Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials of Scaffolds
2.2. Scaffold Preparation Technique
2.3. Characterization of Scaffold
2.3.1. Structure Characterization
2.3.2. Study of Mechanical Properties
2.3.3. Measurement of the Contact Angle
2.4. In Vitro Studies
2.4.1. Cell Culture
2.4.2. Cell Adhesion and Proliferation Analysis
2.5. In Vivo Implantation and Histologic Analysis
2.6. Statistics
3. Results and Discussions
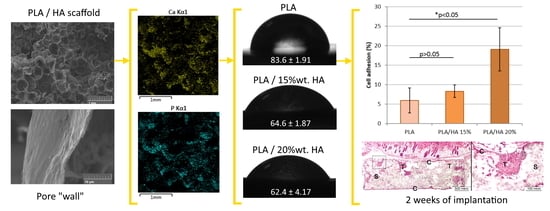
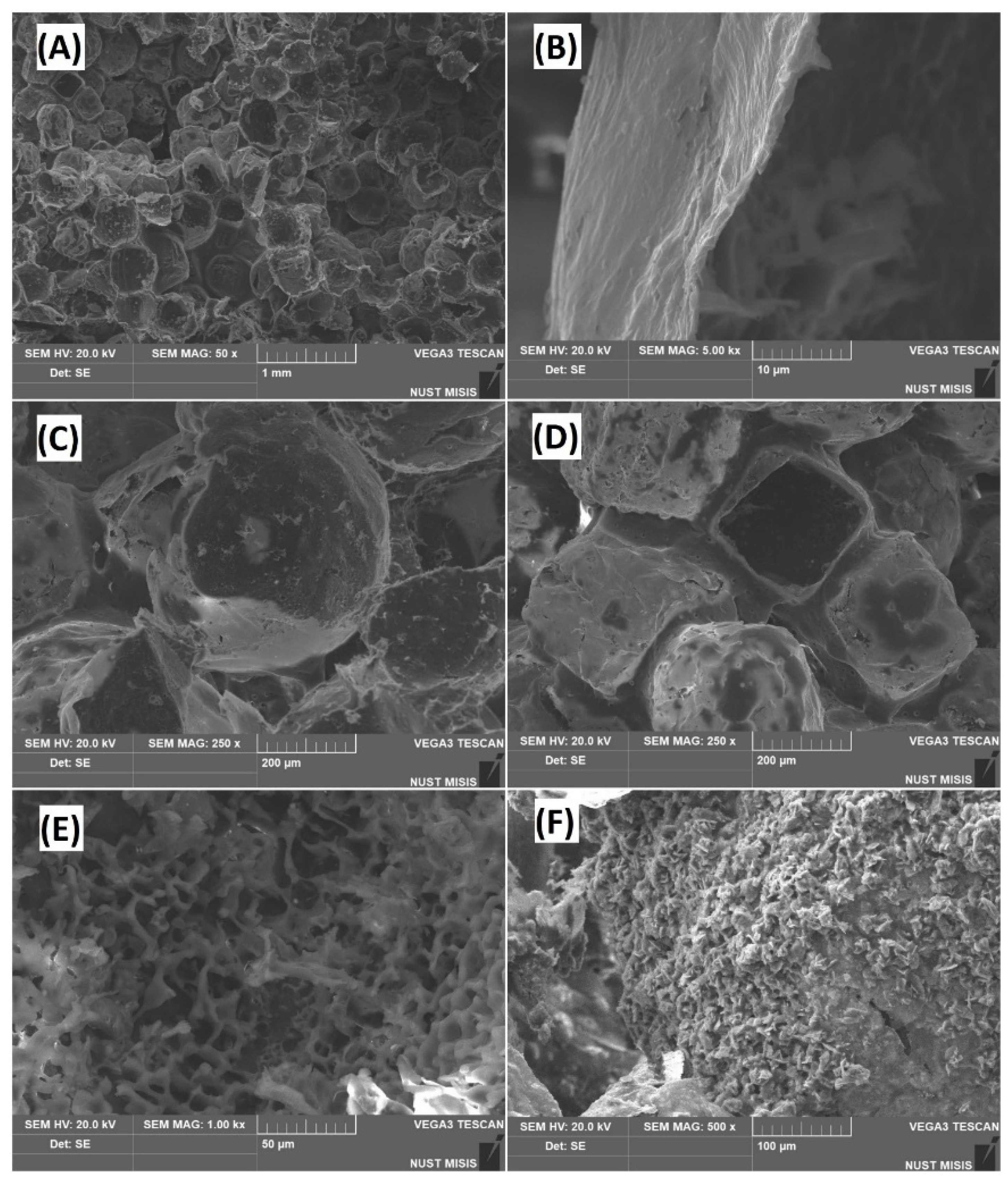
3.1. Microstructure Analysis
3.2. Porosity Calculation
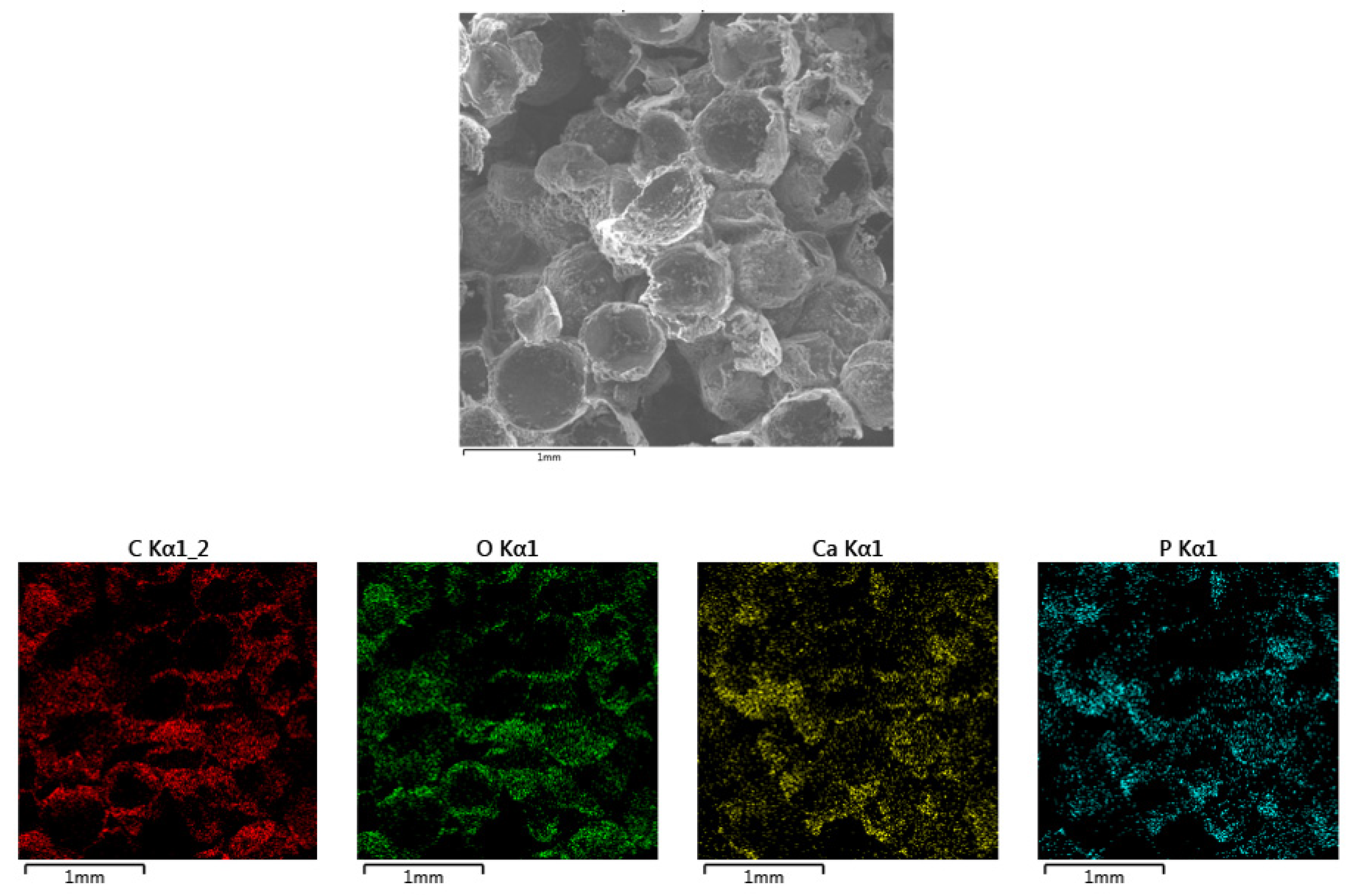
3.3. Sample Composition Analysis
3.4. Mechanical Testing
3.5. FT-IR Analysis
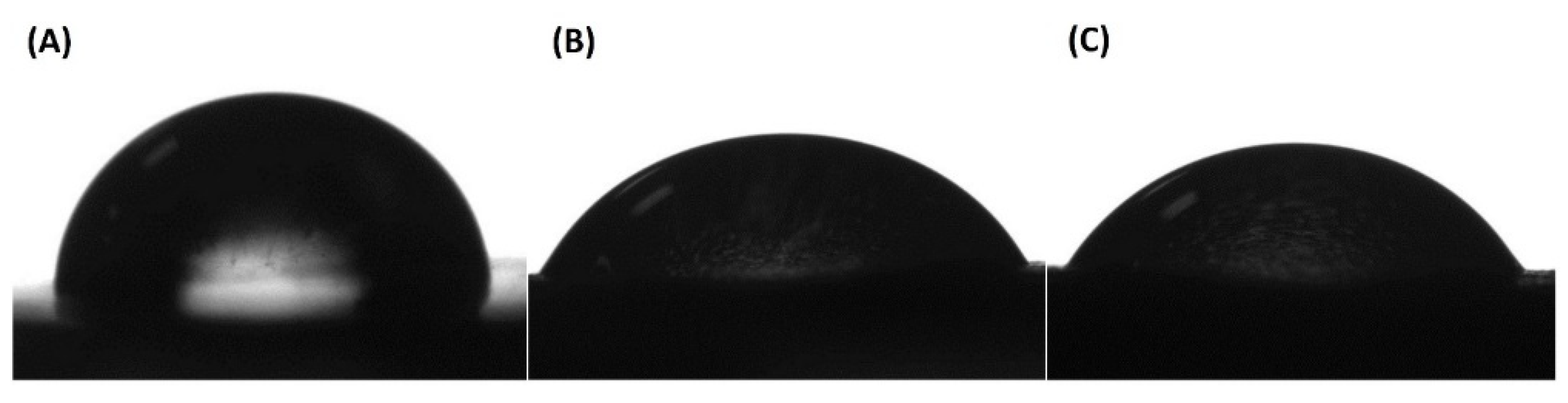
3.6. Assessment of Surface Hydrophilicity
3.7. In Vitro Experiment
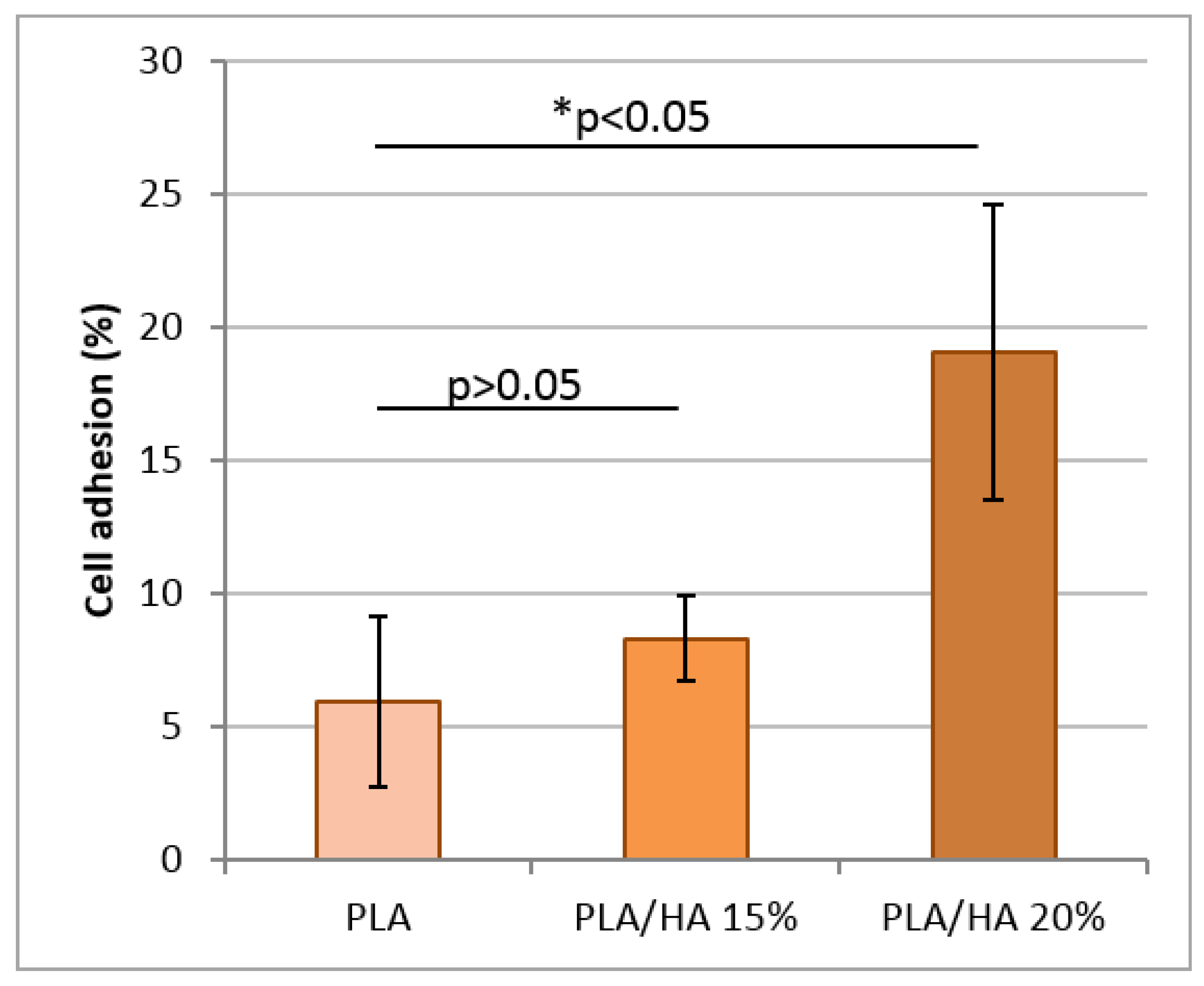
3.7.1. Cell Adhesion
3.7.2. Cell Proliferation
3.7.3. Cell Viability
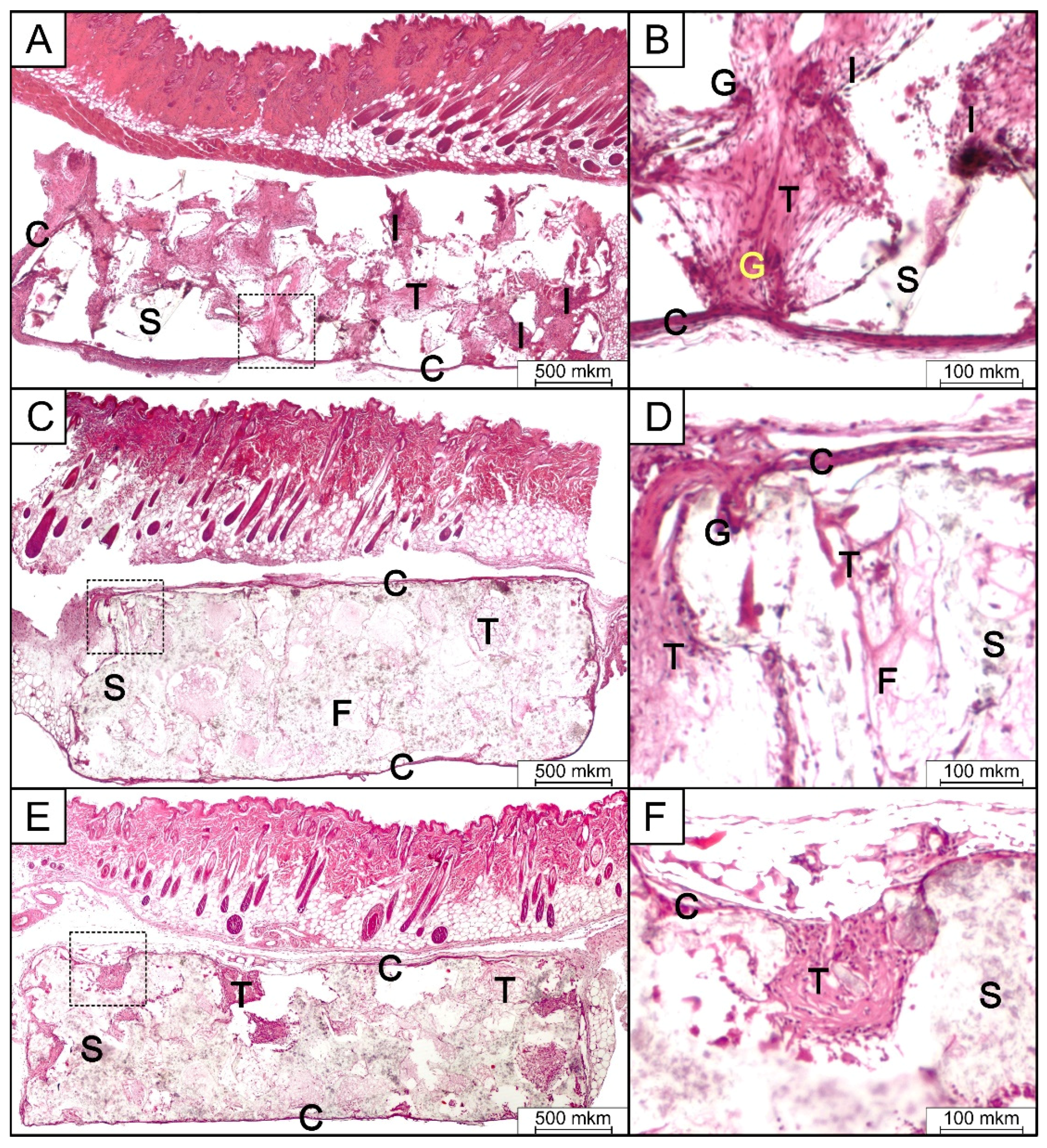
3.8. In Vivo Implantation and Histologic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Kinoshita, Y.; Maeda, H. Recent Developments of Functional Scaffolds for Craniomaxillofacial Bone Tissue Engineering Applications. Sci. World J. 2013, 2013, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Guo, D.; Zhou, X.; Sun, J.; Zhou, Y.; Fan, Y.; Zhou, X.; Wan, M.; Du, W.; Zheng, L. Disturbed Bone Remodelling Activity Varies in Different Stages of Experimental, Gradually Progressive Apical Periodontitis in Rats. Int. J. Oral Sci. 2019, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naenni, N.; Jung, R.E.; Hüsler, J.; Hämmerle, C.H.F.; Thoma, D.S.; Schneider, D. Randomized