Study of High-Density Polyethylene (HDPE) Kinetics Modification Treated by Dielectric Barrier Discharge (DBD) Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
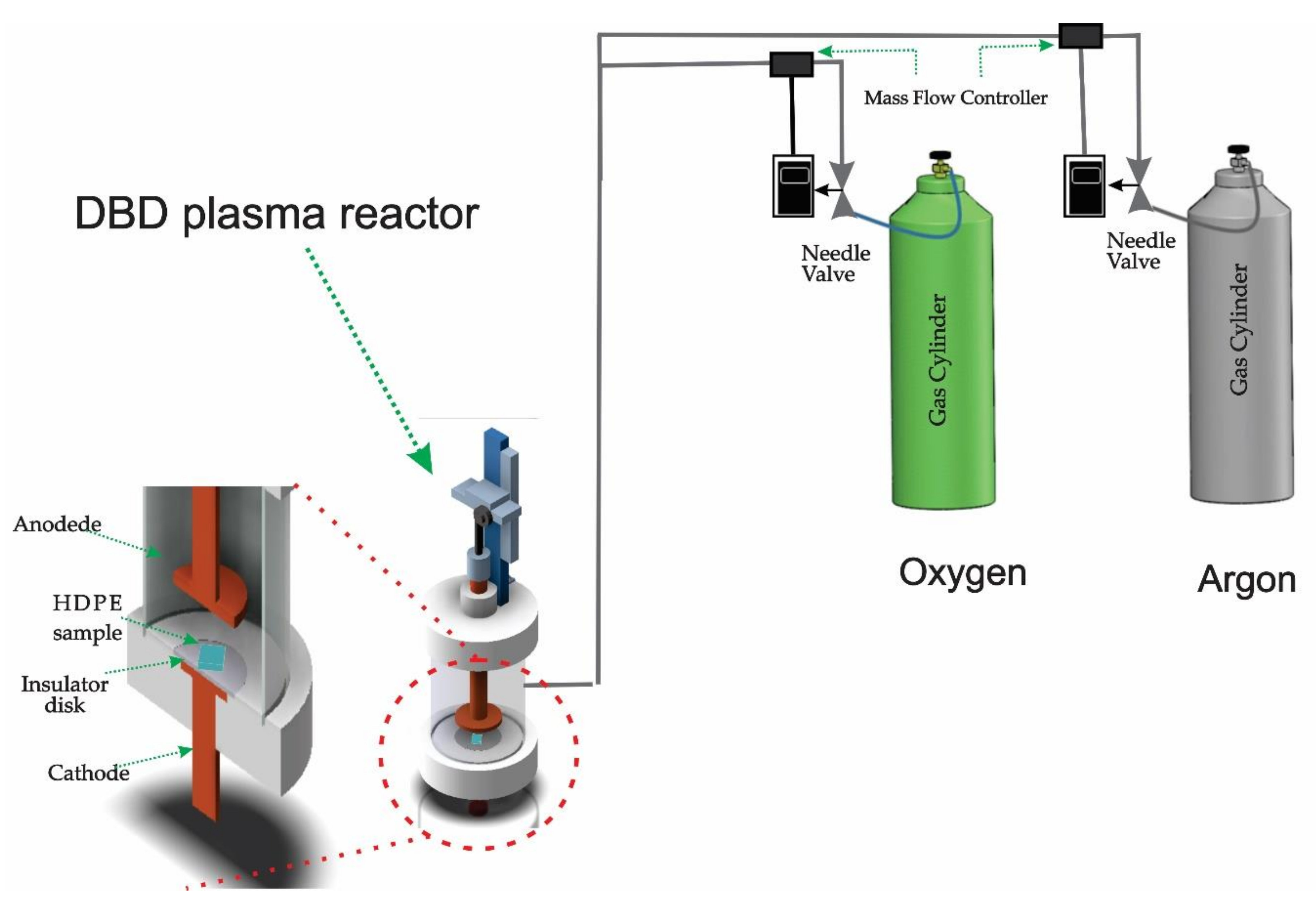
2.2. Plasma Reactor
2.3. Treatment
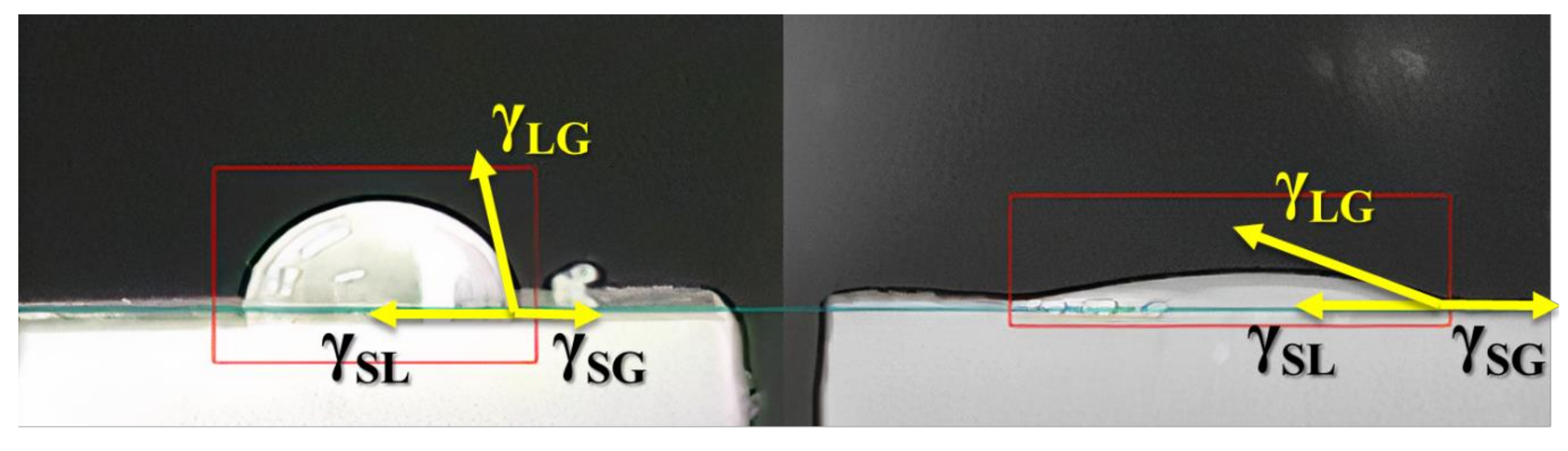
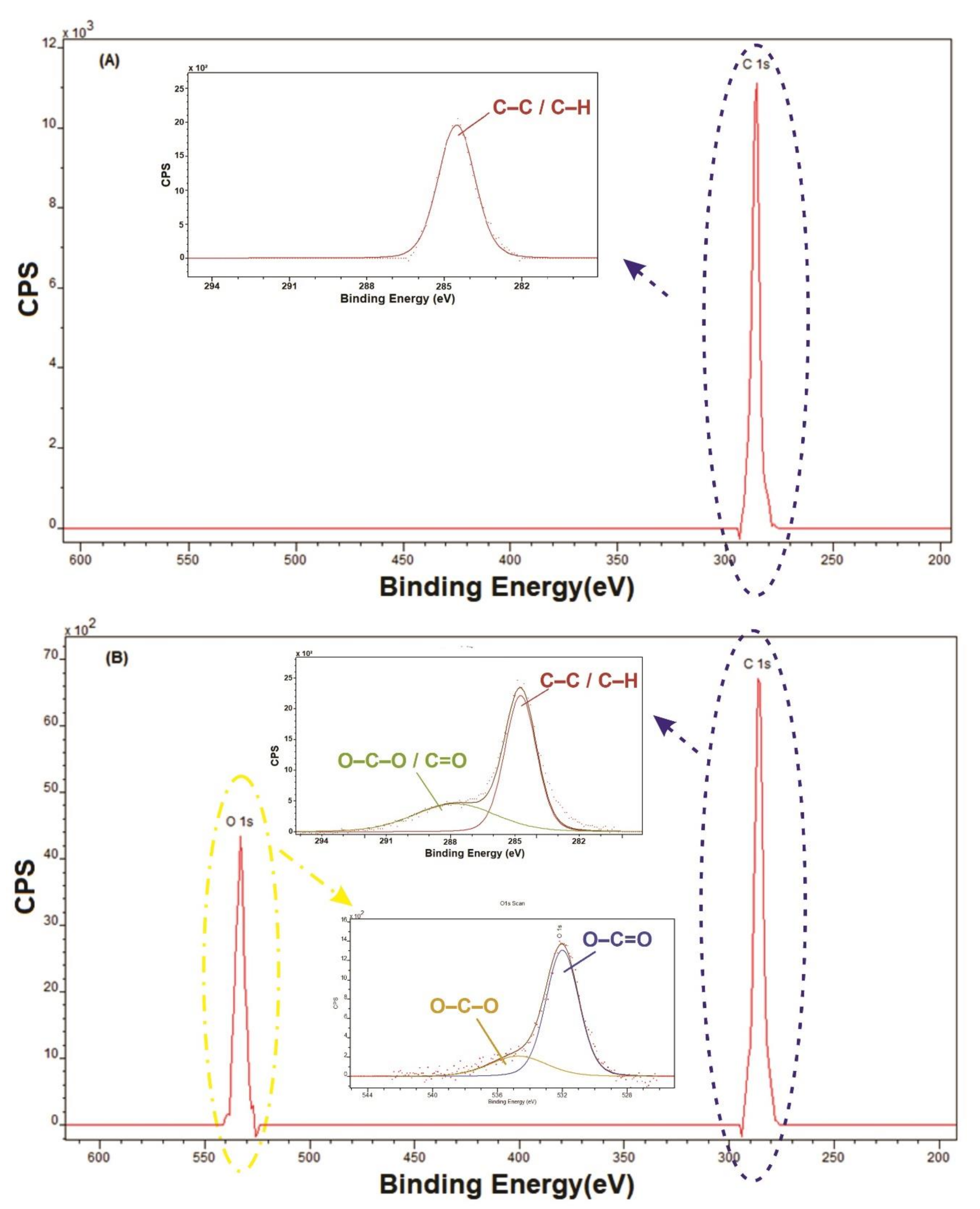
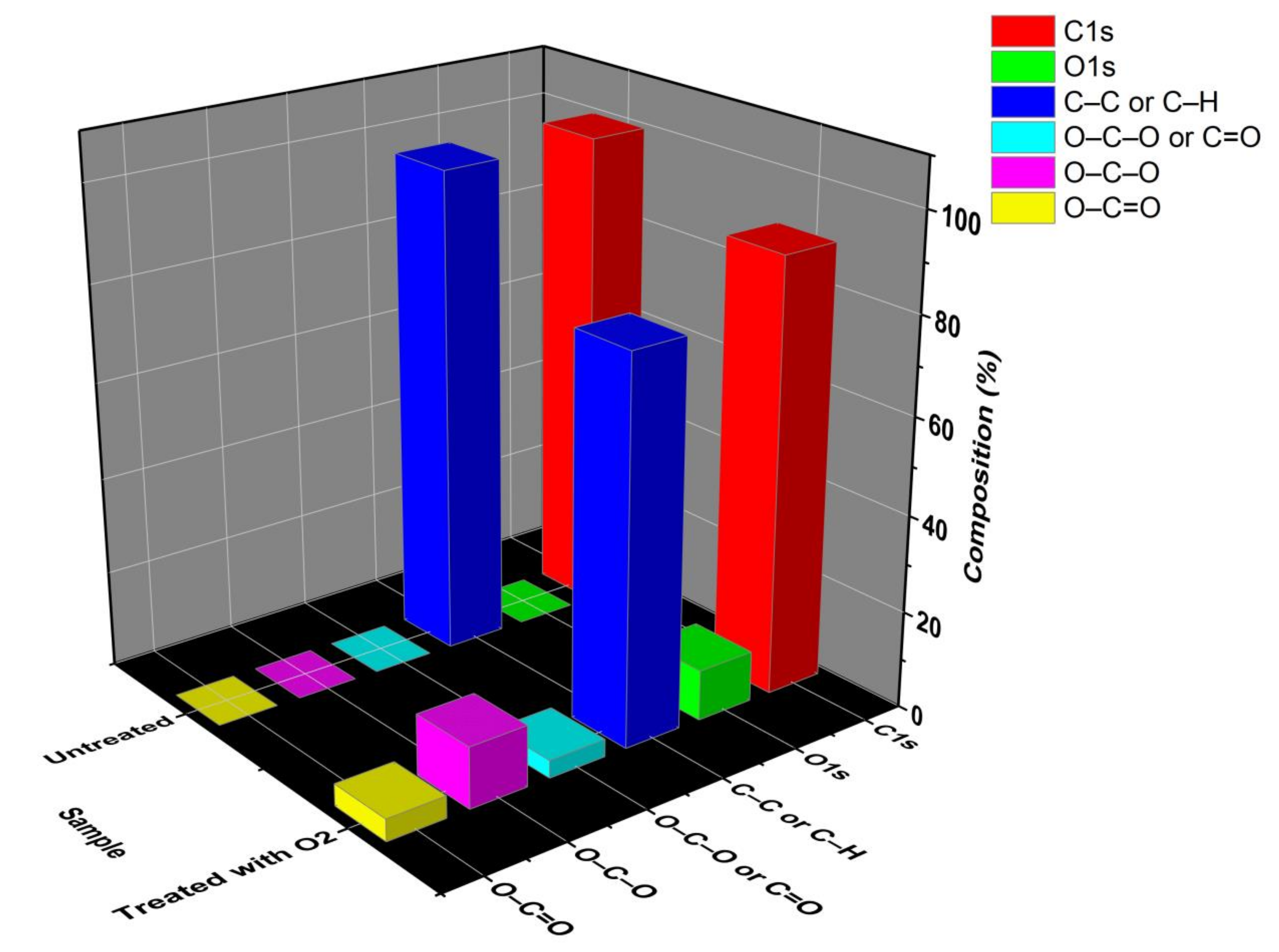
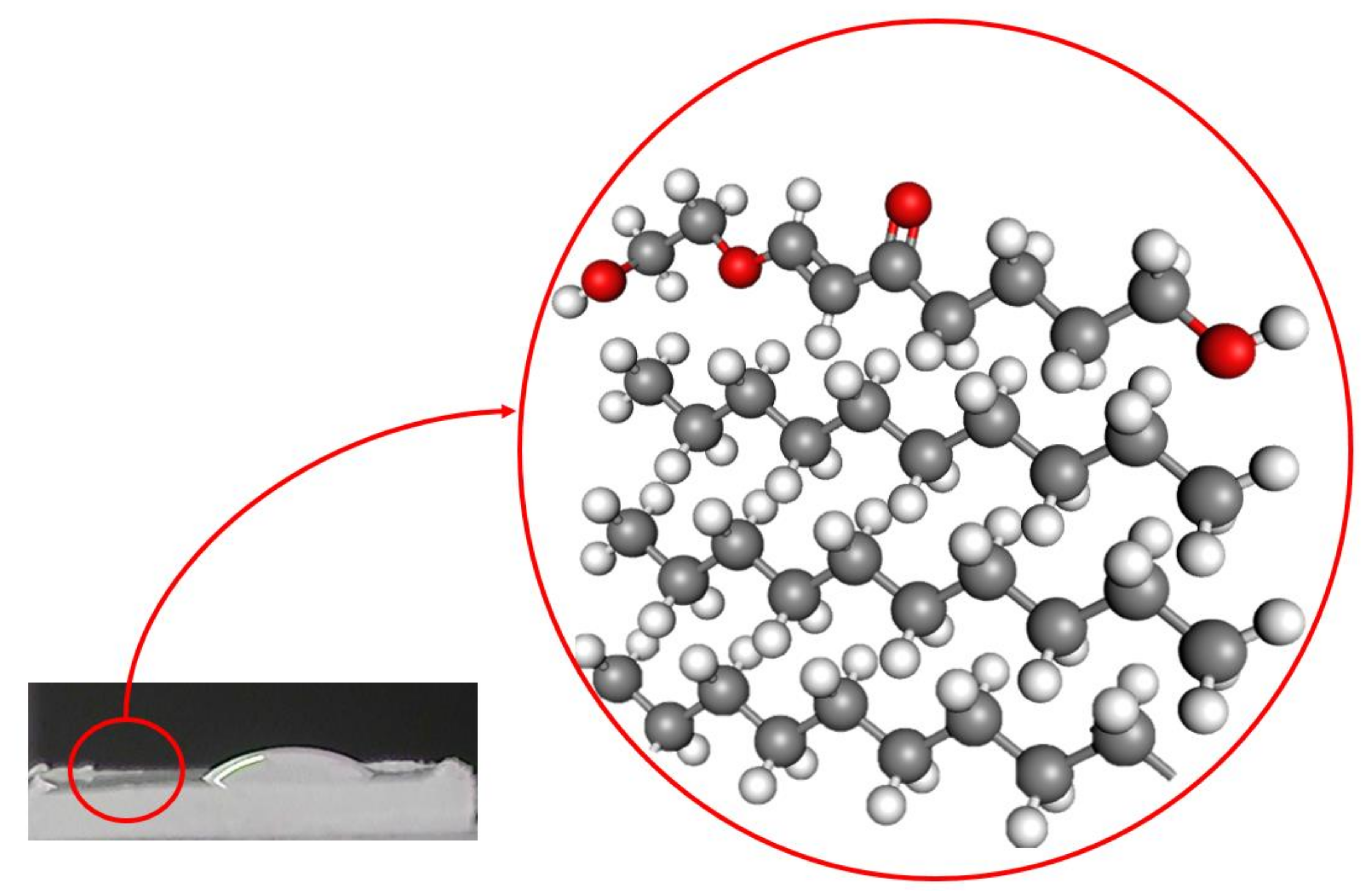
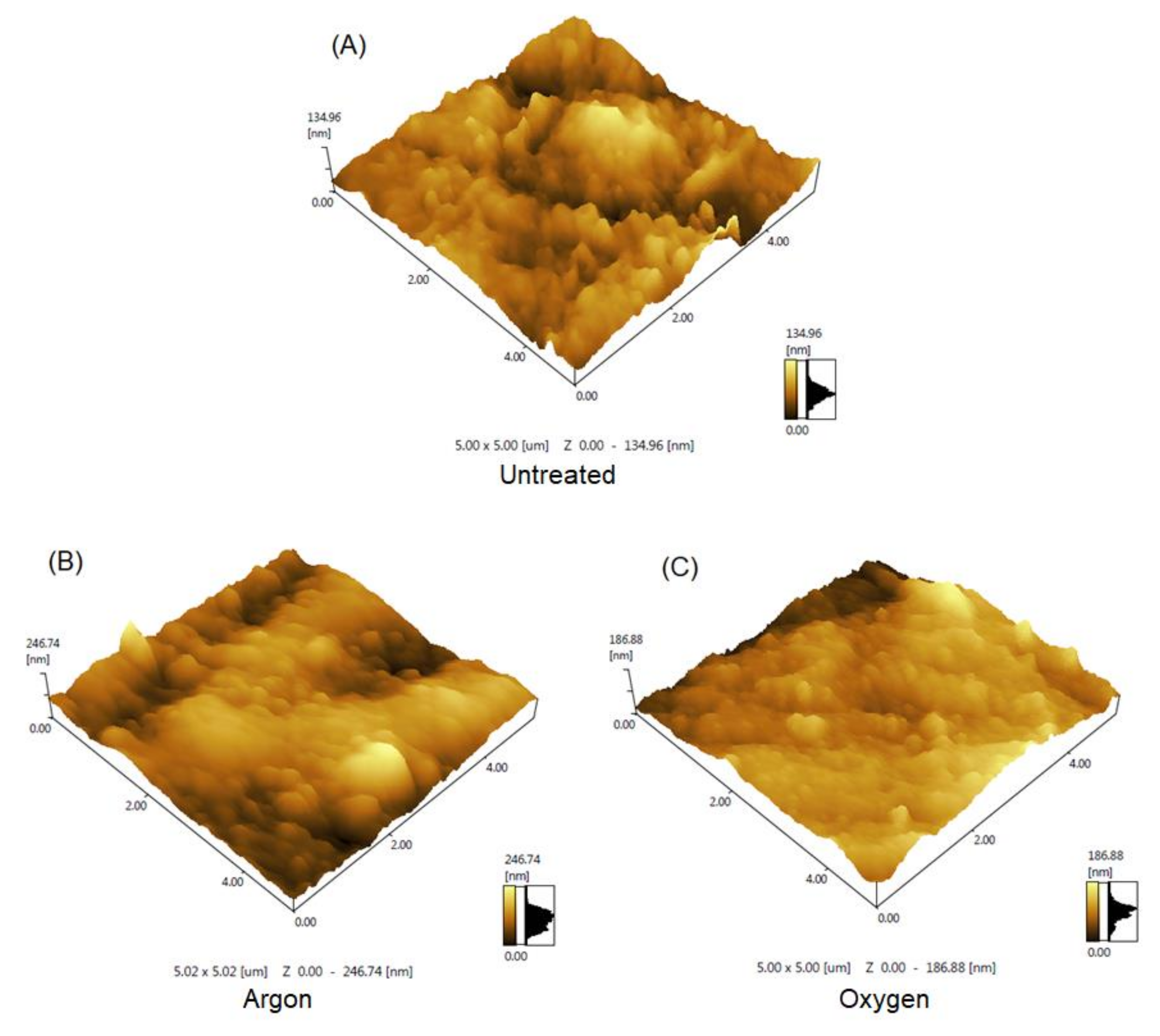
2.4. Characterizations
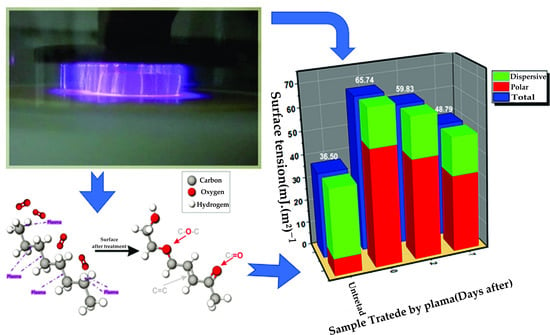
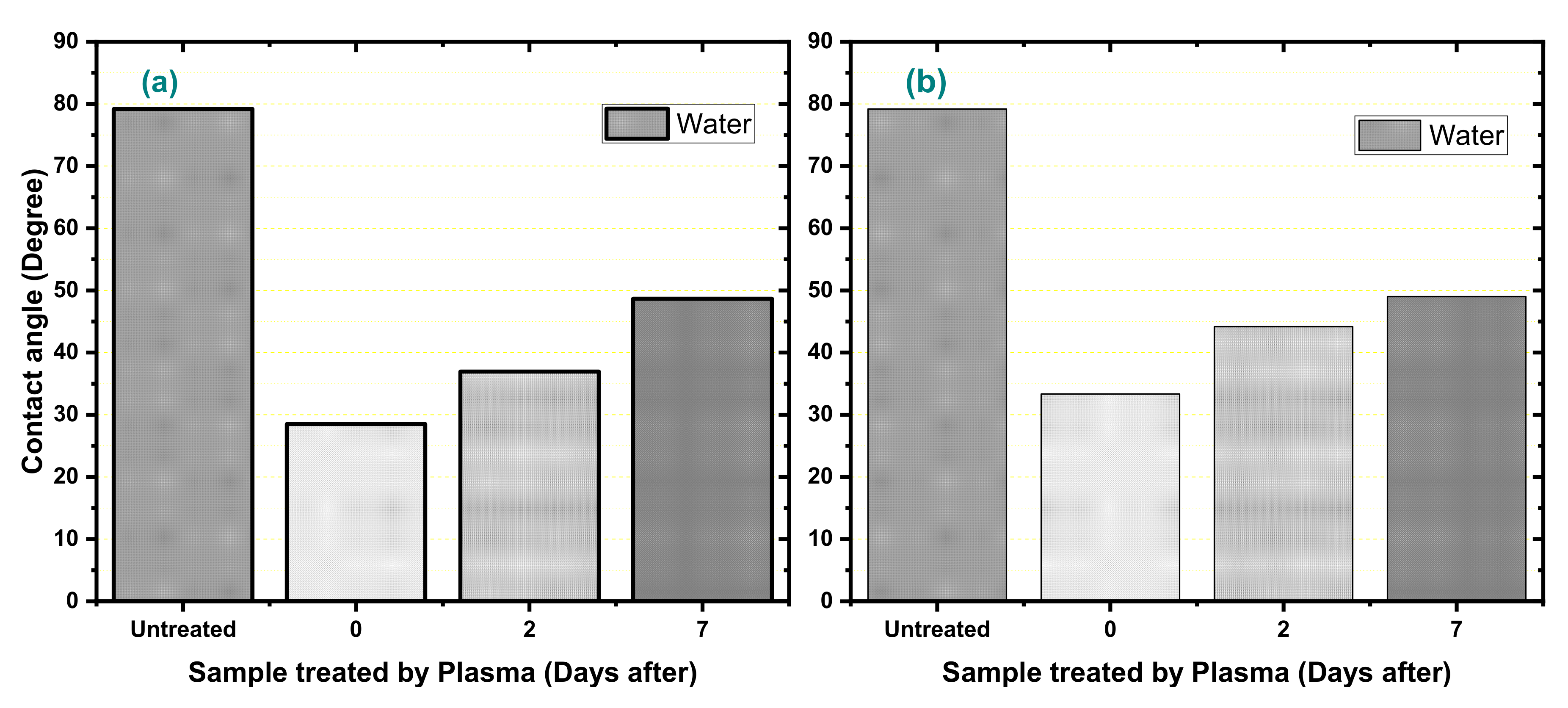
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drnovská, H.; Lapčík, L.; Buršíková, V.; Zemek, J.; Barros-Timmons, A.M. Surface properties of polyethylene after low-temperature plasma treatment. Colloid Polym. Sci. 2003, 281, 1025–1033. [Google Scholar] [CrossRef]
- Roy, A.; Joshi, M.; Butola, B.S. Preparation and antimicrobial assessment of zinc-montmorillonite intercalates based HDPE nanocomposites: A cost-effective and safe bioactive plastic. J. Clean. Prod. 2019, 212, 1518–1525. [Google Scholar] [CrossRef]
- Slepička, P.; Kasálková, N.S.; Stránská, E.; Bačáková, L.; Švorčík, V. Surface characterization of plasma treated polymers for applications as biocompatible carriers. Express Polym. Lett. 2013, 7, 535–545. [Google Scholar] [CrossRef]
- Toosi, S.F.; Moradi, S.; Ebrahimi, M.; Hatzikiriakos, S.G. Microfabrication of polymeric surfaces with extreme wettability using hot embossing. Appl. Surf. Sci. 2016, 378, 426–434. [Google Scholar] [CrossRef]
- Meemusaw, M.; Magaraphan, R. Surface and bulk properties improvement of HDPE by a batch plasma treatment. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Encinas, N.; Pantoja, M.; Abenojar, J.; Martínez, M.A. Control of wettability of polymers by surface roughness modification. J. Adhes. Sci. Technol. 2010, 24, 1869–1883. [Google Scholar] [CrossRef] [Green Version]
- Zaharescu, T.; Râpă, M.; Blanco, I.; Borbath, T.; Borbath, I. Durability of LDPE/UHMWPE Composites under Accelerated Degradation. Polymers 2020, 12, 1241. [Google Scholar] [CrossRef]
