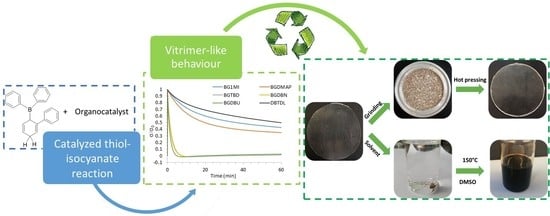
Recyclable Organocatalyzed Poly(Thiourethane) Covalent Adaptable Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Organocatalysts (BG)
2.3. Preparation of the Formulations
2.4. Sample Preparation
2.5. Stress-Relaxation Tests
2.6. Thermal Degradation Studies
2.7. FTIR Analysis
2.8. Gas Chromatography-Mass Spectrometry (GC-MS) Analyses
2.9. Recycling
2.10. Dissolution Experiments
2.11. Kinetic Analysis
3. Results and Discussion
3.1. Study of the Effect of the Catalyst on the Relaxation Behavior
3.2. Study of the Effect of the Stoichiometry of the Formulation
3.3. Study of the Thermal Stability of the PTUs Prepared
3.4. Study of the Recycling Process
3.5. Dissolution Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef]
- Podgórski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward Stimuli-Responsive Dynamic Thermosets through Continuous Development and Improvements in Covalent Adaptable Networks (CANs). Adv. Mater. 2020, 32, 1906876. [Google Scholar] [CrossRef]
- Guerre, M.; Taplan, C.; Winne, J.M.; Du Prez, F.E. Vitrimers: Directing chemical reactivity to control material properties. Chem. Sci. 2020, 11, 4855–4870. [Google Scholar] [CrossRef] [Green Version]
- Elling, B.R.; Dichtel, W.R. Reprocessable Cross-Linked Polymer Networks: Are Associative Exchange Mechanisms Desirable? ACS Cent. Sci. 2020, 6, 1488–1496. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Torkelson, J.M. Reprocessable polymer networks via thiourethane dynamic chemistry: Recovery of cross-link density after recycling and proof of principle solvolysis leading to monomer recovery. Macromolecules 2019, 52, 8207–8216. [Google Scholar] [CrossRef]
- Gamardella, F.; Guerrero, F.; De la Flor, S.; Ramis, X.; Serra, A. A new class of vitrimers based on aliphatic poly(thiourethane) networks with shape memory and permanent shape reconfiguration. Eur. Polym. J. 2020, 122, 109361. [Google Scholar] [CrossRef]
- Gamardella, F.; De la Flor, S.; Ramis, X.; Serra, A. Recyclable poly(thiourethane) vitrimers with high Tg. Influence of the isocyanate structure. React. Funct. Polym. 2020, 151, 104574. [Google Scholar] [CrossRef]
- Wen, Z.; Han, X.; Fairbanks, B.D.; Yang, K.; Bowman, C.N. Development of thiourethanes as robust, reprocessable networks. Polymer 2020, 122715. [Google Scholar] [CrossRef]
- Ireni, N.G.; Narayan, R.; Basak, P.; Raju, K.V.S.N. Poly(thiourethane-urethane-urea) as anticorrosion coatings with impressive optical properties. Polymer 2016, 97, 370–379. [Google Scholar] [CrossRef]
- Jaffrennou, B.; Droger, N.; Mechin, F.; Halary, J.L.; Pascault, J.P. Characterization structural transitions and properties of a tightly crosslinked polythiourethane network for optical applications. e-Polymers 2005, 82, 1618–7229. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, Z.; Zou, W.; Zhao, Q.; Xie, T. Thermoset shape-memory polyurethane with intrinsic plasticity enabled by transcarbamoylation. Angew. Chem. 2016, 128, 11593–11597. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Jin, K.; Torkelson, J.M. Reprocessable polyhydroxyurethane networks exhibiting full property recovery and concurrent associative and dissociative dynamic chemistry via transcarbamoylation and reversible cyclic carbonate aminolysis. Polym. Chem. 2017, 8, 6349–6355. [Google Scholar] [CrossRef]
