Study on Seed Emergence and Seedling Growth of Artemisia Desertorum with Superabsorbent Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Characterization of Superabsorbent Polymer
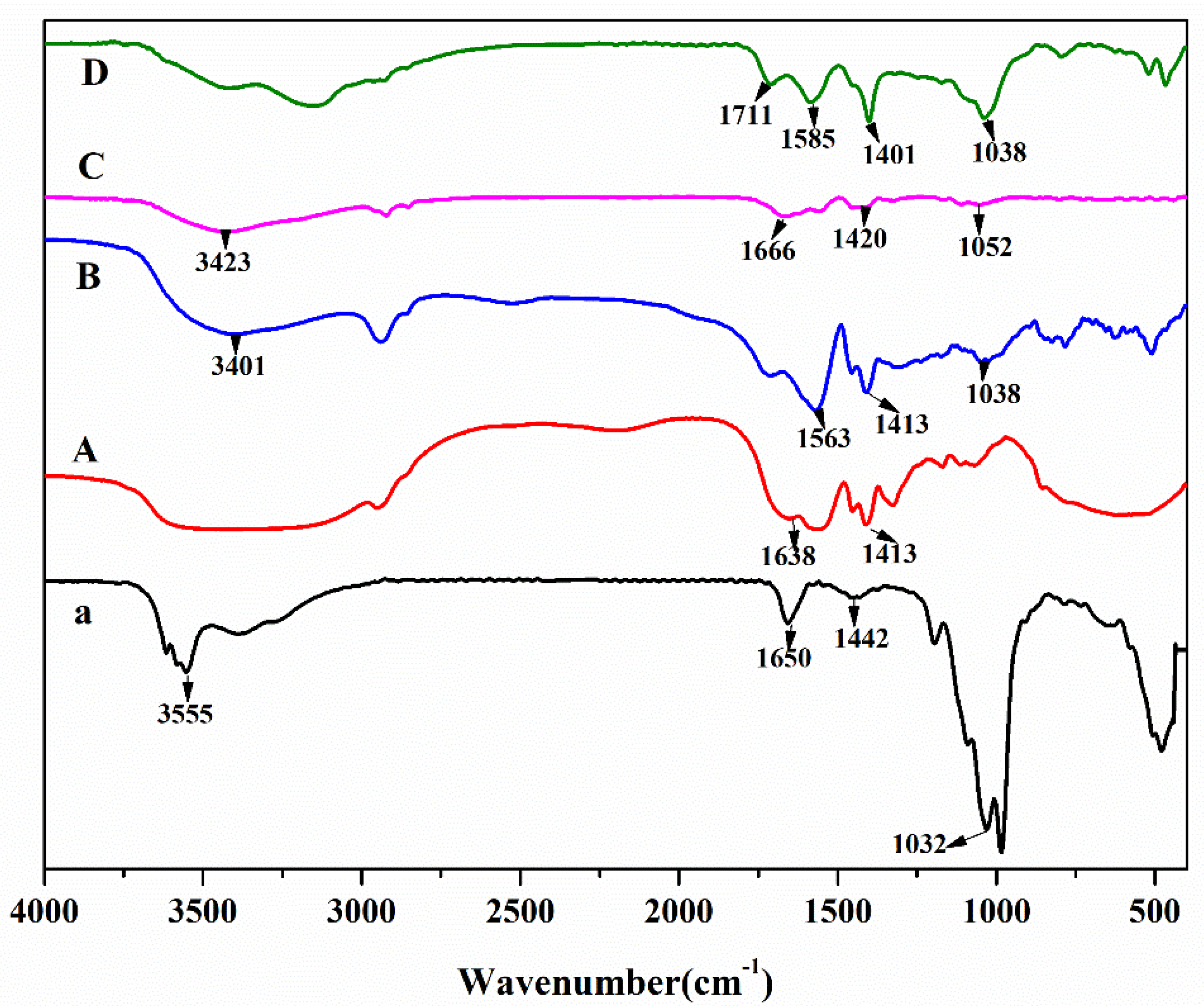
2.2.1. Fourier Transform Infrared Spectroscopy
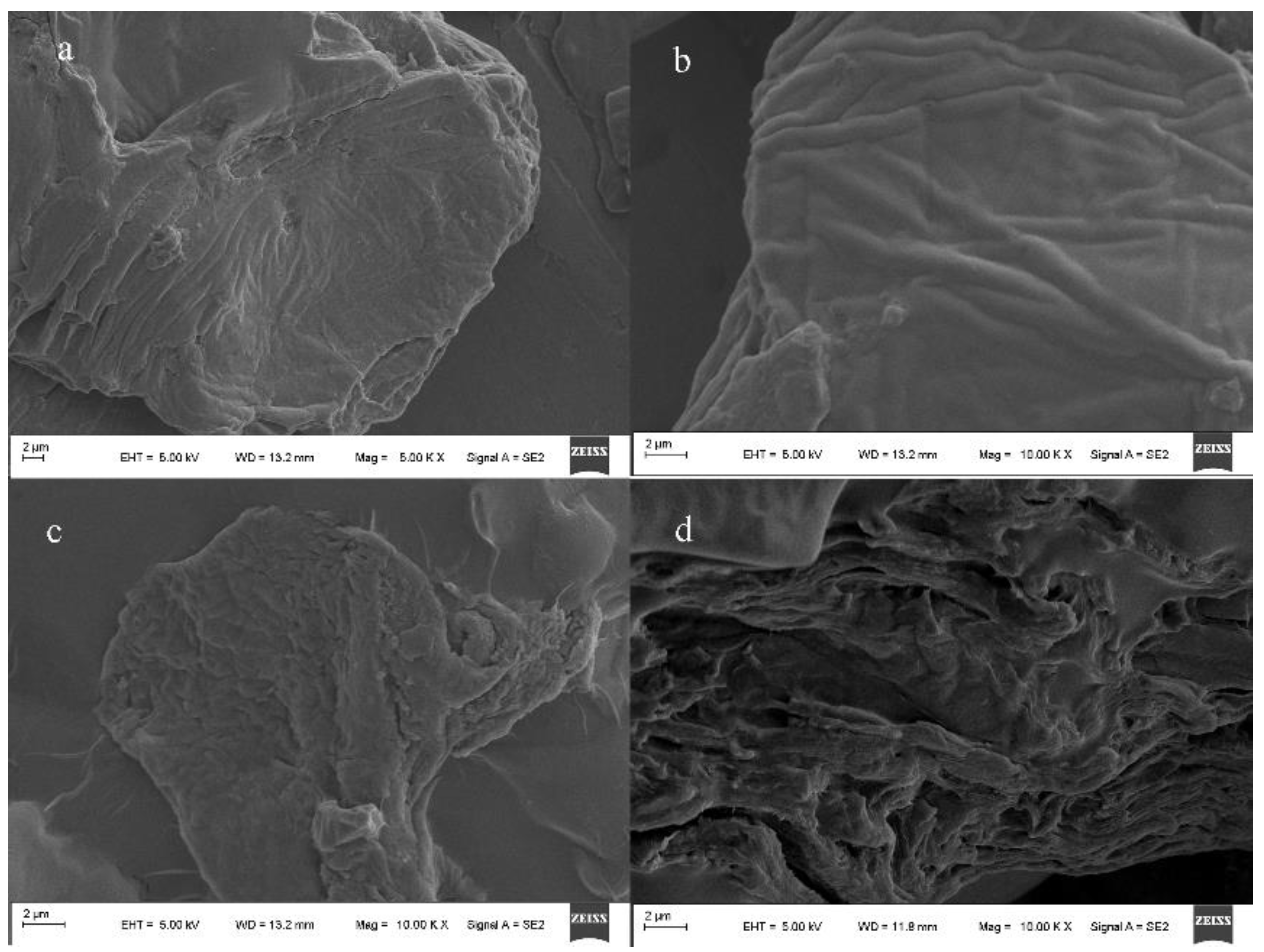
2.2.2. Morphology Analysis
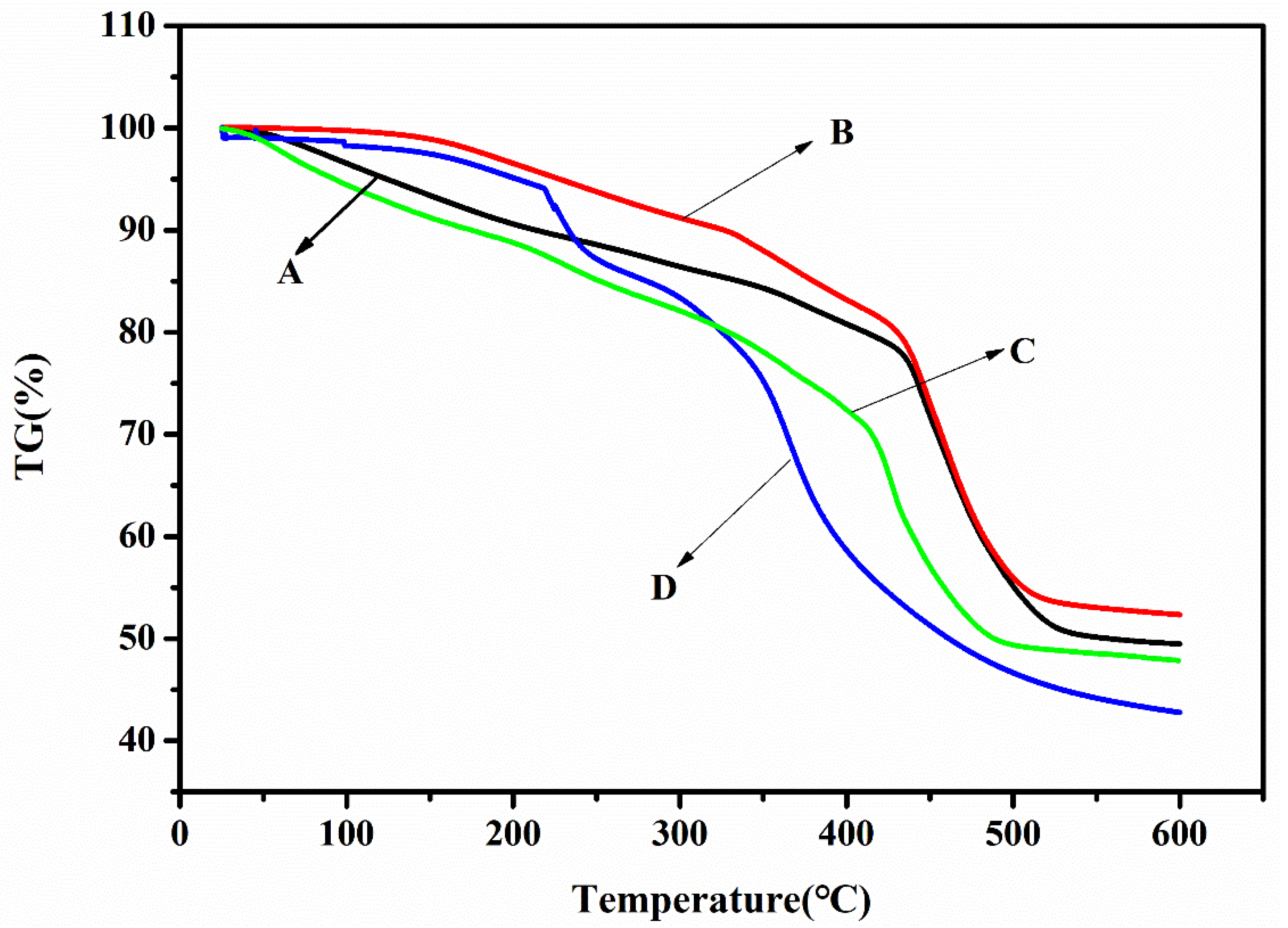
2.2.3. Thermal Gravimetric Analysis of Superabsorbent Polymer
2.3. Experiment Design
2.4. Determination of the Project
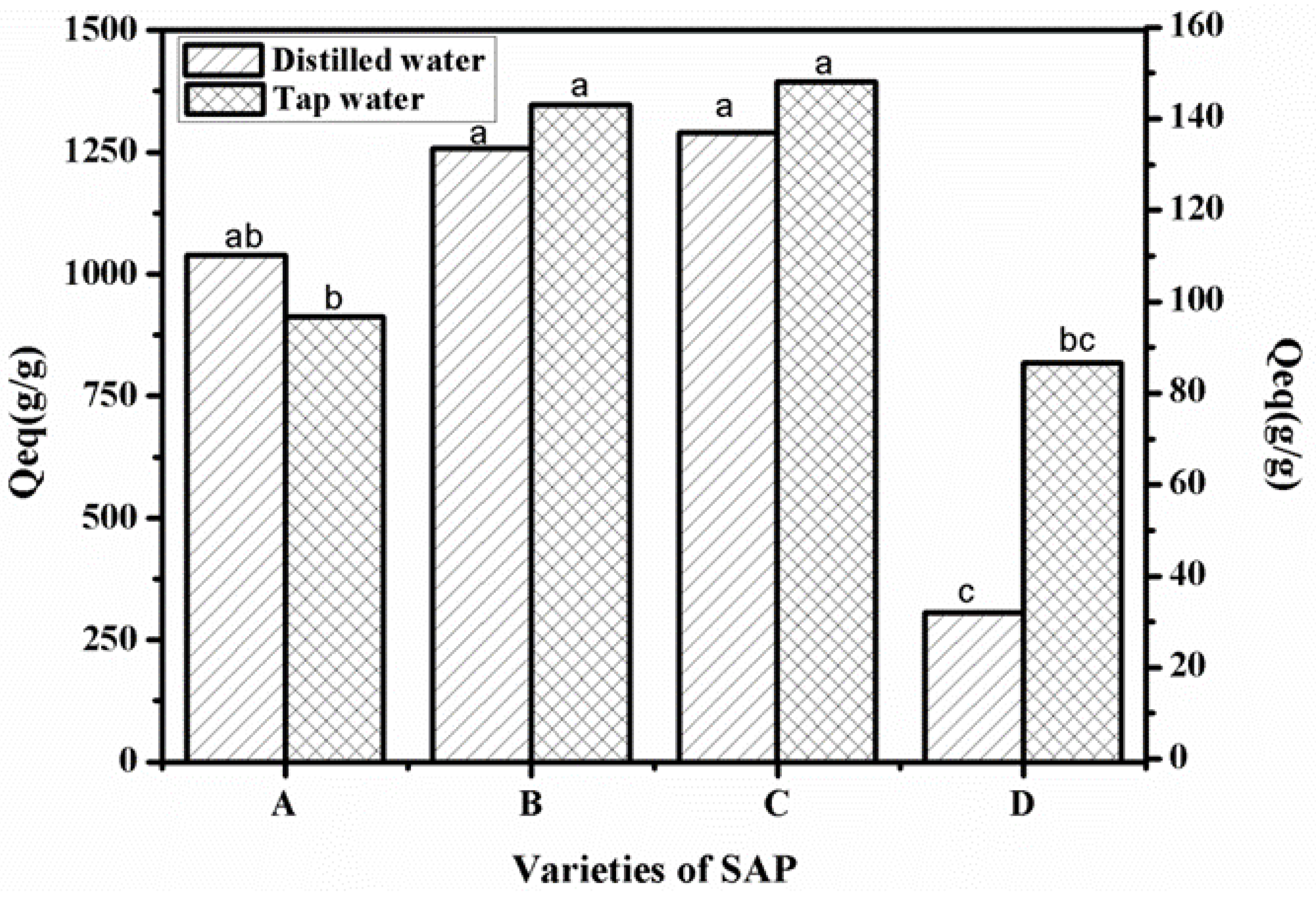
2.4.1. Balanced Swelling Rate Test of Superabsorbent Polymer in Distilled Water and Tap Water
2.4.2. Seed Emergence Rate and Emergence Potential
2.4.3. Water Consumption
2.4.4. Root length, Seedling Height, and Biomass
2.4.5. Data Statistics and Analysis
3. Results and Discussion
3.1. FTIR Analysis
3.2. Scanning Electron Microscope Analysis
3.3. Thermal Gravimetric Analysis
3.4. Water Absorption Capacity of Four Different Superabsorbent Polymers
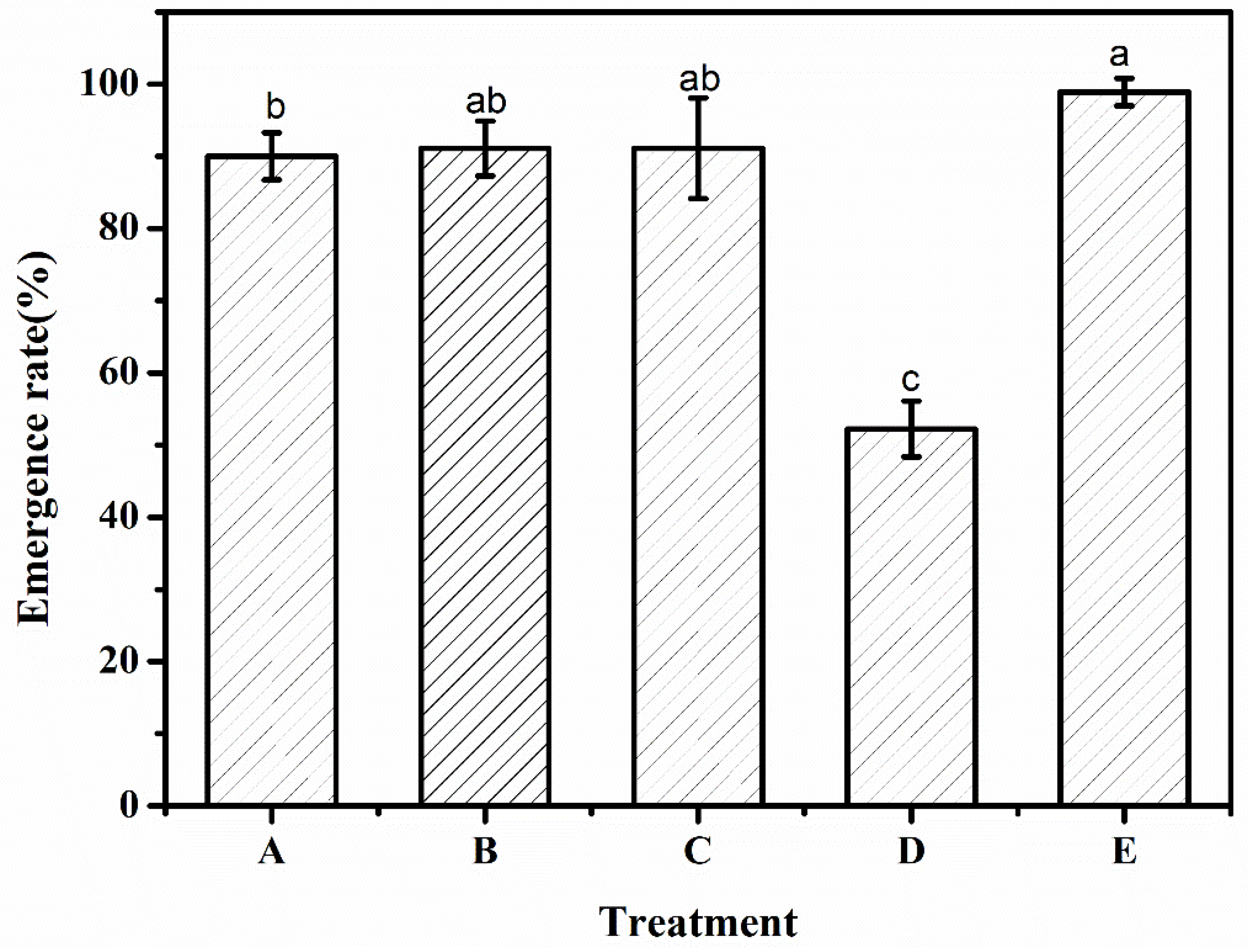
3.5. Emergence Rate
3.6. Emergence Potential
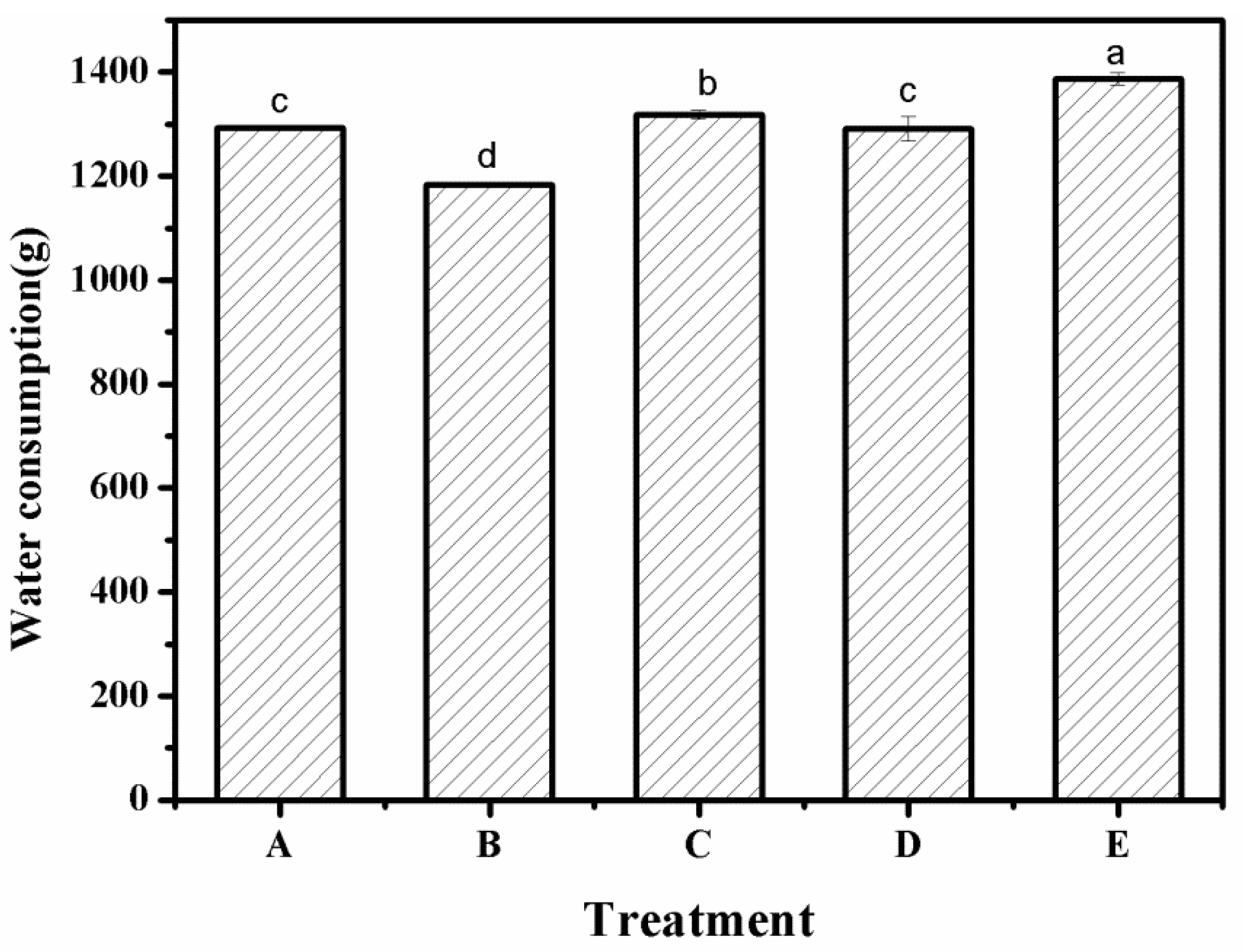
3.7. Water Consumption
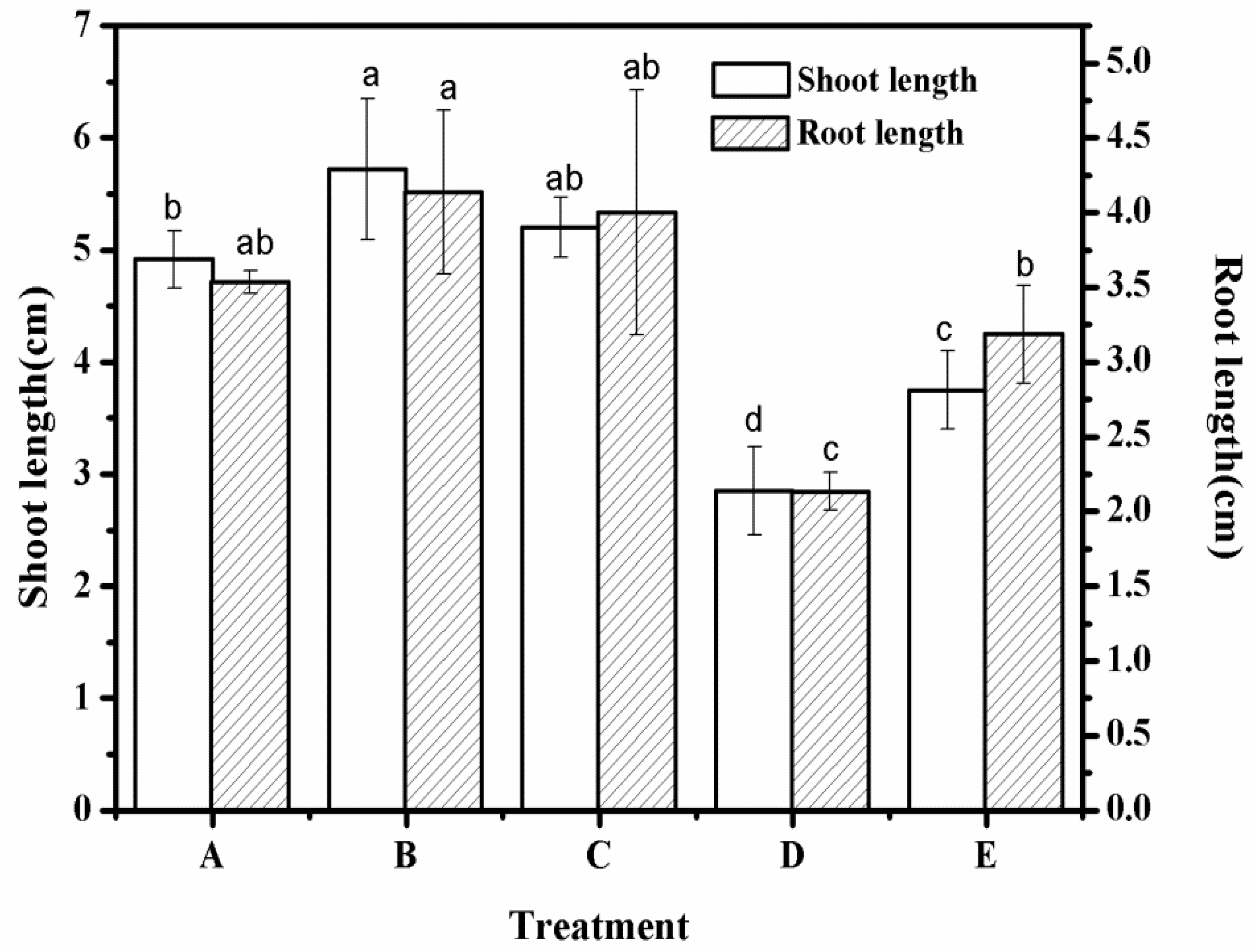
3.8. Root Length and Stem Length of Different Treatment Groups
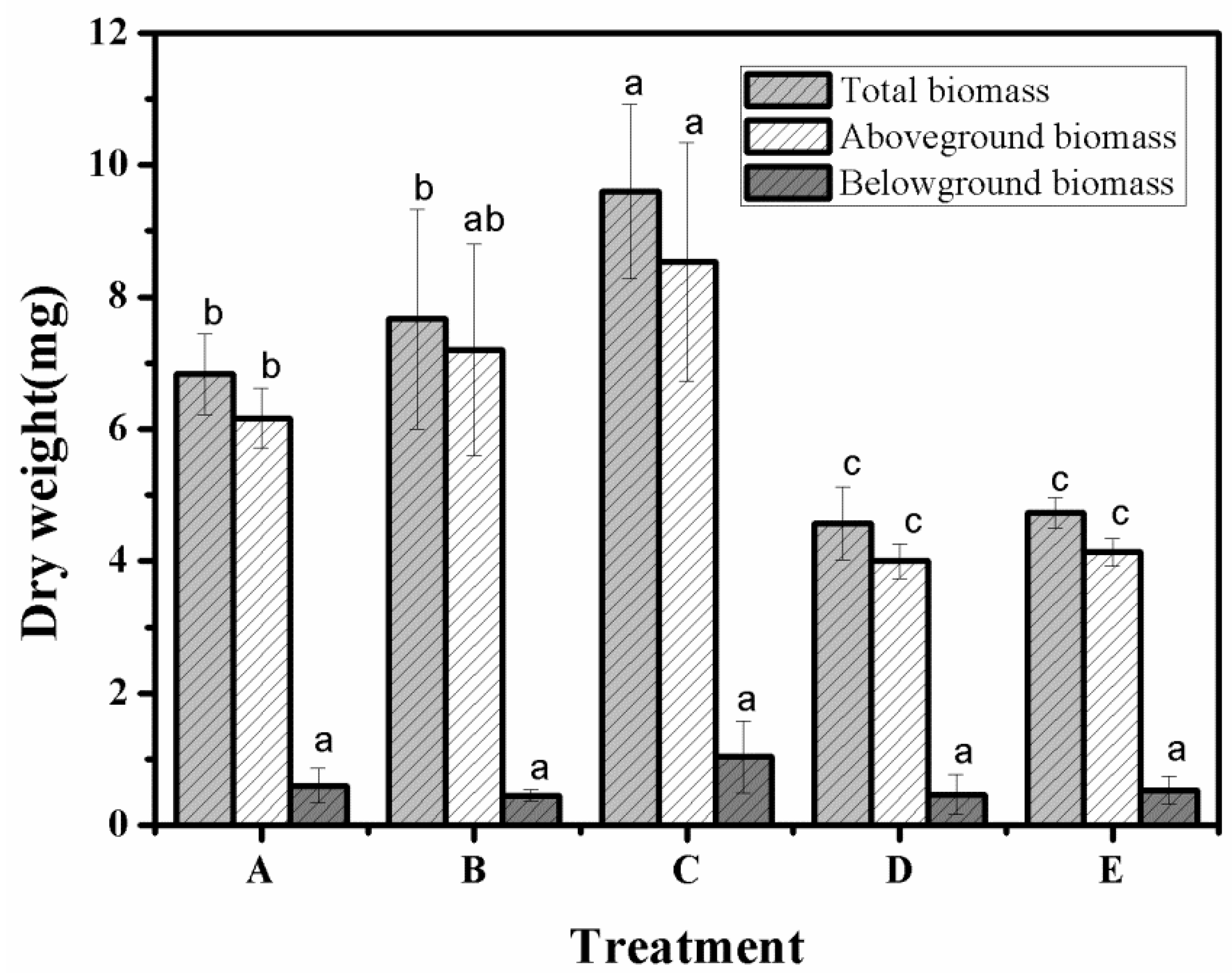
3.9. Aboveground Biomass, Belowground Biomass and Total Biomass in Different Treatment Groups
