Flame-Retardant Performance of Transparent and Tensile-Strength-Enhanced Epoxy Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
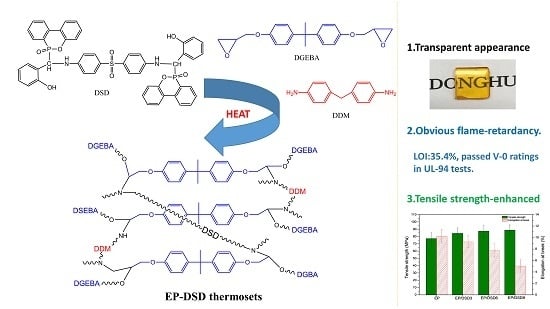
2.2. Synthesis of DSD
2.3. Preparation of Flame-Retardant Epoxy Resin
2.4. Characterization
3. Results
3.1. Characterization of DSD
3.2. Flame-Retardant Properties of Epoxy Thermosets
3.3. Morphology and Structure of Residual Char after the UL-94 Test
3.4. Morphology of Residual Char after Thermal Degradation under Nitrogen Atmosphere
3.5. Thermal Stability of EP and EP/DSD Thermosets
3.6. Py-GC/MS Analysis of DSD, EP, and EP–DSD Thermosets
3.7. Mechanical Properties of Neat EP and EP–DSD Thermosets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jang, J.B.; Kim, T.H.; Kim, T.; Kim, H.J.; Seo, B.; Lim, C.-S.; Lee, W. Modified epoxy resin synthesis from phosphorus-containing polyol and physical changes studies in the synthesized products. Polymers 2019, 11, 2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamciuc, C.; Vlad-Bubulac, T.; Serbezeanu, D.; Carja, I.-D.; Hamciuc, E.; Lisa, G.; Forrat Perez, V. Environmentally friendly fire-resistant epoxy resins based on a new oligophosphonate with high flame retardant efficiency. RSC Adv. 2016, 6, 22764–22776. [Google Scholar] [CrossRef]
- Iddrissu, I.; Zheng, H.; Rowland, S.M. DC electrical tree growth in epoxy resin and the influence of the size of inceptive AC trees. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1965–1972. [Google Scholar] [CrossRef]
- Wang, N.; Gao, H.; Zhang, J.; Qin, Y.; Wang, D. Phytic acid intercalated graphene oxide for anticorrosive reinforcement of waterborne epoxy resin coating. Polymers 2019, 11, 1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, D.; Qiang, H. Study on thermomechanical properties of cross-linked epoxy resin. Mol. Simulat. 2015, 41, 1081–1085. [Google Scholar] [CrossRef]
- Naderi, M.; Hoseinabadi, M.; Najafi, M.; Motahari, S.; Shokri, M. Investigation of the mechanical, thermal, and anticorrosion properties of epoxy nanocomposite coatings: Effect of synthetic hardener and nanoporous graphene. J. Appl. Polym. Sci. 2018, 135, 46201. [Google Scholar] [CrossRef]
- Kireev, V.V.; Bilichenko, Y.V.; Borisov, R.S.; Mu, J.; Kuznetsov, D.A.; Eroshenko, A.V.; Filatov, S.N.; Sirotin, I.S. Synthesis of bisphenol A based phosphazene-containing epoxy resin with reduced viscosity. Polymers 2019, 11, 1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movahedifar, E.; Vahabi, H.; Saeb, M.R.; Thomas, S. Flame retardant epoxy composites on the road of innovation: An analysis with flame retardancy index for future development. Molecules 2019, 24, 3964. [Google Scholar] [CrossRef] [Green Version]
- Vahabi, H.; Laoutid, F.; Movahedifar, E.; Khalili, R.; Rahmati, N.; Vagner, C.; Cochez, M.; Brison, L.; Ducos, F.; Ganjali, M.R.; et al. Description of complementary actions of mineral and organic additives in thermoplastic polymer composites by flame retardancy index. Polym. Adv. Technol. 2019, 30, 2056–2066. [Google Scholar] [CrossRef]
- Vahabi, H.; Saeb, M.R.; Formela, K.; Cuesta, J.-M.L. Flame retardant epoxy/halloysite nanotubes nanocomposite coatings: Exploring low-concentration threshold for flammability compared to expandable graphite as superior fire retardant. Prog. Org. Coat. 2018, 119, 8–14. [Google Scholar] [CrossRef]
- Vahabi, H.; Jouyandeh, M.; Cochez, M.; Khalili, R.; Vagner, C.; Ferriol, M.; Movahedifar, E.; Ramezanzadeh, B.; Rostami, M.; Ranjbar, Z.; et al. Short-lasting fire in partially and completely cured epoxy coatings containing expandable graphite and halloysite nanotube additives. Prog. Org. Coat. 2018, 123, 160–167. [Google Scholar] [CrossRef]
- Sari, M.G.; Saeb, M.R.; Shabanian, M.; Khaleghi, M.; Vahabi, H.; Vagner, C.; Zarrintaj, P.; Khalili, R.; Paran, S.M.R.; Ramezanzadeh, B. Epoxy/starch-modified nano-zinc oxide transparent nanocomposite coatings: A showcase of superior curing behavior. Prog. Org. Coat. 2018, 115, 143–150. [Google Scholar] [CrossRef]