Clinical Study Assessing Two Membranes for Guided Bone Regeneration of Peri-Implant Bone Defects: Clinical and Histological Outcomes at 6 Months. Clin. Oral Implant. Res. 2016, 28, 1309–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; García, A.J. Biomaterial Strategies for Engineering Implants for Enhanced Osseointegration and Bone Repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized Bone Matrix in Bone Repair: History and Use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef]
- McKenzie, J.L.; Webster, T.J. Biomedical Materials; Narayan, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Park, Y.-W. Bioabsorbable Osteofixation for Orthognathic Surgery. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gaba, S.; Jindal, S.; Sharma, P.; Bali, R.K. To Evaluate the Efficacy of Biodegradable Plating System for Fixation of Maxillofacial Fractures: A Prospective Study. Nat. J. Maxillofac. Surg. 2013, 4, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Yolcu, Ü.; Alan, H.; Malkoç, S.; Bozkurt, S.B.; Hakki, S.S. Cytotoxicity Evaluation of Bioresorbable Fixation Screws on Human Gingival Fibroblasts and Mouse Osteoblasts by Real-Time Cell Analysis. J. Oral Maxillofac. Surg. 2015, 73, 1562.e1–1562.e10. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Nagano, D.; Shibata, A.; Sukegawa-Takahashi, Y.; Furuki, Y. The Clinical Feasibility of Newly Developed Thin Flat-Type Bioresorbable Osteosynthesis Devices for the Internal Fixation of Zygomatic Fractures. J. Craniofac. Surg. 2016, 27, 2124–2129. [Google Scholar] [CrossRef] [Green Version]
