Improved Nylon 6,6 Nanofiber Membrane in A Tilted Panel Filtration System for Fouling Control in Microalgae Harvesting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Nylon 6,6 NFM
2.2. Post Treatment Using Solvent Vapor Treatment
2.3. Membrane Characterization
2.4. Membrane Panel Assembly
2.5. Chlorella Vulgaris Feed and Analysis
2.6. Filtration Set-up
3. Results and Discussion
3.1. Effect of Filtration Cycle on Hydraulic Performance of the Pristine NFM
3.2. Impact of Solvent Vapor Treatment of NFM Properties
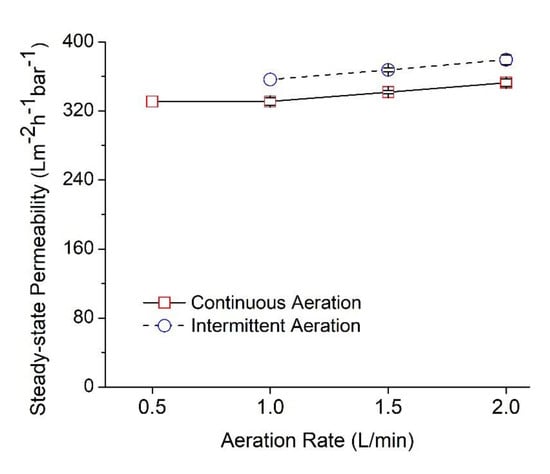
3.3. Hydraulic Performance of the Solvent Vapor Treated NFM
3.4. Stability of the Treated Nylon 6,6 NFM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cunha, A., Jr.; Feddern, V.; De Prá, M.C.; Higarashi, M.M.; de Abreu, P.G.; Coldebella, A. Synthesis and characterization of ethylic biodiesel from animal fat wastes. Fuel 2013, 105, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Banković-Ilić, I.B.; Stojković, I.J.; Stamenković, O.S.; Veljkovic, V.B.; Hung, Y.-T. Waste animal fats as feedstocks for biodiesel production. Renew. Sustain. Energy Rev. 2014, 32, 238–254. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.A.; Aqsha; Putra, Z.A.; Bilad, M.R.; Sapiaa, N.A.H.; Wirzal, M.D.H.; Tijani, M.M. Biogas production from chicken food waste and cow manure via multi-stages anaerobic digestion. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2018. [Google Scholar]
- Lal, R. World crop residues production and implications of its use as a biofuel. Environ. Int. 2005, 31, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Abdurakhman, Y.; Putra, Z.; Bilad, M.; Nordin, N.; Wirzal, M.; Muraza, O. Producing Biodiesel from Waste Cooking Oil with Catalytic Membrane Reactor: Process. Design and Sensitivity Analysis. Arab. J. Sci. Eng. 2018, 43, 6261–6269. [Google Scholar] [CrossRef]
- Abdurakhman, Y.B.; Putra, Z.A.; Bilad, M.R.; Nordin, N.A.H.M.; Wirzal, M.D.H. Techno-economic analysis of biodiesel production process from waste cooking oil using catalytic membrane reactor and realistic feed composition. Chem. Eng. Res. Des. 2018, 134, 564–574. [Google Scholar] [CrossRef]
- Dufey, A. Biofuels Production, Trade and Sustainable Development: Emerging Issues; Iied Press: London, UK, 2006. [Google Scholar]
- Khan, S.; Siddique, R.; Sajjad, W.; Nabi, G.; Hayat, K.M.; Duan, P.; Yao, L. Biodiesel Production From Algae to Overcome the Energy Crisis. HAYATI J. Biosci. 2017, 24, 163–167. [Google Scholar] [CrossRef]
- Roberts, G.W.; Fortier, M.-O.P.; Sturm, B.S.; Stagg-Williams, S.M. Promising pathway for algal biofuels through wastewater cultivation and hydrothermal conversion. Energy Fuels 2013, 27, 857–867. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- López, B.C.; Cerdán, L.E.; Medina, A.R.; López, E.N.; Valverde, L.M.; Peña, E.H.; Moreno, P.A.G.; Grima, E.M. Production of biodiesel from vegetable oil and microalgae by fatty acid extraction and enzymatic esterification. J. Biosci. Bioeng. 2015, 119, 706–711. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Fraeye, I.; Meesschaert, B.; Muylaert, K. Flocculation of Chlorella vulgaris induced by high pH: Role of magnesium and calcium and practical implications. Bioresour. Technol. 