 4-(4-aminophenoxy)-2,6-dimethylaniline. Macromol. Res. 2016, 24, 1091–1097. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Xie, F.; Liu, Y.; Leng, J. A novel low colored and transparent shape memory copolyimide and its durability in space thermal cycling environments. Polymer 2018, 156, 121–127. [Google Scholar] [CrossRef]
- Abdulhamid, M.; Ma, X.; Ghanem, B.S.; Pinnau, I. Synthesis and characterization of organo-soluble polyimides derived from alicyclic dianhydrides and a dihydroxyl-functionalized spirobisindane diamine. ACS Appl. Polym. Mater. 2019, 1, 63–69. [Google Scholar] [CrossRef]
- Susa, A.; Bijleveld, J.; Santana, M.H.; Garcia, S.J. Understanding the effect of the dianhydride structure on the properties of semiaromatic polyimides containing a biobased fatty diamine. ACS Sustainable Chem. Eng. 2018, 6, 668–678. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Yan, J.L.; Wang, Y.; Mu, H.; Wang, Z.; Cheng, H.; Zhao, F.; Wang, Z. Colorless polyimides derived from 2R,5R,7S,10S-naphthanetetracarboxylic dianhydride. Polym. Chem. 2017, 8, 6165–6172. [Google Scholar] [CrossRef]
- Li, H.; Cheng, G.; Xu, G.; Luo, L. Influence of polyimide on thermal stress evolution in polyimide/Cu thick film composite. J. Mater. Sci. Mater. Electron. 2016, 30, 549–560. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qu, L.; Liu, J.; Wu, X.; Zhang, Y.; Zhang, R.; Qi, H.; Zhang, X. Synthesis and characterization of high-temperature-resistant and optically transparent polyimide coatings for potential applications in quartz optical fibers protection. J. Coating Technol. Res. 2019, 16, 511–520. [Google Scholar] [CrossRef]
- Zhuang, Y.B.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sakamoto, Y.; Tanaka, Y.; Kobayashi, Y. Poly(ester imide)s possessing low coefficients of thermal expansion (CTE) and low water absorption (III). Use of bis(4-aminophenyl)terephthalate and effect of substituents. Eur. Polym. J. 2010, 46, 1510–1524. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kasamatsu, K.; Koseki, K. Colorless poly(ester imide)s derived from hydrogenated trimellitic anhydride. Eur. Polym. J. 2012, 48, 483–498. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishigami, T.; Ishii, J.; Sugiura, K.; Fujii, M. Solution-processable transparent polyimides with low coefficients of thermal expansion and self-orientation behavior induced by solution casting. Eur. Polym. J. 2013, 49, 3657–3672. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horiuchi, M.; Kumakura, K.; Koyama, J. Colorless polyimides with low coefficient of thermal expansion derived from alkyl-substituted cyclobutanetetracarboxylic dianhydrides. Polym. Int. 2013, 63, 486–500. [Google Scholar] [CrossRef]
- Hasegawa, M.; Watanabe, Y.; Tsukuda, S.; Ishii, J. Solution-processable colorless polyimides with ultralow coefficients of thermal expansion for optoelectronic applications. Polym. Int. 2016, 65, 1063–1073. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, Q.; Zhu, L.; Liu, S.; Chi, Z.; Chen, X.; Zhang, Y.; Xu, J. The preparations and water barrier properties of polyimide films containing amide moieties. Polymers 2017, 9, 667. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Fujiwara, Y.; Kitaoka, T.; Oishi, Y.; Shibasaki, Y. Synthesis of highly transparent poly (amide–imide) s based on trimellitic acid and dependence of thermal properties on monomer sequence. React. Funct. Polym. 2016, 108, 78–85. [Google Scholar] [CrossRef]
- Rossander, S.S.; Mathieu, H.W. Substituted Benzanilides and the Process for Their Preparation. US Patent 2150190, 14 March 1939. [Google Scholar]
- Subbaiah, D.V.; Sastry, M.S.; Ramamurthy, V. Molecular polarizability in the structural studies of halogenated pyrimidines. J. Mol. Struct. Theochem. 1982, 87, 105–111. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ishiguro, E.; Nakagawa, S.; Kimura, R. Alicyclic polyimides derived from alkanone bis-spironorbornanetetracarboxylic dianhydrides. J. Photopolym. Sci. Technol. 2013, 26, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Ishiguro, E.; Komatsu, S. Low temperature film-fabrication of hardly soluble alicyclic polyimides with high Tg by a combined chemical and thermal imidization method. J. Photopolym. Sci. Technol. 2014, 27, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride with controlled steric structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]







| PI | [η]inh a (dL/g) | Molecular Weight b | Solubility c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn (× 103 g/mol) | Mw (× 103 g/mol) | PDI | NMP | DMAc | GBL | CPA | THF | ||
| PI-Ia | 1.03 | 22.1 | 39.6 | 1.79 | ++ | ++ | ++ | + | − |
| PI-Ib | 1.16 | 37.6 | 67.2 | 1.29 | ++ | ++ | ++ | + | − |
| PI-IIa | 0.92 | 26.3 | 46.7 | 1.78 | ++ | ++ | ++ | ++ | + |
| PI-IIb | 0.97 | 29.8 | 54.6 | 1.83 | ++ | ++ | ++ | ++ | + |
| PI | Optical Properties a | Thermal Properties b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ (nm) | T400 (%) | L* | a* | b* | Haze (%) | Tg (°C) | T5% (°C) | Rw730 (%) | CTE (× 10−6/K) | |
| PI-Ia | 338 | 82.3 | 94.91 | −0.13 | 2.18 | 2.15 | 349 | 438.1 | 43.9 | 33.4 |
| PI-Ib | 347 | 46.2 | 94.28 | −0.42 | 2.54 | 11.05 | 351 | 466.8 | 50.2 | 27.7 |
| PI-IIa | 341 | 85.8 | 95.41 | −0.17 | 1.92 | 1.07 | 265 | 466.3 | 23.9 | 55.3 |
| PI-IIb | 342 | 44.7 | 91.16 | −1.20 | 16.05 | 10.41 | 268 | 453.9 | 28.1 | 53.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.-l.; Wang, D.-y.; Du, H.-p.; Wu, X.; Zhang, Y.; Tan, Y.-y.; Wu, L.; Liu, J.-g.; Zhang, X.-m. Reduced Coefficients of Linear Thermal Expansion of Colorless and Transparent Semi-Alicyclic Polyimide Films via Incorporation of Rigid-Rod Amide Moiety: Preparation and Properties. Polymers 2020, 12, 413. https://doi.org/10.3390/polym12020413
Jiang G-l, Wang D-y, Du H-p, Wu X, Zhang Y, Tan Y-y, Wu L, Liu J-g, Zhang X-m. Reduced Coefficients of Linear Thermal Expansion of Colorless and Transparent Semi-Alicyclic Polyimide Films via Incorporation of Rigid-Rod Amide Moiety: Preparation and Properties. Polymers. 2020; 12(2):413. https://doi.org/10.3390/polym12020413
Chicago/Turabian StyleJiang, Gang-lan, Dong-yang Wang, Hao-peng Du, Xiao Wu, Yan Zhang, Yao-yao Tan, Lin Wu, Jin-gang Liu, and Xiu-min Zhang. 2020. "Reduced Coefficients of Linear Thermal Expansion of Colorless and Transparent Semi-Alicyclic Polyimide Films via Incorporation of Rigid-Rod Amide Moiety: Preparation and Properties" Polymers 12, no. 2: 413. https://doi.org/10.3390/polym12020413