- Pina, S.; Ferreira, J.M.F. Bioresorbable Plates and Screws for Clinical Applications: A Review. J. Healthc. Eng. 2012, 3, 243–260. [Google Scholar] [CrossRef] [Green Version]
- Kanno, T.; Sukegawa, S.; Furuki, Y.; Nariai, Y.; Sekine, J. Overview of Innovative Advances in Bioresorbable Plate Systems for Oral and Maxillofacial Surgery. Jpn. Dent. Sci. Rev. 2018, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.A.; D’Ayala, G.G.; Laurienzo, P.; Malinconico, M.; Della Ragione, F.; Oliva, A. Development of Innovative Biopolymers and Related Composites for Bone Tissue Regeneration: Study of Their Interaction With Human Osteoprogenitor Cells. J. Appl. Biomater. Funct. Mater. 2012, 10, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R. Bioresorbable and Bioactive polymer/Bioglass® Composites With Tailored Pore Structure for Tissue Engineering Applications. Compos. Sci. Technol. 2003, 63, 2417–2429. [Google Scholar] [CrossRef]
- Yannas, I.V.; Burke, J.F. Design of an Artificial Skin. I. Basic Design Principles. J. Biomed. Mater. Res. 1980, 14, 65–81. [Google Scholar] [CrossRef]
- Antoniac, I.V. Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Liao, S.; Wang, W.; Uo, M.; Ohkawa, S.; Akasaka, T.; Tamura, K.; Cui, F.; Watari, F. A Three-Layered Nano-Carbonated hydroxyapatite/Collagen/PLGA Composite Membrane for Guided Tissue Regeneration. Biomaterials 2005, 26, 7564–7571. [Google Scholar] [CrossRef]
- Li, Z.; Yubao, L.; Aiping, Y.; Xuelin, P.; Xuejiang, W.; Xiang, Z. Preparation and in Vitro Investigation of chitosan/Nano-Hydroxyapatite Composite Used As Bone Substitute Materials. J. Mater. Sci. Mater. Electron. 2005, 16, 213–219. [Google Scholar] [CrossRef]