- Encinas, N.; Díaz-Benito, B.; Abenojar, J.; Martínez, M.A. Extreme durability of wettability changes on polyolefin surfaces by atmospheric pressure plasma torch. Surf. Coat. Technol. 2010, 205, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen- And nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Zhang, S.; Awaja, F.; James, N.; McKenzie, D.R.; Ruys, A.J. A comparison of the strength of autohesion of plasma treated amorphous and semi-crystalline PEEK films. Polym. Adv. Technol. 2011, 22, 2496–2502. [Google Scholar] [CrossRef]
- Hünnekens, B.; Peters, F.; Avramidis, G.; Krause, A.; Militz, H.; Viöl, W. Plasma treatment of wood-polymer composites: A comparison of three different discharge types and their effect on surface properties. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Muzammil, I.; Li, Y.; Lei, M. Tunable wettability and pH-responsiveness of plasma copolymers of acrylic acid and octafluorocyclobutane. Plasma Process. Polym. 2017, 14. [Google Scholar] [CrossRef]
- Ji, S.-H.; Choi, K.-H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; Kyung Jung, S.; Choi, E.-H. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef]
- Kim, H.-H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Plasma Catalysis for Environmental Treatment and Energy Applications. Plasma Chem. Plasma Process. 2016, 36, 45–72. [Google Scholar] [CrossRef]
- Souza, I.A.; Nascimento Neto, A.B.; Queiroz, J.C.A.; Matamoros, E.P.; Costa, T.H.; Feitor, M.C.; Souza, J.M.L.; Camara, N.T.; Severiano Sobrinho, V. Study of the Influence of Variation in Distances Between Electrodes in Spectral DBD Plasma Excitation. Mater. Res. 2016, 19, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Arpagaus, C.; Rossi, A.; Rudolf Von Rohr, P. Short-time plasma surface modification of HDPE powder in a Plasma Downer Reactor—Process, wettability improvement and ageing effects. Appl. Surf. Sci. 2005, 252, 1581–1595. [Google Scholar] [CrossRef]
- Felix, T.; Cassini, F.A.; Benetoli, L.O.B.; Dotto, M.E.R.; Debacher, N.A. Morphological study of polymer surfaces exposed to non-thermal plasma based on contact angle and the use of scaling laws. Appl. Surf. Sci. 2017, 403, 57–61. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Hein, L.R.O.; Toth, A. Study of polypropylene surface modification by air dielectric barrier discharge operated at two different frequencies. Surf. Coat. Technol. 2013, 234, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Wagner, H.E.; Brandenburg, R.; Kozlov, K.V.; Sonnenfeld, A.; Michel, P.; Behnke, J.F. The barrier discharge: Basic properties and applications to surface treatment. Vacuum 2003, 71, 417–436. [Google Scholar] [CrossRef] [Green Version]
- Hua, L.I.; Zhengduo, W.; Lizhen, Y.; Qiang, C. Insight into the remaining high surface energy of atmospheric DBD plasma-treated polyethylene web after three months’ aging. Plasma Sci. Technol. 2018, 21, 15504. [Google Scholar]
- Dahle, S.; Gustus, R.; Viöl, W.; Maus-Friedrichs, W. DBD Plasma Treatment of Titanium in O2, N2 and Air. Plasma Chem. Plasma Process. 2012, 32, 1109–1125. [Google Scholar] [CrossRef]
- Misra, N.; Keener, K.; Bourke, P.; Cullen, P. Generation of In-Package Cold Plasma and Efficacy Assessment Using Methylene Blue. Plasma Chem. Plasma Process. 2015, 35, 1043–1056. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, N.W.; Andre, J.; Williamson, J.; Lee, K.-W.; Chen, Z. Plasma treatment effect on polymer buried interfacial structure and property. Phys. Chem. Chem. Phys. 2017, 19, 12144–12155. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Wang, Q.; Chen, P.; Lu, S.; Ren, R. Wettability assessment of plasma-treated PBO fibers based on thermogravimetric analysis. Int. J. Adhes. Adhes. 2017, 74, 123–130. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, M.; Wang, L.; Qu, L.; Men, Y. Surface modification of polyester fabrics by atmospheric-pressure air/He plasma for color strength and adhesion enhancement. Appl. Surf. Sci. 2017, 400, 304–311. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, L.; Wang, C.; Wang, X.; Ding, Z.; Chen, Y. Effect of dielectric barrier discharge treatment on surface nanostructure and wettability of polylactic acid (PLA) nonwoven fabrics. Appl. Surf. Sci. 2017, 426, 612–621. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Kratochvil, J.; Stranak, V. Tailored wettability of plasma polymers made of C–F, C–H, and N–H. Plasma Process. Polym. 2019, 16, 1900076. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Wiącek, A.E.; Jurak, M. Influence of nitrogen plasma treatment on the wettability of polyetheretherketone and deposited chitosan layers. Adv. Polym. Technol. 2018, 37, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Acsente, T.; Ionita, M.D.; Teodorescu, M.; Marascu, V.; Dinescu, G. Surface modification of polymethylmethacrylate foils using an atmospheric pressure plasma jet in presence of water vapors. Thin Solid Films 2016, 614, 25–30. [Google Scholar] [CrossRef]
- Glaser, T.K.; Plohl, O.; Vesel, A.; Ajdnik, U.; Ulrih, N.P.; Hrnčič, M.K.; Bren, U.; Fras Zemljič, L. Functionalization of Polyethylene (PE) and Polypropylene (PP) Material Using Chitosan Nanoparticles with Incorporated Resveratrol as Potential Active Packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberbossel, G.; Probst, C.; Giampietro, V.R.; Rudolf von Rohr, P. Plasma afterglow treatment of polymer powders: Process parameters, wettability improvement, and aging effects. Plasma Process. Polym. 2017, 14, 1600144. [Google Scholar] [CrossRef]
- Popelka, A.; Lehocký, M.; Bílek, F. Antibacterial treatment of LDPE with halogen derivatives via cold plasma. Express Polym. Lett. 2015. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, J.; Li, Y.; Liu, J.; Zhou, J.; Cen, K. Amino-functionalized surface modification of polyacrylonitrile hollow fiber-supported polydimethylsiloxane membranes. Appl. Surf. Sci. 2017, 413, 27–34. [Google Scholar] [CrossRef]
- Rediguieri, C.F.; De Bank, P.A.; Zanin, M.H.A.; Leo, P.; Cerize, N.N.P.; de Oliveira, A.M.; de Jeus Andreoli Pinto, T. The effect of ozone gas sterilization on the properties and cell compatibility of electrospun polycaprolactone scaffolds. J. Biomater. Sci.—Polym. Ed. 2017, 28, 1918–1934. [Google Scholar] [CrossRef]
- Jha, S.; Bhowmik, S.; Bhatnagar, N.; Bhattacharya, N.K.; Deka, U.; Iqbal, H.M.S.; Benedictus, R. Experimental investigation into the effect of adhesion properties of PEEK modified by atmospheric pressure plasma and low pressure plasma. J. Appl. Polym. Sci. 2010, 118, 173–179. [Google Scholar] [CrossRef]