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ying, Z.; Yinqiao, X.; Zheng, W.; Shengyu, L. Advances in Application of Soil Amendments in Desert Control. J. Ludong Univ. 2019, 35, 51–58. [Google Scholar]
- Yuan, Z.; Guang, G.; Shu, Q.; Ming, Y.; Guo, D. Desertification detection and the evaluation indicators: A review. J. Arid Land Resour. Environ. 2019, 33, 81–87. [Google Scholar]
- Yang, Z. Seasonal variation of assembled and naturally recruited plants in a subtropical constructed wetland. Biodivers. Sci. 2005, 13, 527–534. [Google Scholar] [CrossRef]
- Chuan, S. Preliminary Study on Succession of Artemisia ordosica Community in Gonghe Sandy Grassland. Technol. Qinghai Agric. For. 2011. [Google Scholar] [CrossRef]
- Xiao, W.U.; Xue, Z.; Yi, Z. Protecting Cities and Towns from Blown-sand Disasters in Semi-arid Area of China —A case study of Dabqig Town in Uxin Banner of Inner Mongolia. J. Desert Res. 2013, 7. [Google Scholar]
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, K.; Wang, X.; Li, R.; Li, P. Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef]
- Wang, Z.; Ning, A.; Xie, P.; Gao, G.; Xie, L.; Li, X.; Song, A. Synthesis and swelling behaviors of carboxymethyl cellulose-based superabsorbent resin hybridized with graphene oxide. Carbohydr. Polym. 2017, 157, 48–56. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Yuan, M.; Bi, B.; Huang, J.; Zhuo, R.; Jiang, X. Thermosensitive and photocrosslinkable hydroxypropyl chitin-based hydrogels for biomedical applications. Carbohydr. Polym. 