- Fortman, D.J.; Sheppard, D.T.; Dichtel, W.R. Reprocessing cross-linked polyurethanes by catalyzing carbamate exchange. Macromolecules 2019, 52, 6330–6335. [Google Scholar] [CrossRef]
- Brutman, J.P.; Fortman, D.J.; De Hoe, G.X.; Dichtel, W.R.; Hillmyer, M.A. Mechanistic study of stress relaxation in urethane-containing polymer networks. J. Phys. Chem. B 2019, 123, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Kultys, A.; Rogulska, M.; Pikus, S. The synthesis and characterization of new thermoplastic poly (thiourethane−urethane)s. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1770–1782. [Google Scholar] [CrossRef]
- Lu, C.; Guan, C.; Liu, Y.; Cheng, Y.; Yang, B. PbS/Polymer nanocomposite optical materials with high refractive index. Chem. Mater. 2005, 17, 2448–2454. [Google Scholar] [CrossRef]
- Chandrasekaran, S. Click Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Shin, J.; Matsushima, H.; Comer, C.M.; Bowman, C.N.; Hoyle, C.E. Thiol-isocyanate-ene ternary networks by sequential and simultaneous thiol click reactions. Chem. Mater. 2010, 22, 2616–2625. [Google Scholar] [CrossRef]
- Gamardella, F.; Ramis, X.; De la Flor, S.; Serra, A. Preparation of poly(thiourethane) thermosets by controlled thiol-isocyanate click reaction using a latent organocatalyst. React. Funct. Polym. 2019, 134, 174–182. [Google Scholar] [CrossRef]
- Oliveira, V.D.G.; Cardoso, M.F.D.C.; Forezi, L.D.S.M. Organocatalysis: A Brief Overview on Its Evolution and Applications. Catalysts 2018, 8, 605. [Google Scholar] [CrossRef] [Green Version]
- Sardon, H.; Pascual, A.; Mecerreyes, D.; Taton, D.; Cramail, H.; Hedrick, J.L. Synthesis of polyurethanes using organocatalysis: A perspective. Macromolecules 2015, 48, 3153–3165. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020, 104, 101233. [Google Scholar] [CrossRef]
- Denissen, W.; Droesbeke, M.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Chemical control of the viscoelastic properties of vinylogous urethane vitrimers. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Podgórski, M.; Han, X.; Bowman, C.N. Chemical recycling of poly(thiourethane) thermosets enabled by dynamic thiourethane bonds. Polym. Chem. 2020, 11, 6879–6883. [Google Scholar] [CrossRef]
- Maji, B.; Stephenson, D.S.; Mayr, H. Guanidines: Highly Nucleophilic Organocatalysts. ChemCatChem 2012, 4, 993–999. [Google Scholar] [CrossRef]
- Baidya, M.; Mayr, H. Nucleophilicities and carbon basicities of DBU and DBN. Chem. Commun. 2008, 15, 1792–1794. [Google Scholar] [CrossRef]
- Rodima, T.; Kaljurand, I.; Pihl, A.; Mäemets, V.; Leito, I.; Koppel, I.A. Acid-base equilibria in nonpolar media. 2.1 Self-consistent basicity scale in THF solution ranging from 2-methoxypyridine to EtP1(pyrr) phosphazene. J. Org. Chem. 2002, 67, 1873–1881. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; Ramis, X. Latent curing of epoxy-thiol thermosets. Polymer 2017, 116, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Konuray, O.; Liendo, F.; Fernández-Francos, X.; Serra, À.; Sangermano, M.; Ramis, X. Sequential curing of thiol-acetoacetate-acrylate thermosets by latent Michael addition reactions. Polymer 2017, 113, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Gao, J.P.; Wang, Z.Y. Bicyclic guanidinium tetraphenylborate: A photobase Generator and A Photocatalyst for living anionic ring-opening polymerization and cross-linking of polymeric materials containing ester and hydroxy groups. J. Am. Chem. Soc. 2008, 130, 8130–8131. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Birmingham, AL, USA, 2015; pp. 166–231. [Google Scholar]