- Rezaei, F.; Abbasi-Firouzjah, M.; Shokri, B. Investigation of antibacterial and wettability behaviours of plasma-modified PMMA films for application in ophthalmology. J. Phys. D. Appl. Phys. 2014, 47, 85401. [Google Scholar] [CrossRef]
- Ozgen, O.; Aksoy, E.A.; Hasirci, V.; Hasirci, N. Surface characterization and radical decay studies of oxygen plasma-treated PMMA films. Surf. Interface Anal. 2013, 45, 844–853. [Google Scholar] [CrossRef]
- Yalcinkaya, F. Effect of argon plasma treatment on hydrophilic stability of nanofiber webs. J. Appl. Polym. Sci. 2018, 135, 46751. [Google Scholar] [CrossRef]
- Syromotina, D.S.; Surmenev, R.A.; Surmeneva, M.A.; Boyandin, A.N.; Nikolaeva, E.D.; Prymak, O.; Epple, M.; Ulbricht, M.; Oehr, C.; Volova, T.G. Surface wettability and energy effects on the biological performance of poly-3-hydroxybutyrate films treated with RF plasma. Mater. Sci. Eng. C 2016, 62, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Dorey, S.; Gaston, F.; Marque, S.R.; Bortolotti, B.; Dupuy, N. XPS analysis of PE and EVA samples irradiated at different γ-doses. Appl. Surf. Sci. 2018, 427, 966–972. [Google Scholar] [CrossRef]
- Van Deynse, A.; Cools, P.; Leys, C.; Morent, R.; De Geyter, N. Surface modification of polyethylene in an argon atmospheric pressure plasma jet. Surf. Coat. Technol. 2015, 276, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, Q.; Du, X.; Li, L.; Li, P. Surface modification of polyethylene terephthalate films by direct fluorination. AIP Adv. 2018, 8, 125333. [Google Scholar] [CrossRef] [Green Version]
- Popelka, A.; Khanam, P.N.; AlMaadeed, M.A. Surface modification of polyethylene/graphene composite using corona discharge. J. Phys. D. Appl. Phys. 2018, 51, 105302. [Google Scholar] [CrossRef]
- Liu, H.; Pei, Y.; Xie, D.; Deng, X.; Leng, Y.X.; Jin, Y.; Huang, N. Surface modification of ultra-high molecular weight polyethylene (UHMWPE) by argon plasma. Appl. Surf. Sci. 2010, 256, 3941–3945. [Google Scholar] [CrossRef]
- Verkuijlen, R.O.F.; Van Dongen, M.H.A.; Stevens, A.A.E.; Van Geldrop, J.; Bernards, J.P.C. Surface modification of polycarbonate and polyethylene naphtalate foils by UV-ozone treatment and μplasma printing. Appl. Surf. Sci. 2014, 290, 381–387. [Google Scholar] [CrossRef]