2018, 192, 10–18. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Wang, P. Effects of the multilayer structures on Exenatide release and bioactivity in microsphere/thermosensitive hydrogel system. Colloids Surf. B Biointerfaces 2018, 171, 85–93. [Google Scholar] [CrossRef]
- Lee, H.; Wong, H.; Buenfeld, N. Self-sealing of cracks in concrete using superabsorbent polymers. Cem. Concr. Res. 2016, 79, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, Y.; Zhao, J.; Tong, Z.; Jin, S. Preparation of SA-g-(PAA-co-PDMC) polyampholytic superabsorbent polymer and its application to the anionic dye adsorption removal from effluents. Sep. Purif. Technol. 2017, 188, 329–340. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, H. Development of a novel bentonite–acrylamide superabsorbent hydrogel for extinguishing gangue fire hazard. Powder Technol. 2018, 323, 486–494. [Google Scholar] [CrossRef]
- Tubert, E.; Amodeo, G.; Alvarez, M.; Tubert, F.; Baroli, I.; Amodeo, G. Synthesis and evaluation of a superabsorbent-fertilizer composite for maximizing the nutrient and water use efficiency in forestry plantations. J. Environ. Manag. 2018, 210, 239–254. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Liu, J.; Mei, H.; Yong, D.; Li, J. Preparation, swelling behaviors and fertilizer-release properties of sodium humate modified superabsorbent resin. Mater. Today Commun. 2019, 19, 124–130. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Chen, X.; Li, K. Synthesis of chitosan derivative graft acrylic acid superabsorbent polymers and its application as water retaining agent. Int. J. Biol. Macromol. 2018, 115, 754–761. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Su, Y.; Yue, Q.; Gao, B.; Su, Y. A novel wheat straw cellulose-based semi-IPNs superabsorbent with integration of water-retaining and controlled-release fertilizers. J. Taiwan Inst. Chem. Eng. 2015, 55, 170–179. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Y.; Huang, S.; Zhao, Y. Synthesis composite hydrogels from inorganic-organic hybrids based on leftover rice for environment-friendly controlled-release urea fertilizers. Sci. Total. Environ. 2018, 615, 422–430. [Google Scholar] [CrossRef]
- Zhang, H.; Luan, Q.; Huang, Q.; Tang, H.; Huang, F.-H.; Li, W.; Wan, C.; Liu, C.; Xu, J.; Guo, P.; et al. A facile and efficient strategy for the fabrication of porous linseed gum/cellulose superabsorbent hydrogels for water conservation. Carbohydr. Polym. 2017, 157, 1830–1836. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Satriani, A.; Catalano, M.; Scalcione, E. The role of superabsorbent hydrogel in bean crop cultivation under deficit irrigation conditions: A case-study in Southern Italy. Agric. Water Manag. 2018, 195, 114–119. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhang, F.-S. Recycling waste polyethylene film for amphoteric superabsorbent resin synthesis. Chem. Eng. J. 2018, 331, 169–176. [Google Scholar] [CrossRef]