- Rastin, H.; Saeb, M.R.; Nonahal, M.; Shabanian, M.; Vahabi, H.; Formela, K.; Gabrion, X.; Seidi, F.; Zarrintaj, P.; Sari, M.G. Transparent nanocomposite coatings based on epoxy and layered double hydroxide: Nonisothermal cure kinetics and viscoelastic behavior assessments. Prog. Org. Coat. 2017, 113, 126–135. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2009, 21, 1–26. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Doering, M. Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Jiang, J.; Jiang, P.; Yu, J. Synthesis, characteristic of a novel flame retardant containing phosphorus, silicon and its application in ethylene vinyl-acetate copolymer (EVM) rubber. J. Polym. Res. 2010, 17, 891–902. [Google Scholar] [CrossRef]
- Wang, X.; Feng, N.; Liu, L.; Wang, Z.; Wen, Y. Preparation of Lig-based intumescent flame retardant and its effect on flame resistance of TPO. China Synth. Resin Plast. 2017, 34, 6–10. [Google Scholar]
- Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Lanzi, M.; Saraga, F.; Sambri, L.; Franchini, M.C.; Giorgini, L. New nitrogen-rich heterocycles for organo-modified bentonites as flame retardant fillers in epoxy resin nanocomposites. Polym. Eng. Sci. 2017, 57, 621–630. [Google Scholar] [CrossRef]
- Duan, H.; Ji, S.; Yin, T.; Tao, X.; Chen, Y.; Ma, H. Phosphorus–nitrogen-type fire-retardant vinyl ester resin with good comprehensive properties. J. Appl. Polym. Sci. 2019, 136, 47997. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Tai, Q.; Wang, X.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, R.K.K. Comparative study on the flame retarded efficiency of melamine phosphate, melamine phosphite and melamine hypophosphite on poly(butylene succinate) composites. Polym. Degrad. Stab. 2014, 105, 248–256. [Google Scholar] [CrossRef]
- Jian, R.; Wang, P.; Xia, L.; Yu, X.; Zheng, X.; Shao, Z. Low-flammability epoxy resins with improved mechanical properties using a Lewis base based on phosphaphenanthrene and 2-aminothiazole. J. Mater. Sci. 2017, 52, 1–15. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, S.; Ding, Y.; Wang, F.; Liu, P.; Tang, H.; Wang, Y.; Yang, M. Synthesis of a heat-resistant DOPO derivative and its application as flame-retardant in engineering plastics. J. Appl. Polym. Sci. 2017, 134, 44892. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, B. Synthesis of a new phosphorus-nitrogen flame retardant GAP-DOPO and its flame retardant performance for epoxy resin. Fine Chem. 2017, 34, 382–388. [Google Scholar]
- Shi, Y.; Yu, B.; Zheng, Y.; Yang, J.; Hu, Y. Design of reduced graphene oxide decorated with DOPO-phosphanomidate for enhanced fire safety of epoxy resin. J. Colloid Interf. Sci. 2018, 521, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhou, X.; Xing, Z.; Wu, Y. Flame retardant performance of a carbon source containing DOPO derivative in PET and epoxy. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Xu, M.-J.; Xu, G.-R.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stabil. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Ping, C.; Shengping, L.; Dezhong, W. Epoxy Resin and Its Application; Chemical Industry Press: Beijing, China, 2011. [Google Scholar]
- Tan, Y.; Shao, Z.-B.; Chen, X.-F.; Long, J.-W.; Chen, L.; Wang, Y.-Z. A novel multifunctional organic-inorganic hybrid curing agent with high flame-retardant efficiency for epoxy resin. ACS Appl. Mater. Interfaces 2015, 7, 17919–17928. [Google Scholar] [CrossRef]
- Yang, J.P.; Chen, Z.-K.; Yang, G.; Fu, S.-Y.; Ye, L. Simultaneous improvements in the cryogenic tensile strength, ductility and impact strength of epoxy resins by a hyperbranched polymer. Polymer 2008, 49, 3168–3175. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Yuan, C.H.; Song, C.F.; Chang, Y.; Chen, G.R.; Xu, Y.T.; Dai, L.Z. Modification of epoxy resin through the self-assembly of a surfactant-like multi-element flame retardant. J. Mater. Chem. A 2016, 4, 3462–3470. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yang, R. Pyrolysis and fire behaviour of epoxy resin composites based on a phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS). Polym. Degrad. Stabil. 2011, 96, 1821–1832. [Google Scholar] [CrossRef]