2012, 105, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanotu, J.; Bandulasena, H.H.; Zimmerman, W.B. Microflotation performance for algal separation. Biotechnol. Bioeng. 2012, 109, 1663–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dassey, A.J.; Theegala, C.S. Harvesting economics and strategies using centrifugation for cost effective separation of microalgae cells for biodiesel applications. Bioresour. Technol. 2013, 128, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Bilad, M.R.; Discart, V.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F. Harvesting microalgal biomass using a magnetically induced membrane vibration (MMV) system: Filtration performance and energy consumption. Bioresour. Technol. 2013, 138, 329–338. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X; Hu, Q. ; Sommerfeld, M.; Puruhito, E.; Chen, Y. Harvesting algal biomass for biofuels using ultrafiltration membranes. Bioresour. Technol. 2010, 101, 5297–5304.
- Yang, L.; Wang, L.; Ren, S.; Pan, B.; Li, J.; Zhang, X.; Chen, Y.; Hu, Q. Harvesting of Scenedesmus acuminatus using ultrafiltration membranes operated in alternative feed directions. J. Biosci. Bioeng. 2019, 128, 103–109. [Google Scholar] [CrossRef]
- Bilad, M.R.; Azizo, A.S.; Wirzal, M.D.H.; Jia, L.J.; Putra, Z.A.; Nordin, N.A.H.M.; Mavukkandy, M.O.; Jasni, M.J.F.; Yusoff, A.R.M. Tackling membrane fouling in microalgae filtration using nylon 6,6 nanofiber membrane. J. Environ. Manag. 2018, 223, 23–28. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Hydrophilic nylon 6, 6 nanofibers supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2014, 457, 162–169. [Google Scholar] [CrossRef]
- Al-Husaini, I.; Yusoff, A.; Lau, W.; Ismail, A.; Al-Abri, M.; Al-Ghafri, B.; Wirzal, M. Fabrication of polyethersulfone electrospun nanofibrous membranes incorporated with hydrous manganese dioxide for enhanced ultrafiltration of oily solution. Sep. Purif. Technol. 2019, 212, 205–214. [Google Scholar] [CrossRef]
- Park, M.J.; Gonzales, R.R.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Hydrophilic polyvinyl alcohol coating on hydrophobic electrospun nanofiber membrane for high performance thin film composite forward osmosis membrane. Desalination 2018, 426, 50–59. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Fane, A.G. Fabrication of bioinspired composite nanofiber membranes with robust superhydrophobicity for direct contact membrane distillation. Environ. Sci. Technol. 2014, 48, 6335–6341. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Woo, Y.C.; Tijing, L.D.; Shim, W.-G.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Effect of heat-press conditions on electrospun membranes for desalination by direct contact membrane distillation. Desalination 2016, 378, 80–91. [Google Scholar] [CrossRef]
- Yao, M.; Woo, Y.; Tijing, L.; Cesarini, C.; Shon, H. Improving nanofiber membrane characteristics and membrane distillation performance of heat-pressed membranes via annealing post-treatment. Appl. Sci. 2017, 7, 78. [Google Scholar] [CrossRef]