- Simeng, B.; Xiaoran, S. Preparation and Structure Characterization of Graphene Nanoplatelets based on Humic Acid. Humic Acid 2019. [Google Scholar] [CrossRef]
- Shuwei, S.; Huiling, Z.; Caiyan, Y.U.; Ying, B.J.E.S. Technology. Experimental measurement and analysis of Raman/infrared methods for lithium batteries. Energy storage Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Pouria, S.; Meisam, N.; Gunter, B. Effect of UV-C Radiation on Seed Germination and Seedling Growth of Maize. Mol. Plant Breed. 2018, 16, 3312–3316. [Google Scholar] [CrossRef]
- Gao, J.; Yang, Q.; Ran, F.; Ma, G.; Lei, Z. Preparation and properties of novel eco-friendly superabsorbent composites based on raw wheat bran and clays. Appl. Clay Sci. 2016, 739–747. [Google Scholar] [CrossRef]
- Gharekhani, H.; Olad, A.; Mirmohseni, A.; Baybordi, A. Superabsorbent hydrogel made of NaAlg-g-poly(AA-co-AAm) and rice husk ash: Synthesis, characterization, and swelling kinetic studies. Carbohydr. Polym. 2017, 168, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Essawy, H.A.; Ghazy, M.B.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Montesano, F.F.; Parente, A.; Santamaria, P.; Sannino, A.; Di Serio, F. Biodegradable Superabsorbent Hydrogel IncreasesWater Retention Properties of Growing Media and Plant Growth. Agric. Agric. Sci. Procedia 2015, 4, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Hasija, V.; Sharma, K.; Kumar, V.; Sharma, S.; Sharma, V. Green synthesis of agar/Gum Arabic based superabsorbent as an alternative for irrigation in agriculture. Vacuum 2018, 157, 458–464. [Google Scholar] [CrossRef]
- Olad, A.; Gharekhani, H.; Mirmohseni, A.; Baybordi, A. Superabsorbent nanocomposite based on maize bran with integration of water-retaining and slow-release NPK fertilizer. Adv. Polym. Technol. 2017, 37, 1682–1694. [Google Scholar] [CrossRef]
- Hüttermann, A.; Orikiriza, L.J.B.; Agaba, H. Application of Superabsorbent Polymers for Improving the Ecological Chemistry of Degraded or Polluted Lands. CLEAN Soil Air Water 2009, 37, 517–526. [Google Scholar] [CrossRef]
- Naz, F.S.; Yusuf, M.; Khan, T.A.; Fariduddin, Q.; Ahmad, A. Low level of selenium increases the efficacy of 24-epibrassinolide through altered physiological and biochemical traits of Brassica juncea plants. Food Chem. 2015, 185, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tong, Z.; Geng, Y.; Li, Y.; Zhang, M. Biobased Polymer Composites Derived from Corn Stover and Feather Meals as Double-Coating Materials for Controlled-Release and Water-Retention Urea Fertilizers. J. Agric. Food Chem. 2013, 61, 8166–8174. [Google Scholar] [CrossRef]
- Lei, X.; Hai-xia, X.; Qing, L.; Ting, G. Scaling from leaf to whole plant in biomass and nitrogen content of Nitraria tangutorum seedlings. J. Beijing For. Univ. 2018, 40, 76–81. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Shu, X.; Yan, L.; Tan, Z.; Wang, J.; Tan, G.; Yu, W. Biomass variation, allocation pattern and heredity stability of Magnolia officinalis seedlings from different provenances. J. Fujian Agric. For. Univ. 2011, 40, 351–359. [Google Scholar]
- Dorraji, S.S.; Golchin, A.; Ahmadi, S. The Effects of Hydrophilic Polymer and Soil Salinity on Corn Growth in Sandy and Loamy Soils. CLEAN Soil Air Water 2010, 38, 584–591. [Google Scholar] [CrossRef]
- Islam, M.R.; Hu, Y.; Mao, S.; Mao, J.; Eneji, A.E.; Xue, X. Effectiveness of a water-saving super-absorbent polymer in soil water conservation for corn (Zea mays L.) based on eco-physiological parameters. J. Sci. Food Agric. 2011, 91, 1998–2005. [Google Scholar] [CrossRef]

| Varieties of SAP | Treatment | Sand (g) | Water (g) | SAPs (g) | Source |
|---|---|---|---|---|---|
| SAP1 | A | 1535.8 | 350.2 | 3.70 | Laboratory-Prepared |
| SAP2 | B | 1535.0 | 350.0 | 2.52 | Laboratory-Prepared |
| SAPRX | C | 1521.9 | 347.0 | 2.40 | Purchased |
| SAPHDB | D | 1536.7 | 350.4 | 4.17 | Purchased |
| 0 | E | 1533.3 | 349.6 | 0 | 0 |
| Setting Parameters | Part One | Part Two |
|---|---|---|
| Temperature | 20 °C | 30 °C |
| Humidity | 40% | 25% |
| Light intensity | 0 | 4 |
| Time | 720 min | 720 min |
| A | B | C | D | E | |
|---|---|---|---|---|---|
| Emergence rate (%) | 90.0 | 91.1 | 91.1 | 52.2 | 98.9 |
| Er1 | 3.300 | 3.811 | 6.966 | 3.868 | 1.905 |
| Emergence potential (%) | 80.0 | 82.2 | 86.6 | 37.7 | 91.1 |
| Er2 | 3.300 | 8.404 | 3.350 | 5.085 | 6.966 |
| Root length (cm) | 3.52 | 4.12 | 3.98 | 2.12 | 3.17 |
| Er3 | 0.076 | 0.548 | 0.822 | 0.126 | 0.325 |
| Shoot length (cm) | 4.92 | 5.72 | 5.20 | 2.85 | 3.75 |
| Er4 | 0.257 | 0.629 | 0.265 | 0.391 | 0.350 |
| Total biomass (mg) | 6.83 | 7.67 | 9.60 | 4.57 | 4.73 |
| Er5 | 0.611 | 1.662 | 1.323 | 0.551 | 0.231 |
| Aboveground biomass (mg) | 6.17 | 7.20 | 8.53 | 4.00 | 4.13 |
| Er6 | 0.451 | 1.609 | 1.801 | 0.265 | 0.208 |
| Belowground biomass (mg) | 0.60 | 0.45 | 1.03 | 0.47 | 0.53 |
| Er7 | 0.265 | 0.087 | 0.551 | 0.306 | 0.208 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Liu, Q.; Liu, S.; Chen, J.; Guo, L.; Wang, P.; Lei, Z. Study on Seed Emergence and Seedling Growth of Artemisia Desertorum with Superabsorbent Polymers. Polymers 2020, 12, 2873. https://doi.org/10.3390/polym12122873
Zhang W, Liu Q, Liu S, Chen J, Guo L, Wang P, Lei Z. Study on Seed Emergence and Seedling Growth of Artemisia Desertorum with Superabsorbent Polymers. Polymers. 2020; 12(12):2873. https://doi.org/10.3390/polym12122873
Chicago/Turabian StyleZhang, Wenxu, Qian Liu, Shengfang Liu, Jing Chen, Lulu Guo, Peng Wang, and Ziqiang Lei. 2020. "Study on Seed Emergence and Seedling Growth of Artemisia Desertorum with Superabsorbent Polymers" Polymers 12, no. 12: 2873. https://doi.org/10.3390/polym12122873




