Larch Wood Residues Valorization through Extraction and Utilization of High Value-Added Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wood Material
2.2. Chemicals
2.3. Solid-Liquide Extraction
2.4. GC-MS Characterization
2.5. Fourier Transform-RAMAN Spectroscopy
2.6. Fourier Transform Infrared Spectroscopy
2.7. Fourier Transform Near-Infrared Spectroscopy
2.8. Data Analysis
3. Results and Discussion
3.1. GC-MS Characterization
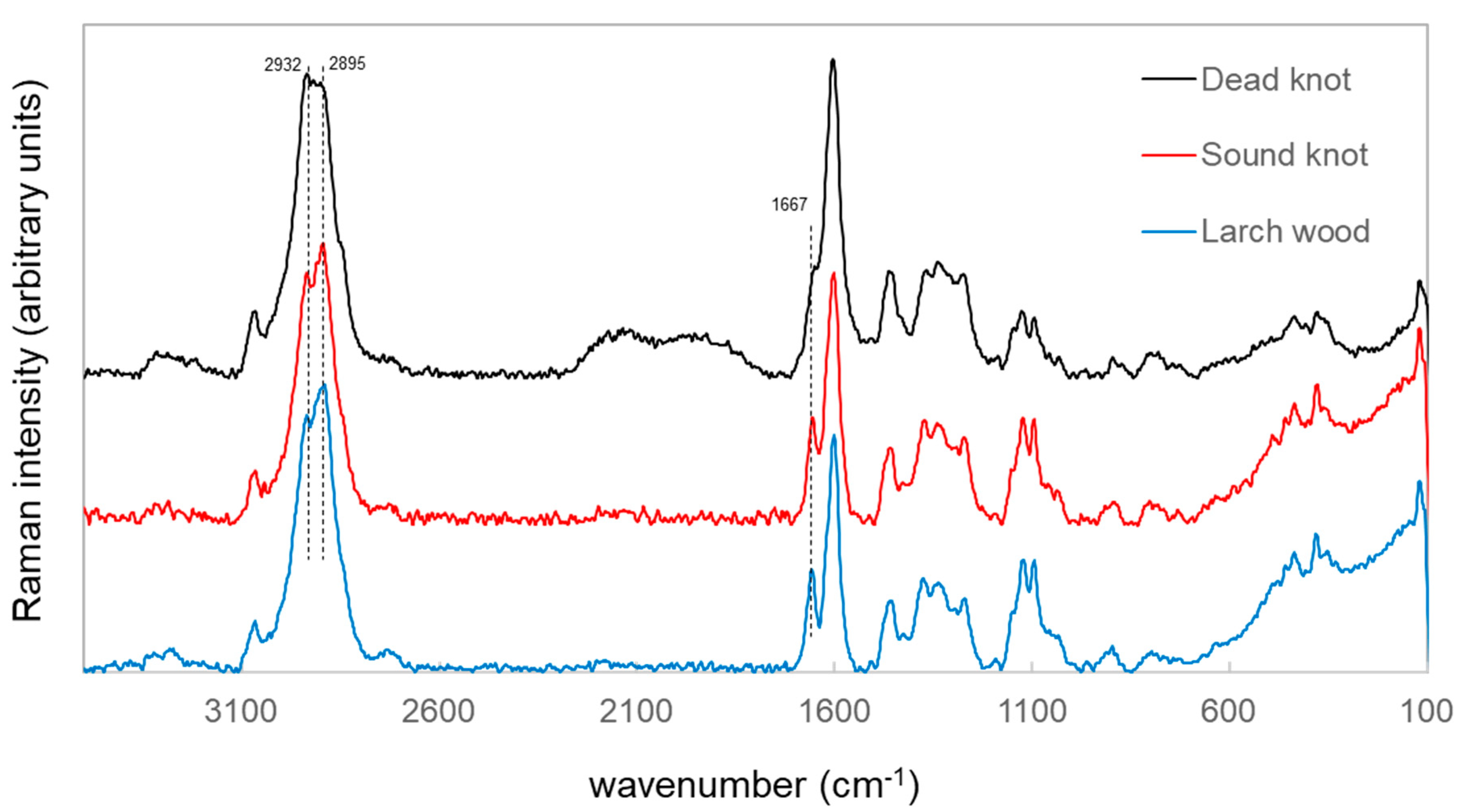
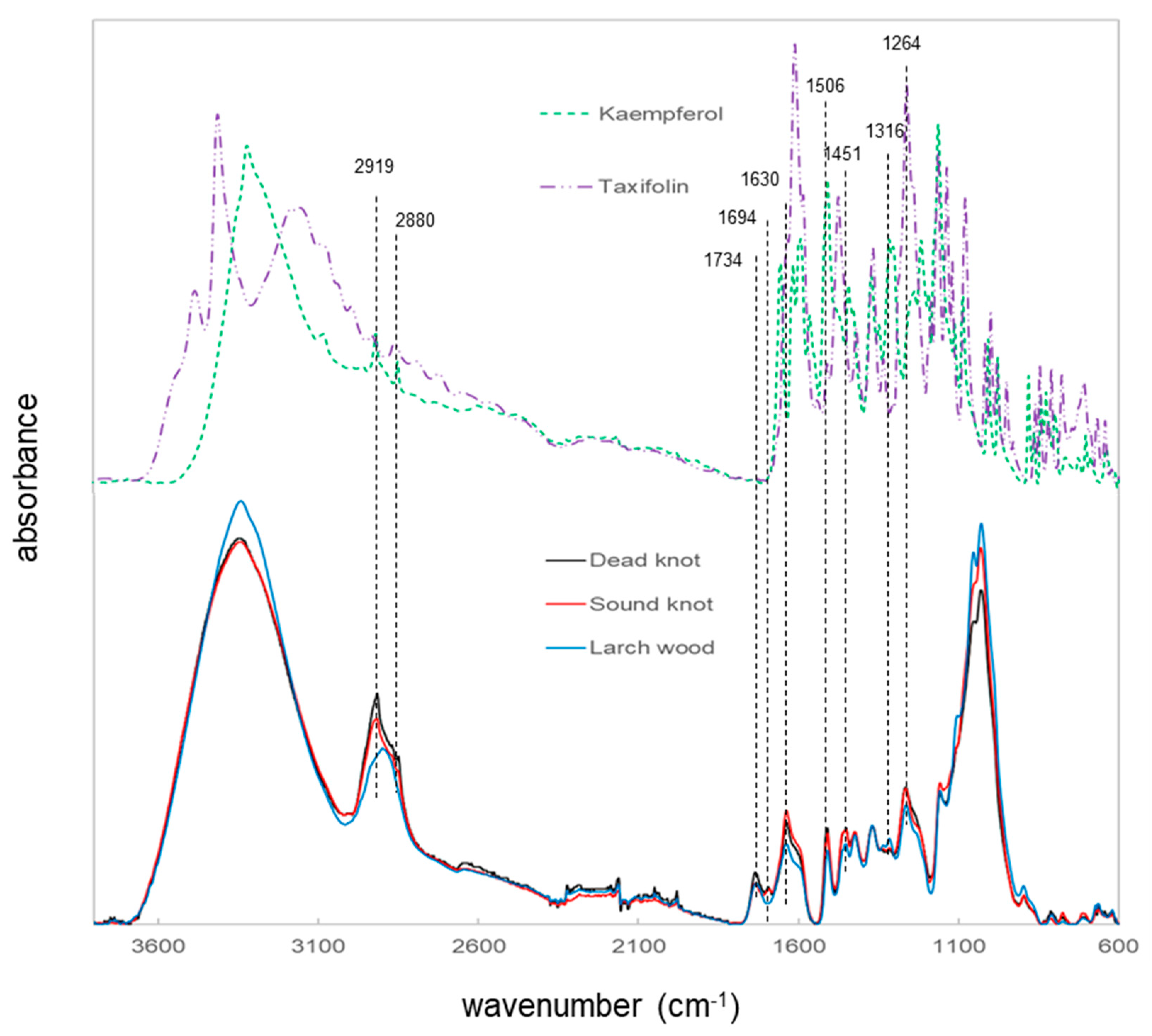
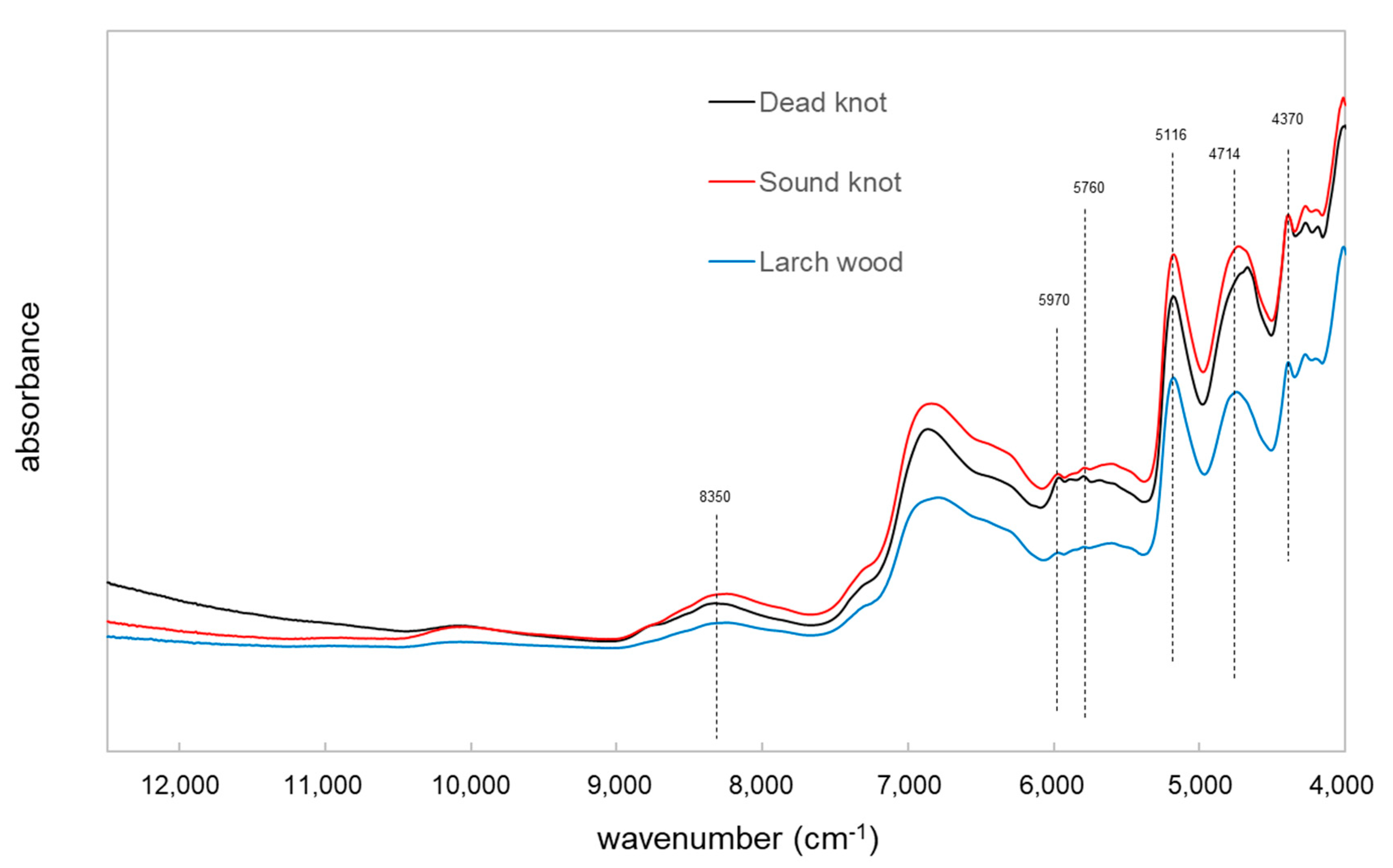
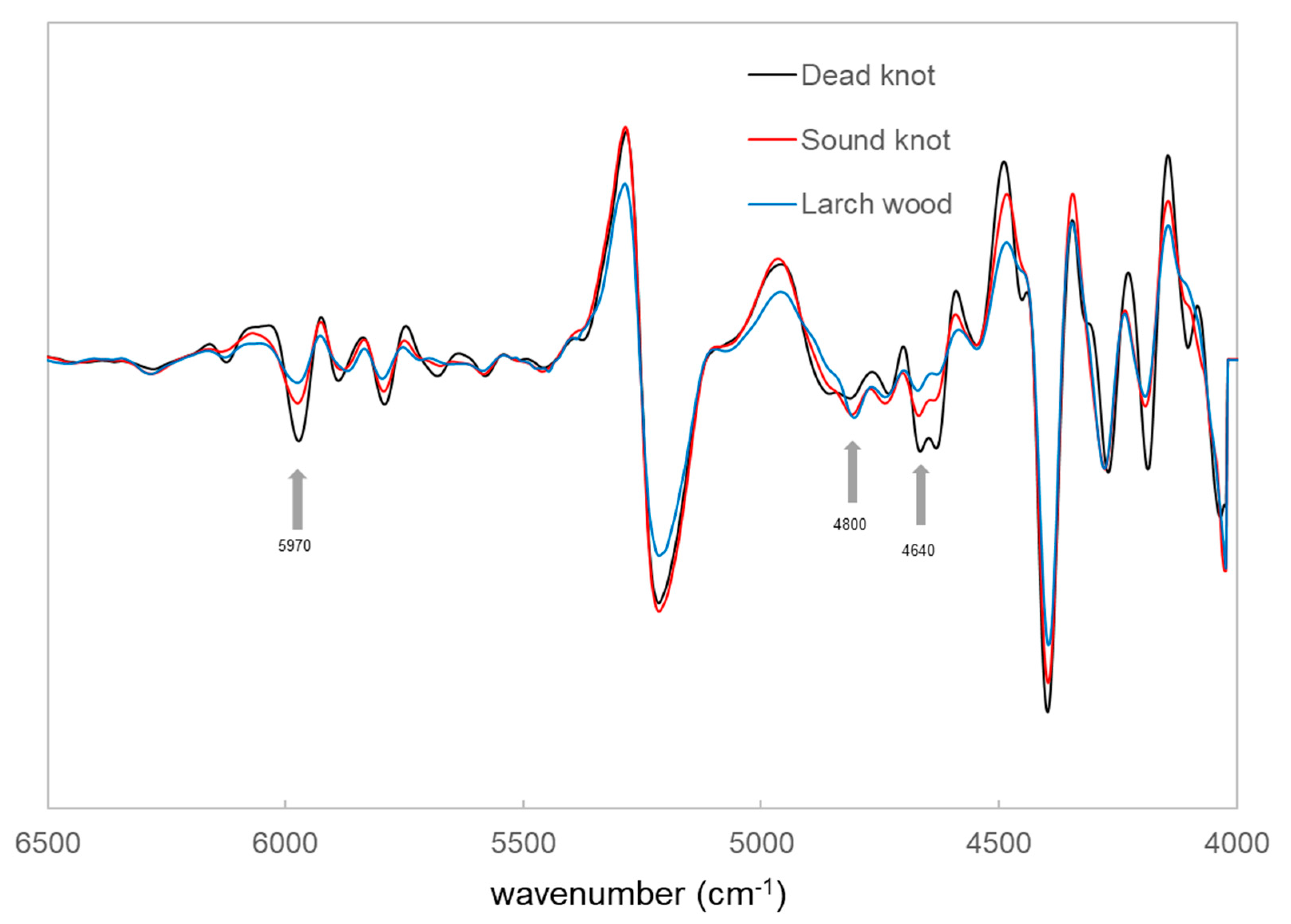
3.2. Univariate Analyis Method of Different Vibrational Spectrosocpy Methods
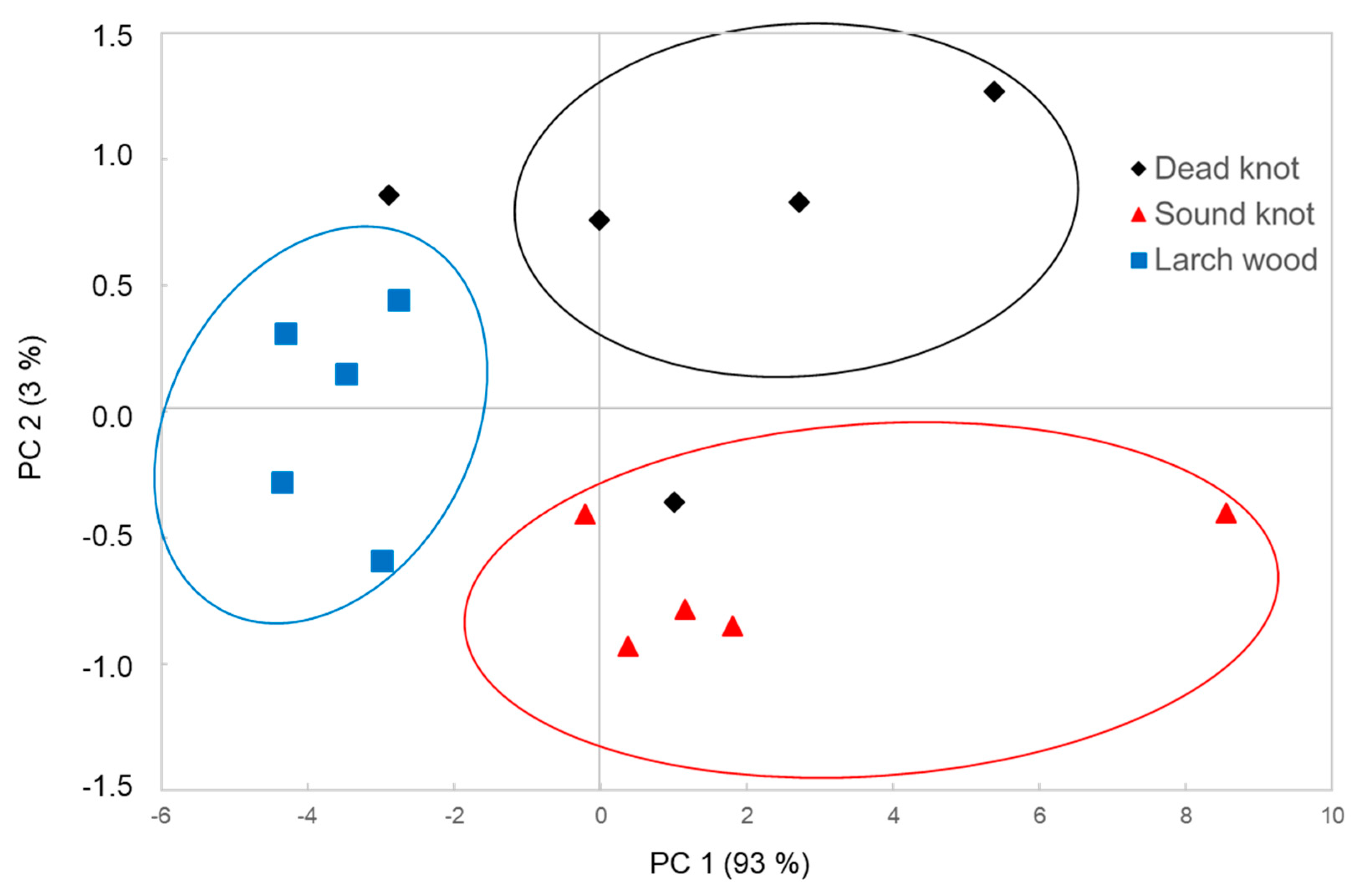
3.3. Multivariate Data Analysis of FT-NIR Spetra
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pieratti, E.; Paletto, A.; Atena, A.; Bernardi, S.; Palm, M.; Patzelt, D.; Romagnoli, M.; Teston, F.; Voglar, G.E.; Grebenc, T.; et al. Environmental and climate change impacts of eighteen biomass-based plants in the alpine region: A comparative anaylsis. J. Clean. Prod. 2019, 242. [Google Scholar] [CrossRef]