- He, X.; Zhang, W.; Yang, R. The characterization of DOPO/MMT nanocompound and its effect on flame retardancy of epoxy resin. Compos. Part A Appl. Sci. Manuf. 2017, 98, 124–135. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Kalali, E.N.; Wang, X.; Wang, D.-Y. A sustainable, eugenol-derived epoxy resin with high biobased content, modulus, hardness and low flammability: Synthesis, curing kinetics and structure-property relationship. Chem. Eng. J. 2016, 284, 1080–1093. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Chou, Y.C.; Shiao, W.F.; Wang, M.W. High temperature, flame-retardant, and transparent epoxy thermosets prepared from an acetovanillone-based hydroxyl poly(ether sulfone) and commercial epoxy resins. Polymer 2016, 97, 300–308. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W.; Zhong, Y.; Ruan, M.; Hui, L.L. Flame-retardant materials based on phosphorus-containing polyhedral oligomeric silsesquioxane and bismaleimide/diallylbisphenol a with improved thermal resistance and dielectric properties. J. Appl. Polym. Sci. 2015, 132, 41545. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Bai, Z.-M.; Tang, G.; Song, L.; Stec, A.A.; Hull, T.R.; Hu, Y.; Hu, W.-Z. Synthesis of mesoporous silica@Co–Al layered double hydroxide spheres: Layer-by-layer method and their effects on the flame retardancy of epoxy resins. ACS Appl. Mater. Interfaces 2014, 6, 14076–14086. [Google Scholar] [CrossRef]
- Schartel, B.; Braun, U.; Balabanovich, A.I.; Artner, J.; Ciesielski, M.; Doering, M.; Perez, R.M.; Sandler, J.K.W.; Altstaedt, V. Pyrolysis and fire behaviour of epoxy systems containing a novel 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-(DOPO)-based diamino hardener. Eur. Polym. J. 2008, 44, 704–715. [Google Scholar] [CrossRef]
- Kopwyhoba, А.Н.; Korshunova, A.N. The peculiarities of polaron motion in the molecular polynucleotide chains of finite length in the presence of localized excitations in the chain. Mat. Biolog. Bioinform. 2017, 11, 141–158. [Google Scholar] [CrossRef]











| Samples | Composition (g) | DSD (wt %) | P (wt %) | N (wt %) | ||
|---|---|---|---|---|---|---|
| DGEBA | DDM | DSD | ||||
| EP0 | 100 | 22 | 0 | 0 | 0 | 2.54 |
| EP–DSD3 | 100 | 21.16 | 3.75 | 3 | 0.21 | 2.49 |
| EP–DSD6 | 100 | 19.38 | 7.62 | 6 | 0.42 | 2.35 |
| EP–DSD9 | 100 | 19.37 | 11.81 | 9 | 0.63 | 2.37 |
| Samples | LOI (%) | UL-94, 3.0 mm bar | |||
|---|---|---|---|---|---|
| t1 + t2 a (s) | Ignition of Cotton | Dripping | Rating | ||
| EP | 24.8 | NR b | Yes | Yes | No rating |
| EP–DSD3 | 30.7 | 11.5 + 5.8 | NO | NO | V-1 |
| EP–DSD6 | 34.2 | 5.5 + 4.2 | NO | NO | V-0 |
| EP–DSD9 | 35.4 | 3.1 + 2.3 | NO | NO | V-0 |
| Sample | T5% (°C) | Tmax (°C) | CYTmax (%) | Vmax (%/°C) | CY700 (%) |
|---|---|---|---|---|---|
| EP | 369.7 | 384.9 | 79.84 | −1.47 | 1.21 |
| EP–DSD3 | 361.3 | 375.9 | 79.92 | −1.25 | 1.54 |
| EP–DSD6 | 351.1 | 369.7 | 80.28 | −1.14 | 2.49 |
| EP–DSD9 | 346.4 | 364.5 | 82.97 | −1.14 | 3.98 |
| Sample | T5% (°C) | Tmax (°C) | CYTmax (%) | Vmax (%/°C) | CY700 (%) |
|---|---|---|---|---|---|
| EP | 367.2 | 387.2 | 75.78 | −1.76 | 16.58 |
| EP–DSD3 | 362.2 | 377.2 | 79.49 | −1.39 | 20.48 |
| EP–DSD6 | 354.6 | 372.1 | 81.73 | −1.25 | 22.74 |
| EP–DSD9 | 349.7 | 364.7 | 82.13 | −1.21 | 22.87 |
| Sample | Tg (°C) | νe (mol/m3) |
|---|---|---|
| EP | 171.2 | 1958 |
| EP–DSD3 | 173.6 | 2015 |
| EP–DSD6 | 174.9 | 2067 |
| EP–DSD9 | 176.5 | 2105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Cai, Z. Flame-Retardant Performance of Transparent and Tensile-Strength-Enhanced Epoxy Resins. Polymers 2020, 12, 317. https://doi.org/10.3390/polym12020317
Li L, Cai Z. Flame-Retardant Performance of Transparent and Tensile-Strength-Enhanced Epoxy Resins. Polymers. 2020; 12(2):317. https://doi.org/10.3390/polym12020317
Chicago/Turabian StyleLi, Liang, and Zaisheng Cai. 2020. "Flame-Retardant Performance of Transparent and Tensile-Strength-Enhanced Epoxy Resins" Polymers 12, no. 2: 317. https://doi.org/10.3390/polym12020317