- McManus, A.J.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Evaluation of Cytocompatibility and Bending Modulus of nanoceramic/Polymer Composites. J. Biomed. Mater. Res. 2004, 72, 98–106. [Google Scholar] [CrossRef]
- Kaito, T.; Myoui, A.; Takaoka, K.; Saito, N.; Nishikawa, M.; Tamai, N.; Ohgushi, H.; Yoshikawa, H. Potentiation of the Activity of Bone Morphogenetic Protein-2 in Bone Regeneration by a PLA–PEG/Hydroxyapatite Composite. Biomaterials 2005, 26, 73–79. [Google Scholar] [CrossRef]
- Enderle, J.; Blanchard, S.; Bronzino, J. Introduction to Biomedical Engineering; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Salerno, A.; Fernández-Gutiérrez, M.; Del Barrio, J.S.R.; Pascual, C.D. Macroporous and Nanometre Scale Fibrous PLA and PLA–HA Composite Scaffolds Fabricated by a Bio Safe Strategy. RSC Adv. 2014, 4, 61491–61502. [Google Scholar] [CrossRef] [Green Version]
- Si, J.; Lin, J.; Su, C.; Yu, S.; Cui, Z.; Wang, Q.; Chen, W.; Turng, L.-S. Ultrasonication-Induced Modification of Hydroxyapatite Nanoparticles onto a 3D Porous Poly(lactic Acid) Scaffold With Improved Mechanical Properties and Biocompatibility. Macromol. Mater. Eng. 2019, 304. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D Printed Porous PLA/NHA Composite Scaffolds With Enhanced Osteogenesis and Osteoconductivity in Vivo for Bone Regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Spanou, A.; Diez-Escudero, A.; Persson, C. 3D-Printed PLA/HA Composite Structures as Synthetic Trabecular Bone: A Feasibility Study Using Fused Deposition Modeling. J. Mech. Behav. Biomed. Mater. 