- Chen, M.; Luo, J.; Shi, R.; Zhang, J.; Gao, Q.; Li, J. Improved adhesion performance of soy protein-based adhesives with a larch tannin-based resin. Polymers 2017, 9, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarre, M.; Petit-Boix, A.; Priefer, C.; Meyer, R.; Leipold, S. Transforming the bio-based sector towards a circular economy–What can we learn from wood cascading? For. Policy Econ. 2020, 101872. [Google Scholar] [CrossRef]
- Keegan, D.; Kretschmer, B.; Elbersen, B.; Panoutsou, C. Cascading use: A systematic approach to biomass beyond the energy sector. Biofuels Bioprod. Biorefin. 2013, 7, 193–206. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood Chemistry Ultrastruture Rections; Verlag Kessel: Remagen, Germany, 2003. [Google Scholar]
- Zule, J.; Čufar, K.; Tišler, V. Hydrophilic extractives in heartwood of European larch (Larix decidua Mill.). Drvna Ind. 2016, 67, 363–370. [Google Scholar] [CrossRef]
- Hörhammer, H.; van Heiningen, A. A larch biorefinery: Influence of washing and PS charge on pre-extraction PSAQ pulping. BioResources 2012, 7, 3539–3554. [Google Scholar]
- Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Reunanen, M.H.T.; Eklund, P.C.; Sjöholm, R.E.; Eckerman, C.S.E.; Pohjamo, S.P.; Holmbom, B.R. Antioxidant activity of knotwood extractives and phenolic compounds of selected tree species. J. Agric. Food Chem. 2003, 51, 7600–7606. [Google Scholar] [CrossRef]
- Willför, S.M.; Hemming, J.; Reunanen, M.; Eckerman, C.; Holmbom, B. Lignans and lipophilic extractives in Norway spruce knots and steamwood. Holzforschung 2003, 57, 27–36. [Google Scholar] [CrossRef]
- Willför, S.M.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and lipophilic extractives in Scots pine knots and stemwood. Holzforschung 2003, 57, 359–372. [Google Scholar] [CrossRef]
- Laireiter, C.M.; Schnabel, T.; Köck, A.; Stalzer, P.; Petutschnigg, A.; Oostingh, G.J.; Hell, M. Active anti-microbial effects of larch and pine wood on four bacterial strains. BioRescources 2013, 9, 273–281. [Google Scholar] [CrossRef]