- Xiang, C.; Frey, M. Increasing mechanical properties of 2-D-structured electrospun nylon 6 non-woven fiber mats. Materials 2016, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Manickam, S.S.; McCutcheon, J.R. Increasing strength of electrospun nanofiber membranes for water filtration using solvent vapor. J. Membr. Sci. 2013, 436, 213–220. [Google Scholar] [CrossRef]
- Wu, J.; Le-Clech, P.; Stuetz, R.M.; Fane, A.G.C.; Vicki. Effect of relaxation and backwashing conditions on fouling in membrane bioreactor. J. Membr. Sci. 2008, 324, 26–32. [Google Scholar] [CrossRef]
- Zhao, F.; Chu, H.; Tan, X.; Zhang, Y.; Yang, L.; Zhou, X.; Zhao, J. Comparison of axial vibration membrane and submerged aeration membrane in microalgae harvesting. Bioresour. Technol. 2016, 208, 178–183. [Google Scholar] [CrossRef]
- Bilad, M.; Marbelia, L.; Naik, P.; Laine, C.; Vankelecom, I. Direct comparison of aerated and vibrated filtration systems for harvesting of Chlorella vulgaris. Algal Res. 2014, 6, 32–38. [Google Scholar] [CrossRef]
- Chen, X.; Huang, C.; Liu, T. Harvesting of microalgae Scenedesmus sp. using polyvinylidene fluoride microfiltration membrane. Desalin. Water Treat. 2012, 45, 177–181. [Google Scholar] [CrossRef]
- Eliseus, A.; Bilad, M.; Nordin, N.; Putra, Z.; Wirzal, M. Tilted membrane panel: A new module concept to maximize the impact of air bubbles for membrane fouling control in microalgae harvesting. Bioresour. Technol. 2017, 241, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chu, H.; Su, Y.; Tan, X.; Zhang, Y.; Yang, L.; Zhou, X. Microalgae harvesting by an axial vibration membrane: The mechanism of mitigating membrane fouling. J. Membr. Sci. 2016, 508, 127–135. [Google Scholar] [CrossRef]
- Kanchanatip, E.; Su, B.-R.; Tulaphol, S.; Den, W.; Grisdanurak, N.; Kuo, C.-C. Fouling characterization and control for harvesting microalgae Arthrospira (Spirulina) maxima using a submerged, disc-type ultrafiltration membrane. Bioresour. Technol. 2016, 209, 23–30. [Google Scholar] [CrossRef]
- Alipourzadeh, A.; Mehrnia, M.R.; Sani, A.H.; Babaei, A. Application of response surface methodology for investigation of membrane fouling behaviours in microalgal membrane bioreactor: The effect of aeration rate and biomass concentration. RSC Adv. 2016, 6, 111182–111189. [Google Scholar] [CrossRef]
- Zhao, F.; Chu, H.; Tan, X.; Yang, L.; Su, Y.; Zhou, X.; Zhao, J.; Zhang, Y. Using axial vibration membrane process to mitigate membrane fouling and reject extracellular organic matter in microalgae harvesting. J. Membr. Sci. 2016, 517, 30–38. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L.; Fontananova, E. Comprehensive Membrane Science and Engineering; Elsevier Press: Oxford, UK, 2017. [Google Scholar]
- Bilad, M.; Arafat, H.A.; Vankelecom, I.F. Membrane technology in microalgae cultivation and harvesting: A review. Biotechnol. Adv. 2014, 32, 1283–1300. [Google Scholar] [CrossRef]
- Ueda, T.; Hata, K.; Kikuoka, Y.; Seino, O. Effects of aeration on suction pressure in a submerged membrane bioreactor. Water Res. 1997, 31, 489–494. [Google Scholar] [CrossRef]
- Tian, J.-Y.; Xu, Y.-P.; Chen, Z.-L.; Nan, J.; Li, G.-B. Air bubbling for alleviating membrane fouling of immersed hollow-fiber membrane for ultrafiltration of river water. Desalination 2010, 260, 225–230. [Google Scholar] [CrossRef]