2020, 103, 103608. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Li, Q.; Bai, N.; Dong, H.; Li, D. Porous Stable poly(lactic acid)/ethyl cellulose/Hydroxyapatite Composite Scaffolds Prepared by a Combined Method for Bone Regeneration. Carbohydr. Polym. 2018, 180, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Hammouti, B.; Akartasse, N.; Berrabah, M.; Elidrissi, A.; Jodeh, S.; Hamed, O.; Abidi, N. Novel Tricomponenets Composites Films from Polylactic Acid/Hydroxyapatite/Poly-Caprolactone Suitable for Biomedical Applications. J. Mater. Environ. Sci. 2016, 7, 761–769. [Google Scholar]
- Pandele, A.M.; Constantinescu, A.E.; Radu, I.C.; Miculescu, F.; Voicu, Ș.I.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-Structured Hydroxyapatite Composite Films. Materials 2020, 13, 274. [Google Scholar] [CrossRef] [Green Version]
- Senatov, F.; Anisimova, N.; Kiselevskiy, M.; Kopylov, A.; Tcherdyntsev, V.; Maksimkin, A. Polyhydroxybutyrate/Hydroxyapatite Highly Porous Scaffold for Small Bone Defects Replacement in the Nonload-Bearing Parts. J. Bionic Eng. 2017, 14, 648–658. [Google Scholar] [CrossRef]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; et al. Osteoinduction of Porous Ti Implants with a Channel Structure Fabricated by Selective Laser Melting. Acta Biomater. 2011, 7, 2327–2336. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D.L. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Jing, D.; Ding, J. A Comparative Study of Porous Scaffolds With Cubic and Spherical Macropores. Polymers 2005, 46, 4979–4985. [Google Scholar] [CrossRef]