- Wagner, K.; Roth, C.; Willför, S.; Musso, M.; Petutschnigg, A.; Oostingh, G.J.; Schnabel, T. Identification of antibmicrobial compounds in different hydrophilic larch bark extracts. BioRescources 2019, 14, 5807–5815. [Google Scholar]
- Välimaa, A.-L.; Honkalampi-Hämäläinen, U.; Pietarinen, S.; Willför, S.; Holmbom, B.; von Wright, A. Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms. Int. J. Food Microbiol. 2007, 115, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, J.L.; Shmulsky, R.; Haygreen, J.G. Forest Products and Wood Science: An Introduction; Iowa State Press: Ames, IA, USA, 2003. [Google Scholar]
- Nisula, L. Wood extractives in conifers. A Study of Stemwood and Knots of Industrially Important Species. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 2018. [Google Scholar]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Schwanninger, M.; Hinterstoisser, B.; Gradingler, C.; Messner, K.; Fackler, K. Examination of spruce wood biodegradaed by Ceriporisopsis subvermispora using near and mid infrared spectroscopy. J. Near Infrared Spectrosc. 2004, 12, 397–409. [Google Scholar] [CrossRef]
- Schnabel, T.; Musso, M.; Tondi, G. Univariate and multivariate analysis of tannin-impregnated wood species using vibrational spectroscopy. Appl. Spectroc. 2014, 68, 488–494. [Google Scholar] [CrossRef]
- Wagner, K.; Schnabel, T.; Barbu, M.-C.; Petutschigg, A. Analysis of selected properties of fibreboard panels manufactured from wood and leather using the near infrared spectroscopy. Int. J. Spectrosc. 2015. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, U.P.; Atalla, R.H. Vibrational spectroscopy. In Lignin and Lignans. Advances in Chemistry; Heiter, C., Dimmel, D.R., Schmidt, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 103–136. [Google Scholar]
- Musso, M.; Oehem, K.L. Raman spectroscopy. In Lasers in Chemistry: Probing and Influencing Matter; Lackner, M., Ed.; Wiley-VCM: Weinheim, Germany, 2008; pp. 531–591. [Google Scholar]
- Belt, T.; Keplinger, T.; Hänninen, T.; Rautkari, L. Cellular level distributions of Scots pine heartwood and knot heartwood extractives revealed by Raman spectroscopy imaging. Ind. Crop. Prod. 2017, 108, 327–335. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds, 4th ed.; Springer: Berling/Heidlberg, Germany, 2009; pp. 207–335. [Google Scholar]