- Tajima, S.; Komvopoulos, K. Effect of ion energy fluence on the topography and wettability of low-density polyethylene exposed to inductively coupled argon plasma. J. Phys. D Appl. Phys. 2006, 39, 1084. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M. Evolution of the Surface Wettability of PET Polymer upon Treatment with an Atmospheric-Pressure Plasma Jet. Polymers 2020, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Panousis, E.; Clément, F.; Loiseau, J.F.; Spyrou, N.; Held, B.; Larrieu, J.; Lecoq, E.; Guimon, C. Titanium alloy surface treatment using an atmospheric plasma jet in nitrogen pulsed discharge conditions. Surf. Coat. Technol. 2007, 201, 7292–7302. [Google Scholar] [CrossRef]
- Zhang, X.W.; Han, G.R. Microporous textured titanium dioxide films deposited at atmospheric pressure using dielectric barrier discharge assisted chemical vapor deposition. Thin Solid Films 2008, 516, 6140–6144. [Google Scholar] [CrossRef]
- Jie-Rong, C.; Wakida, T. Studies on the surface free energy and surface structure of PTFE film treated with low temperature plasma. J. Appl. Polym. Sci. 1997, 63, 1733–1739. [Google Scholar] [CrossRef]
- Fowkes, F.M. Determination of interfacial tensions, contact angles, and dispersion forces in surfaces by assuming additivity of intermolecular interactions in surfaces. J. Phys. Chem. 1962, 66, 382. [Google Scholar] [CrossRef]
- Matykina, E.; Garcia, I.; de Damborenea, J.J.; Arenas, M.A. Comparative determination of TiO2 surface free energies for adhesive bonding application. Int. J. Adhes. Adhes. 2011, 31, 832–839. [Google Scholar] [CrossRef]
- Chun, K.S.; Husseinsyah, S.; Syazwani, N.F. Properties of kapok husk-filled linear low-density polyethylene ecocomposites: Effect of polyethylene-grafted acrylic acid. J. Thermoplast. Compos. Mater. 2016, 29, 1641–1655. [Google Scholar] [CrossRef]
- Zięba-Palus, J. The usefulness of infrared spectroscopy in examinations of adhesive tapes for forensic purposes. Forensic Sci. Criminol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Singh, R.K.; Thomas, J.; Sunil, S. Parametric study of expanding plasma plume formed by laser-blow-off of thin film using triple Langmuir probe. J. Appl. Phys. 2009, 106, 43306. [Google Scholar] [CrossRef]