- Vadgama, P. Surfaces and Interfaces for Biomaterials; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Akindoyo, J.O.; Beg, M.D.; Ghazali, S.; Heim, H.P.; Feldmann, M. Effects of Surface Modification on Dispersion, Mechanical, Thermal and Dynamic Mechanical Properties of Injection Molded PLA-Hydroxyapatite Composites. Compos. Part A Appl. Sci. Manuf. 2017, 103, 96–105. [Google Scholar] [CrossRef]
- Liuyun, J.; Chengdong, X.; Lixin, J.; Dongliang, C.; Qing, L. Effect of N-HA Content on the Isothermal Crystallization, Morphology and Mechanical Property of N-HA/PLGA Composites. Mater. Res. Bull. 2013, 48, 1233–1238. [Google Scholar] [CrossRef]
- Zare, R.N.; Doustkhah, E.; Assadi, M.H.N. Three-Dimensional Bone Printing Using Hydroxyapatite-PLA Composite. Mater. Today Proc. 2019. [Google Scholar] [CrossRef]
- Larrañaga, A.; Diamanti, E.; Rubio, E.; Palomares, T.; Alonso-Varona, A.; Aldazabal, P.; Martín, F.; Sarasua, J.-R. A Study of the Mechanical Properties and Cytocompatibility of Lactide and Caprolactone Based Scaffolds Filled With Inorganic Bioactive Particles. Mater. Sci. Eng. C 2014, 42, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Anthony, P.; Chowdhury, A. High Molecular Weight Poly(lactic Acid) Synthesized With Apposite Catalytic Combination and Longer Time. Orient. J. Chem. 2018, 34, 1984–1990. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/Poly(ethylene Glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Ganeles, J.; Zöllner, A.; Jackowski, J.; Bruggenkate, C.T.; Beagle, J.; Guerra, F. Immediate and Early Loading of Straumann Implants With a Chemically Modified Surface (SLActive) in the Posterior Mandible and Maxilla: 1-Year Results from a Prospective Multicenter Study. Clin. Oral Implant. Res. 2008, 19, 1119–1128. [Google Scholar] [CrossRef]