- Wienhaus, H.; Pilz, W.; Seibt, H.; Dässler, H.-G. Die Diterpene Larixylacetat and Larixol. Chem. Berichte 1960, 93, 2625–2630. [Google Scholar] [CrossRef]
- Gierlinger, N.; Schwanninger, M.; Hnterstoisser, B.; Wimmer, R. Rapid determination of heartwood extractives in Larix sp. by means of Fourier transform near infrared spectroscopy. J. Near Infrared Spectrosc. 2002, 10, 203–214. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Facker, K. A review of band assignsments in near infrared spectra of wodo and wood components. J. Near Infrared Spectrosc. 2011, 19, 287–308. [Google Scholar] [CrossRef]
- Michell, A.J.; Schimleck, L.R. NIR spectroscopy of wood from Eucalyptus globulus. Appita J. 1996, 49, 23–26. [Google Scholar]
- Barton II, F.E.; Himmelbach, D.S.; Duckworth, J.H.; Smith, M.J. Two-dimensional vibration spectroscopy: Correlation of Mid- and Near-Infrared region. Appl. Spectrosc. 1992, 46, 420–429. [Google Scholar] [CrossRef]



| Component Groups | Larch Wood Mixture (mg/g) | Sound Knotwood (mg/g) | Dead Knotwood (mg/g) |