- Qayyum, A.; Ahmad, N.; Ahmad, S.; Deeba, F.; Ali, R.; Hussain, S. Time-resolved measurement of plasma parameters by means of triple probe. Rev. Sci. Instrum. 2013, 84, 123502. [Google Scholar] [CrossRef]
- Král, P.; Ráhel’, J.; Stupavská, M.; Šrajer, J.; Klímek, P.; Mishra, P.K.; Wimmer, R. XPS depth profile of plasma-activated surface of beech wood (Fagus sylvatica) and its impact on polyvinyl acetate tensile shear bond strength. Wood Sci. Technol. 2015, 49, 319–330. [Google Scholar] [CrossRef]
- Zanini, S.; Barni, R.; Della Pergola, R.; Riccardi, C. Modification of the PTFE wettability by oxygen plasma treatments: Influence of the operating parameters and investigation of the ageing behaviour. J. Phys. D Appl. Phys. 2014, 47, 325202. [Google Scholar] [CrossRef]
- Farooq, M.U.; Ali, A.; Qayyum, A.; Naz, M.Y.; Khan, Y.; Shukrullah, S.; Ghaffar, C.A. Time function triple Langmuir probe measurements in low frequency pulsed DC discharge plasma. High Energy Chem. 2015, 49, 286–293. [Google Scholar] [CrossRef]
- De Souza, I.A.; de Medeiros Neto, J.F.; Nascimento, I.O.; Matamoros, E.P.; Feitor, M.C.; Fernandes de Melo, F.; Magalhães Sousa, R.R.; de Carvalho Costa, T.H. Triple langmuir probe, optical emission spectroscopy and lissajous figures for diagnosis of plasma produced by dielectric barrier discharge of parallel plates in atmospheric pressure. Int. J. Appl. Electromagn. Mech. 2020, 63, 315–325. [Google Scholar] [CrossRef]
- Biswas, S.; Chowdhury, S.; Palivela, Y.; Pal, R. Effect of fast drifting electrons on electron temperature measurement with a triple Langmuir probe. J. Appl. Phys. 2015, 118, 63302. [Google Scholar] [CrossRef]
- Ebnesajjad, S.; Ebnesajjad, C. Surface Treatment of Materials for Adhesive Bonding; William Andrew: Norwich, NY, USA, 2013; ISBN 0323265049. [Google Scholar]
- Ishida, N.; Matsuo, K.; Imamura, K.; Craig, V.S.J. Hydrophobic Attraction Measured between Asymmetric Hydrophobic Surfaces. Langmuir 2018, 34, 3588–3596. [Google Scholar] [CrossRef] [PubMed]