- Rani, V.D.; Vinoth-Kumar, L.; Anitha, V.; Manzoor, K.; Deepthy, M.; Shantikumar, V.N. Osteointegration of Titanium Implant Is Sensitive to Specific Nanostructure Morphology. Acta Biomater. 2012, 8, 1976–1989. [Google Scholar] [CrossRef]
- Zhao, L.; Hua, L.; Huo, K.; Zhang, Y.; Wu, Z.; Chu, P.K. Mechanism of Cell Repellence on Quasi-Aligned Nanowire Arrays on Ti Alloy. Biomaterials 2010, 31, 8341–8349. [Google Scholar] [CrossRef]
- Mitra, J.; Tripathi, G.; Sharma, A.; Basu, B. Scaffolds for Bone Tissue Engineering: Role of Surface Patterning on Osteoblast Response. RSC Adv. 2013, 3, 11073–11094. [Google Scholar] [CrossRef]
- Chang, H.-I.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffold. Regen. Med. Tissue Eng. Cells Biomater. 2011. [Google Scholar] [CrossRef] [Green Version]
- Ramot, Y.; Nyska, A.; Markovitz, E.; Dekel, A.; Klaiman, G.; Zada, M.H.; Domb, A.J.; Maronpot, R.R. Long-Term Local and Systemic Safety of Poly(l-Lactide-Co-Epsilon-Caprolactone) After Subcutaneous and Intra-Articular Implantation in Rats. Toxicol. Pathol. 2015, 43, 1127–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyska, A.; Schiffenbauer, Y.S.; Brami, C.T.; Maronpot, R.R.; Ramot, Y. Histopathology of Biodegradable Polymers: Challenges in Interpretation and the Use of a Novel Compact MRI for Biocompatibility Evaluation. Polym. Adv. Technol. 2014, 25, 461–467. [Google Scholar] [CrossRef]