|---|---|---|---|
| Carboxylic acids | 0.026 | 0.139 | 0.011 |
| Phenolic acids | 0.116 | 1.666 | 0.081 |
| Sugar | 0.206 | 0.472 | 0.045 |
| Fatty acids | 0.140 | 0.483 | 0.047 |
| Resin acids | 0.079 | 0.186 | 0.014 |
| Polyphenols | 2.726 | 23.766 | 1.305 |
| Sterols | 0.016 | 0.235 | 0.011 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, K.; Musso, M.; Kain, S.; Willför, S.; Petutschnigg, A.; Schnabel, T. Larch Wood Residues Valorization through Extraction and Utilization of High Value-Added Products. Polymers 2020, 12, 359. https://doi.org/10.3390/polym12020359
Wagner K, Musso M, Kain S, Willför S, Petutschnigg A, Schnabel T. Larch Wood Residues Valorization through Extraction and Utilization of High Value-Added Products. Polymers. 2020; 12(2):359. https://doi.org/10.3390/polym12020359
Chicago/Turabian StyleWagner, Kerstin, Maurizio Musso, Stefan Kain, Stefan Willför, Alexander Petutschnigg, and Thomas Schnabel. 2020. "Larch Wood Residues Valorization through Extraction and Utilization of High Value-Added Products" Polymers 12, no. 2: 359. https://doi.org/10.3390/polym12020359