- Vyazovkin, S.; Sbirrazzuoli, N. Kinetic methods to study isothermal and nonisothermal epoxy-anhydride cure. Macromol. Chem. Phys. 1999, 200, 2294–2303. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Ramis, X.; Cadenato, A.; Salla, J.M.; Morancho, J.M.; Vallés, A.; Contat, L.; Ribes, A. Thermal degradation of polypropylene/starch-based materials with enhanced biodegradability. Polym. Degrad. Stab. 2004, 86, 483–491. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethanes. Chem. Rev. 2012, 113, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Rogulska, M.; Kultys, A.; Olszewska, E. New thermoplastics poly(thiourethane-urethane) elastomers based on hexane-1,6-diyl diisocyanate (HDI). J. Therm. Anal. Calorim. 2013, 114, 903–916. [Google Scholar] [CrossRef] [Green Version]













| Formulation | Diisocyanate (g) | Thiol (g) | BGDBU 0.1% (mg) |
|---|---|---|---|
| 10% exc HDI | 2.09 | 3.00 | 9.30 |
| Stochiometric | 1.90 | 3.00 | 9.30 |
| 10% exc S3 | 1.90 | 3.30 | 9.30 |
| Samples | τ0.37 (min) 1 |
|---|---|
| BG1MI | Not reached in 1 h |
| BGDMAP | 45 |
| BGTBD | 1.7 |
| BGDBN | 1.7 |
| BGDBU | 1.3 |
| DBTDL | Not reached in 1 h |
| Samples | Εa (kJ/mol) | Tv (°C) | τ0.37−130 °C (min) | τ0.37−140 °C (min) | τ0.37−150 °C (min) | τ0.37−160 °C (min) | τ0.37−170 °C (min) |
|---|---|---|---|---|---|---|---|
| 10% exc HDI | 132 | 94 | 27.8 | 11.7 | 4.7 | 1.9 | 0.8 |
| Stoichiometric | 117 | 87 | 21.5 | 13.0 | 4.8 | 1.9 | 1.1 |
| 10% exc S3 | 127 | 96 | 45.2 | 16.0 | 6.9 | 2.7 | 1.5 |
| Samples | T2% (°C) | Εa I peak (kJ/mol) | Εa II peak (kJ/mol) | Εa III peak (kJ/mol) |
|---|---|---|---|---|
| 10% exc HDI | 209 | 127 | 170 | 257 |
| Stoichiometric | 209 | 130 | 158 | 275 |
| 10% exc S3 | 212 | 139 | 149 | 259 |
| Original | ||||||
|---|---|---|---|---|---|---|
| Samples | E1 (GPa) | σmax2 (MPa) | εmax3 (%) | Ttanδ4 (°C) | FWHM 5 (°C) | E’ 6 (MPa) |
| 10% exc HDI | 1.7 | 30.4 | 3.0 | 56.3 | 9.6 | 11.4 |
| Stoichiometric | 2.5 | 45.1 | 2.5 | 57.4 | 9.9 | 12.7 |
| 10% exc S3 | 1.8 | 31.4 | 2.7 | 49.0 | 10.8 | 9.6 |
| Recycled | ||||||
| Samples | E1 (GPa) | σmax2 (MPa) | κmax3 (%) | Ttanδ4 (°C) | FWHM 5 (°C) | E’ 6 (MPa) |
| 10% exc HDI | 1.6 | 28.9 | 3.1 | 56.3 | 10.7 | 11.3 |
| Stoichiometric | 2.1 | 43.3 | 2.8 | 57.5 | 9.2 | 13.0 |
| 10% exc S3 | 1.8 | 32.2 | 2.5 | 49.2 | 10.9 | 9.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamardella, F.; Muñoz, S.; De la Flor, S.; Ramis, X.; Serra, A. Recyclable Organocatalyzed Poly(Thiourethane) Covalent Adaptable Networks. Polymers 2020, 12, 2913. https://doi.org/10.3390/polym12122913
Gamardella F, Muñoz S, De la Flor S, Ramis X, Serra A. Recyclable Organocatalyzed Poly(Thiourethane) Covalent Adaptable Networks. Polymers. 2020; 12(12):2913. https://doi.org/10.3390/polym12122913
Chicago/Turabian StyleGamardella, Francesco, Sara Muñoz, Silvia De la Flor, Xavier Ramis, and Angels Serra. 2020. "Recyclable Organocatalyzed Poly(Thiourethane) Covalent Adaptable Networks" Polymers 12, no. 12: 2913. https://doi.org/10.3390/polym12122913