- Maiborodin, I.; Kuznetsova, I.V.; Beregovoi, E.A.; Shevela, A.I.; Barannik, M.I.; Maiborodina, V.I.; Manaev, A.A. Reaction of Rat Tissues to Implantation of Lactic Acid-Based Biodegradable Polymer. Bull. Exp. Biol. Med. 2014, 156, 874–879. [Google Scholar] [CrossRef] [PubMed]




| Samples | Ultimate Strength, MPa | Young’s Modulus, MPa | Elongation at Break, % |
|---|---|---|---|
| Cancellous Bone | 80–90 | 50–500 | <1 |
| PLA | 1.53 ± 0.22 | 89.01 ± 12.74 | 6.42 ± 2.0 |
| PLA/HA 15% | 2.47 ± 0.19 | 177.24 ± 53.62 | 2.23 ± 0.54 |
| PLA/HA 20% | 1.41 ± 0.37 | 183.56 ± 39.60 | 1.15 ± 0.55 |
| Sample | The Value of the Wetting Angle, ° |
|---|---|
| PLA | 83.6 ± 1.91 |
| PLA/HA 15% | 64.6 ± 1.87 |
| PLA/HA 20% | 62.4 ± 4.17 |
| PLA | PLA/HA 15% | PLA/HA 20% | |
|---|---|---|---|
| Polymorphonuclear cells | 2 * | 0 | 1 |
| Lymphocytes | 3 | 2 | 1 |
| Plasma cells | 1 | 1 | 1 |
| Macrophages | 1 | 1 | 1 |
| Giant cells | 2 | 1 | 1 |
| Necrosis | 0 | 0 | 0 |
| Fibrosis | 1 | 1 | 1 |
| Fatty infiltrate | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers 2020, 12, 2938. https://doi.org/10.3390/polym12122938
Zimina A, Senatov F, Choudhary R, Kolesnikov E, Anisimova N, Kiselevskiy M, Orlova P, Strukova N, Generalova M, Manskikh V, et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers. 2020; 12(12):2938. https://doi.org/10.3390/polym12122938
Chicago/Turabian StyleZimina, Anna, Fedor Senatov, Rajan Choudhary, Evgeniy Kolesnikov, Natalya Anisimova, Mikhail Kiselevskiy, Polina Orlova, Natalia Strukova, Mariya Generalova, Vasily Manskikh, and et al. 2020. "Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction" Polymers 12, no. 12: 2938. https://doi.org/10.3390/polym12122938