- Kozbial, A.; Trouba, C.; Liu, H.; Li, L. Characterization of the Intrinsic Water Wettability of Graphite Using Contact Angle Measurements: Effect of Defects on Static and Dynamic Contact Angles. Langmuir 2017, 33, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.J.P.; Silva, G.S.; Feitor, M.C.; Costa, T.H.C.; Ito, E.N.; Melo, J.D.D. Surface modification of kapok fibers by cold plasma surface treatment. J. Mater. Res. Technol. 2019, 9, 2467–2476. [Google Scholar] [CrossRef]
- Song, X.; Cvelbar, U.; Strazar, P.; Vossebein, L.; Zille, A. Chemical, Thermo-Mechanical and Antimicrobial Properties of DBD Plasma Treated Disinfectant-Impregnated Wipes during Storage. Polymers 2019, 11, 1769. [Google Scholar] [CrossRef] [Green Version]
- De Geyter, N.; Morent, R.; Leys, C. Surface characterization of plasma-modified polyethylene by contact angle experiments and ATR-FTIR spectroscopy. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2008, 40, 608–611. [Google Scholar] [CrossRef]
- Qureshi, A.; Shah, S.; Pelagade, S.; Singh, N.L.; Mukherjee, S.; Tripathi, A.; Despande, U.P.; Shripathi, T. Surface modification of polycarbonate by plasma treatment. J. Phys. Conf. Ser. 2010, 208, 12108. [Google Scholar] [CrossRef]
- Tsvetkova, T.; Balabanov, S.; Bischoff, L.; Krastev, V.; Stefanov, P.; Avramova, I. X-ray photoelectron study of Si+ ion implanted polymers. J. Phys. Conf. Ser. 2010, 253, 12070. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.-H.; Ji, J.-H.; Zhao, J.; Yan, Q.; Chu, P.K. Antimicrobial properties of copper plasma-modified polyethylene. Polymer 2006, 47, 7441–7445. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, Y.; Shi, M.; Liu, Y. Effect of an Atmospheric Pressure Plasma Jet on the Structure and Physicochemical Properties of Waxy and Normal Maize Starch. Polymers 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borah, D.; Rasappa, S.; Senthamaraikannan, R.; Holmes, J.; Morris, M. Block Co-Polymers for Nanolithography: Rapid Microwave Annealing for Pattern Formation on Substrates. Polymers 2015, 7, 592–609. [Google Scholar] [CrossRef] [Green Version]
- Ting, Y.-H.; Liu, C.-C.; Park, S.-M.; Jiang, H.; Nealey, P.F.; Wendt, A.E. Surface Roughening of Polystyrene and Poly(methyl methacrylate) in Ar/O2 Plasma Etching. