Using Genipin to Immobilize Bone Morphogenetic Protein-2 on Zirconia Surface for Enhancing Cell Adhesion and Mineralization in Dental Implant Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Surface Characterization
2.3. Cell Responses
2.3.1. Cell Culture
2.3.2. Cell Adhesion
2.3.3. Cell Proliferation
2.3.4. Cell Mineralization
2.4. Statistical Analysis
3. Results and Discussion
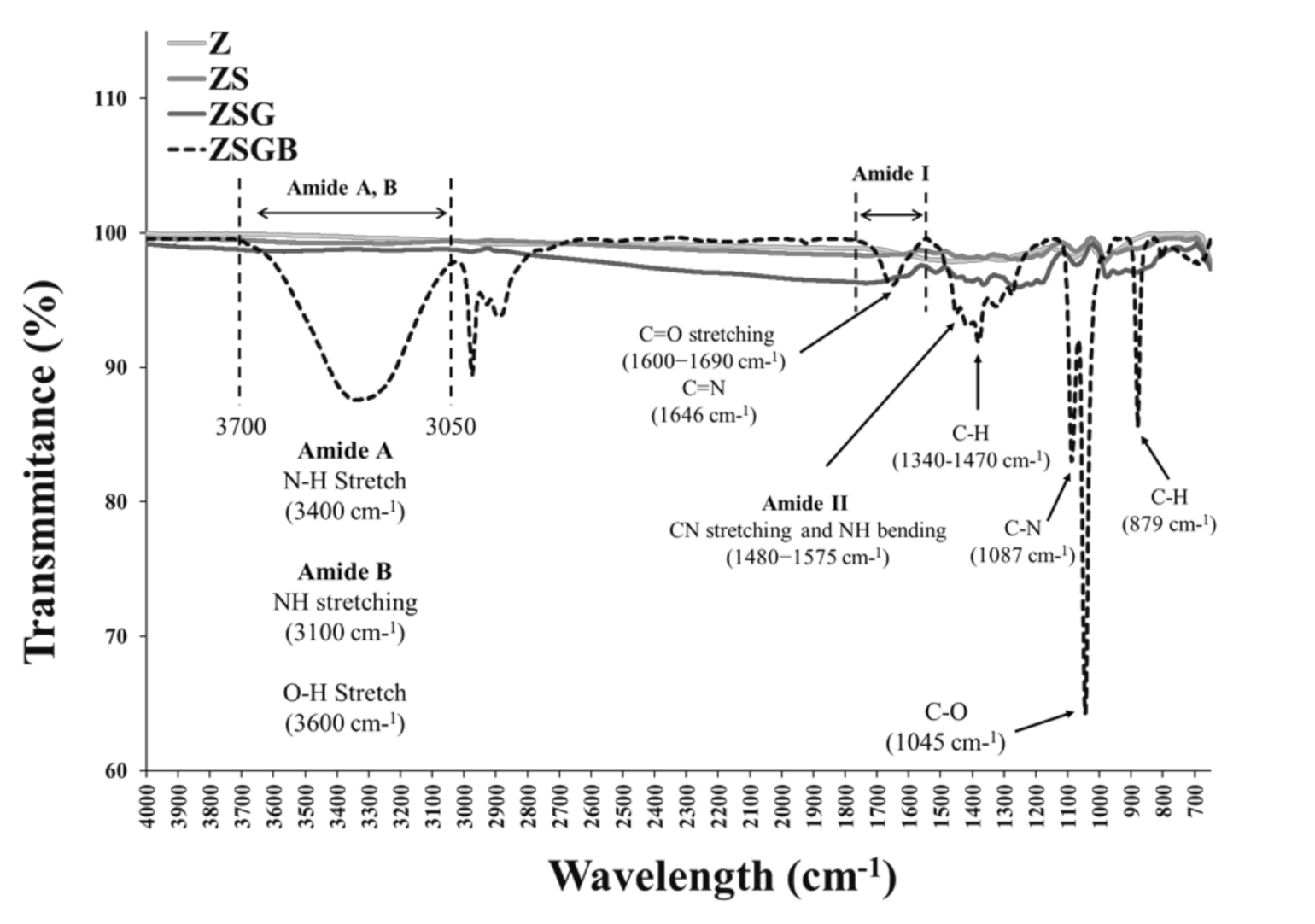
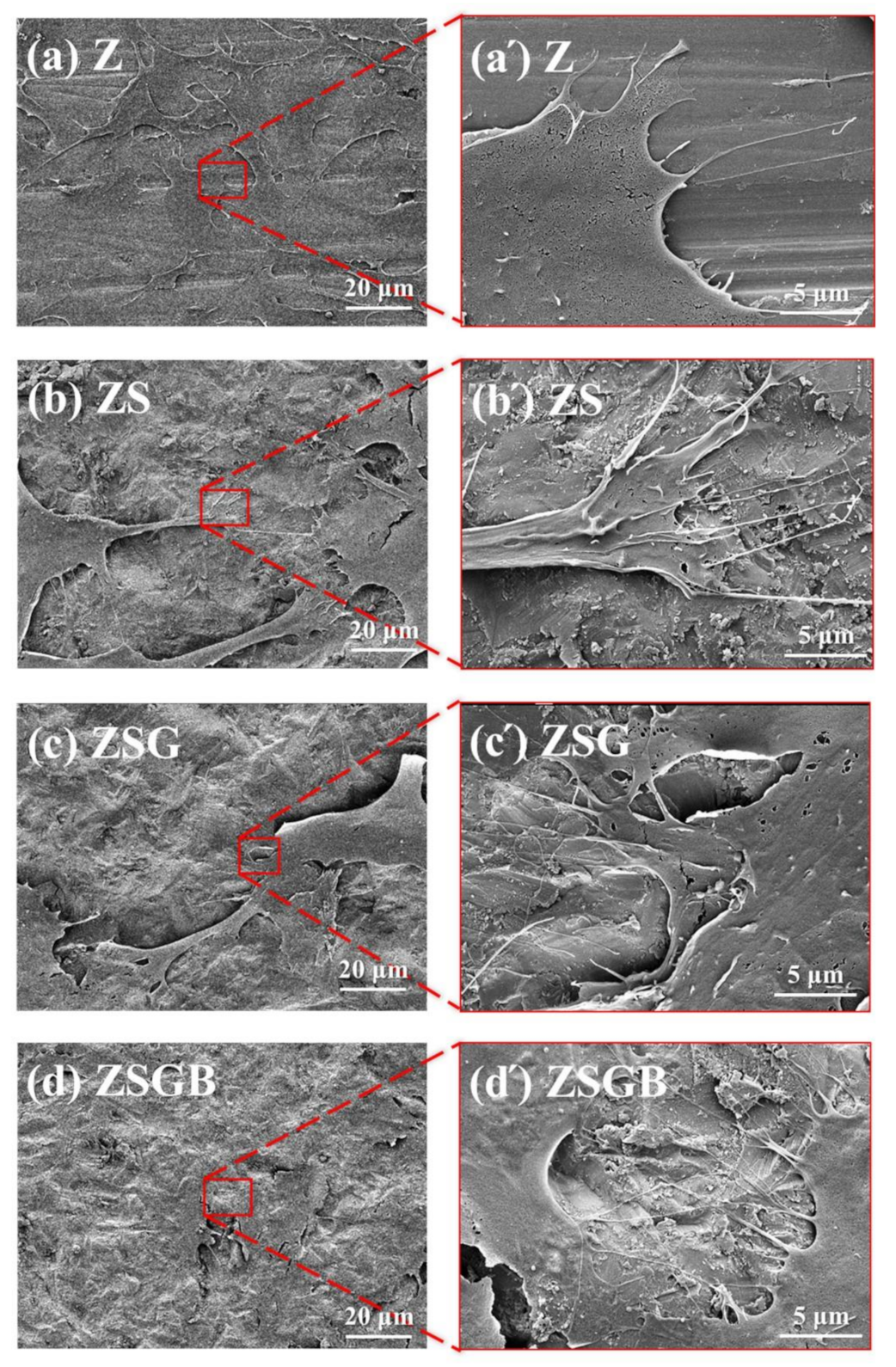
3.1. Surface Characterization
3.2. Cell Responses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, D.B. The use of a zirconia custom implant-supported fixed partial denture prosthesis to treat implant failure in the anterior maxilla: A clinical report. J. Prosthet. Dent. 2008, 100, 415–421. [Google Scholar] [CrossRef]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef]
- Huang, Y.S.; Huang, H.H. Effects of clinical dental implant abutment materials and their surface characteristics on initial bacterial adhesion. Rare Met. 2019, 38, 512–519. [Google Scholar] [CrossRef]
- Shalabi, M.M.; Gortemaker, A.; Van’t Hof, M.A.; Jansen, J.A.; Creugers, N.H. Implant surface roughness and bone healing: A systematic review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Al Qahtani, W.M.; Schille, C.; Spintzyk, S.; Al Qahtani, M.S.; Engel, E.; Geis-Gerstorfer, J.; Rupp, F.; Scheideler, L. Effect of surface modification of zirconia on cell adhesion, metabolic activity and proliferation of human osteoblasts. Biomed. Tech. 2017, 62, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign body reaction to biomaterials: On mechanisms for buildup and breakdown of osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Schickle, K.; Spitz, J.; Neuss, S.; Telle, R. Biomimetic in situ nucleation of calcium phosphates by protein immobilization on high strength ceramic materials. J. Eur. Ceram. Soc. 2018, 38, 271–277. [Google Scholar] [CrossRef]
- Bächle, M.; Butz, F.; Hübner, U.; Bakalinis, E.; Kohal, R.J. Behavior of cal72 osteoblast—Like cells cultured on zirconia ceramics with different surface topographies. Clin. Oral Implant. Res. 2007, 18, 53–59. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Zhang, H.; Bradley, A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 1996, 122, 2977–2986. [Google Scholar]
- Ducy, P.; Karsenty, G. The family of bone morphogenetic proteins. Kidney Int. 2000, 57, 2207–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009, 20, 475–480. [Google Scholar] [CrossRef]
- Singh, A.P.; Castranio, T.; Scott, G.; Guo, D.; Harris, M.A.; Ray, M.; Harris, S.E.; Mishina, Y. Influences of reduced expression of maternal bone morphogenetic protein 2 on mouse embryonic development. Sex. Dev. 2008, 2, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Matin, K.; Senpuku, H.; Hanada, N.; Ozawa, H.; Ejiri, S. Bone regeneration by recombinant human bone morphogenetic protein-2 around immediate implants: A pilot study in rats. Int. J. Oral Maxillofac. Surg. 2003, 18, 211–217. [Google Scholar]
- Muthukuru, M. Bone Morphogenic Protein--2 Induces Apoptosis and Cytotoxicity in Periodontal Ligament Cells. J. Periodontol. 2013, 84, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Schuckert, K.H.; Jopp, S.; Osadnik, M. The use of platelet rich plasma, bone morphogenetic protein-2 and different scaffolds in oral and maxillofacial surgery-literature review in comparison with own clinical experience. J. Oral Maxillofac. Res. 2011, 2, e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legendre, F.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Gruchy, N.; Gerin, F.M.; Leclercq, S.; Demoor, M.; Galéra, P. Enhanced chondrogenesis of bone marrow-derived stem cells by using a combinatory cell therapy strategy with BMP-2/TGF-β1, hypoxia, and COL1A1/HtrA1 siRNAs. Sci. Rep. 2007, 7, 3406. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Sapkota, D.; Xue, Y.; Sun, Y.; Finne-Wistrand, A.; Bruland, O.; Mustafa, K. Adenoviral mediated expression of BMP2 by bone marrow stromal cells cultured in 3D copolymer scaffolds enhances bone formation. PLoS ONE 2016, 11, e0147507. [Google Scholar] [CrossRef]
- Bi, L.; Cao, Z.; Hu, Y.; Song, Y.; Yu, L.; Yang, B.; Mu, J.; Huang, Z.; Han, Y. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 51–62. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Z.; Shen, Y. Influence of chemical cross-linking on properties of gelatin/chitosan microspheres. Polym. Plast. Technol. Eng. 2012, 51, 381–385. [Google Scholar] [CrossRef]
- Zhang, C.; Parton, L.E.; Ye, C.P.; Krauss, S.; Shen, R.; Lin, C.T.; Porco, J.A.; Lowell, B.B. Genipin inhibits ucp2-mediated proton leak and acutely reverses obesity-and high glucose-induced β cell dysfunction in isolated pancreatic islets. Cell Metab. 2006, 3, 417–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Miura, N.; Ohtake, N.; Amagaya, S.; Ishige, A.; Sasaki, H.; Komatsu, Y.; Fukuda, K.; Ito, T.; Terasawa, K. Genipin, a metabolite derived from th herbal medicine inchin-ko-to, and suppression of fas-induced lethal live apoptosis in mice. Gastroenterology 2000, 118, 380–389. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar. Drugs. 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.S.; Huang, C.Y.; Chen, C.S.; Chang, J.H.; Hou, W.T.; Li, S.J.; Hao, Y.L.; Pan, H.; Huang, H.H. Bone cell responses to a low elastic modulus titanium alloy surface immobilized with the natural cross-linker genipin. Surf. Coat. Technol. 2018, 350, 918–924. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Inc. Thermo Scientific™ Avantage™ Data System for XPS; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2020; Available online: https://xpssimplified.com/elements/nitrogen.php (accessed on 1 November 2020).
- Sun, Y.S.; Chang, J.H.; Huang, H.H. Enhancing the biological response of titanium surface through the immobilization of bone morphogenetic protein-2 using the natural cross-linker genipin. Surf. Coat. Technol. 2016, 303, 289–297. [Google Scholar] [CrossRef]
- Solorio, L.; Zwolinski, C.; Lund, A.W.; Farrell, M.J.; Stegemann, J.P. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J. Tissue Eng. Regen. Med. 2010, 4, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Orsini, G.; Assenza, B.; Scarano, A.; Piatelli, M.; Piattelli, A. Surface analysis of machined versus sandblasted and acid-etched titanium implants. Int. J. Oral Maxillofac. Implants 2000, 15, 779–784. [Google Scholar]
- Shah, A.K.; Lazatin, J.; Sinha, R.K.; Lennox, T.; Hickok, N.J.; Tuan, R.S. Mechanism of BMP-2 stimulated adhesion of osteoblastic cells to titanium alloy. Biol. Cell. 1999, 91, 131–142. [Google Scholar] [CrossRef]
- Qi, S.; Yi, C.; Ji, S.; Fong, C.-C.; Yang, M. Cell adhesion and spreading behavior on vertically aligned silicon nanowire arrays. ACS Appl. Mater. Interfaces 2009, 1, 30–34. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-S.; Lin, Y.-A.; Huang, H.-H. Using Genipin to Immobilize Bone Morphogenetic Protein-2 on Zirconia Surface for Enhancing Cell Adhesion and Mineralization in Dental Implant Applications. Polymers 2020, 12, 2639. https://doi.org/10.3390/polym12112639
Sun Y-S, Lin Y-A, Huang H-H. Using Genipin to Immobilize Bone Morphogenetic Protein-2 on Zirconia Surface for Enhancing Cell Adhesion and Mineralization in Dental Implant Applications. Polymers. 2020; 12(11):2639. https://doi.org/10.3390/polym12112639
Chicago/Turabian StyleSun, Ying-Sui, Yu-An Lin, and Her-Hsiung Huang. 2020. "Using Genipin to Immobilize Bone Morphogenetic Protein-2 on Zirconia Surface for Enhancing Cell Adhesion and Mineralization in Dental Implant Applications" Polymers 12, no. 11: 2639. https://doi.org/10.3390/polym12112639