- Puspitasari, V.; Granville, A.; Le-Cletch, P.; Chen, V. Cleaning and ageing effect of sodium hypochlorite on polyvinylidene fluoride (PVDF) membrane. Sep. Purif. Technol. 2010, 72, 301–308. [Google Scholar] [CrossRef]






| Membrane Type/Fouling Control System | Membrane | Feed | Flux (Lm−2·h−1) | Permeance (Lm−2·h−1·bar−1) | Refs |
|---|---|---|---|---|---|
| Untreated NFM in tilted panel | Pristine nylon 6,6 nanofiber | 1.1 g L−1 of Chlorella vulgaris | 40.2 | 402.3 | This study |
| Solvent vapor treated NFM in tilted panel | Treated nylon 6,6 nanofiber | 1.1 g L−1 of Chlorella vulgaris | 37.9 | 379.5 | This study |
| Axial vibration and aeration | PVDF | 0.3 g L−1 of Chlorella pyrenoidosa | 238.4 | 340.6 | [31] |
| Vibration and aeration | PVDF | 0.08g L−1 of Chlorella vulgaris | 32.5 | 325 | [32] |
| Pristine NFM | Pristine nylon 6,6 nanofiber | 1g L−1 of Euglena sp. | 30.0 | 300.0 | [21] |
| Backwashing and ventilation | PVDF | Scenedesmus sp. | 130.0 | 260.0 | [33] |
| Tilted panel | 15% wt PVDF | 1g L−1 of Euglena sp. | 22.5 | 225.0 | [34] |
| Membrane vibrations | 9% and 12% wt PVDF | 0.25 g L−1 of Phaeodactylum tricornutum 0.21 g L−1 of Chlorella vulgaris | ± .21.25–42.5 ± 25.5–42.5 | 212.5–425.0 * 25–425.0 * | [16] |
| Axial vibration | PVDF | 0.55 g L−1 of Chlorella pyrenoidosa | 22.0–64.0 ** | 220–640.0 | [35] |
| Disc type panel | PVDF | 10 g L−1 of Arthrospira (Spirulina) maxima | 57.0–142.9 | 95–238.3 | [36] |
| Aeration in vertical panel | Cellulose ester | 0.65 g L−1 of Chlorella vulgaris | 11.6–20.5 | 23.2–41.0 | [37] |
| Axial vibration membrane | PVDF | 0.3 g L−1 of Chlorella pyrenoidosa | 60.0 | 85.7 | [38] |
| Parameters | Pristine NFM | Treated NFM |
|---|---|---|
| Thickness (mm) | 0.22 ± 0.08 | 0.18 ± 0.02 |
| Porosity (%) | 71.30 ± 2.00 | 68.75 ± 0.45 |
| Mean Pore Size (µm) | 0.20 ± 0.03 | 0.12 ± 0.05 |
| Average fiber diameters | 138.5 ± 45.01 | 187 ± 141.3 |
| Tensile strength (MPa) | 737.56 ± 10.24 | 2373.27 ± 15.32 |
| Surface roughness (nm) | 231.10 ± 3.61 | 85.43 ± 2.30 |
| Contact Angle (°) | 56.01 ± 5.91 | 40.56 ± 5.29 |
| Clean water permeability (Lm−2·h−1·bar−1) | 18,701 ± 603 | 16,538 ± 254 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mat Nawi, N.I.; Abd Halim, N.S.; Lee, L.C.; Wirzal, M.D.H.; Bilad, M.R.; Nordin, N.A.H.; Putra, Z.A. Improved Nylon 6,6 Nanofiber Membrane in A Tilted Panel Filtration System for Fouling Control in Microalgae Harvesting. Polymers 2020, 12, 252. https://doi.org/10.3390/polym12020252
Mat Nawi NI, Abd Halim NS, Lee LC, Wirzal MDH, Bilad MR, Nordin NAH, Putra ZA. Improved Nylon 6,6 Nanofiber Membrane in A Tilted Panel Filtration System for Fouling Control in Microalgae Harvesting. Polymers. 2020; 12(2):252. https://doi.org/10.3390/polym12020252
Chicago/Turabian StyleMat Nawi, Normi Izati, Nur Syakinah Abd Halim, Leong Chew Lee, Mohd Dzul Hakim Wirzal, Muhammad Roil Bilad, Nik Abdul Hadi Nordin, and Zulfan Adi Putra. 2020. "Improved Nylon 6,6 Nanofiber Membrane in A Tilted Panel Filtration System for Fouling Control in Microalgae Harvesting" Polymers 12, no. 2: 252. https://doi.org/10.3390/polym12020252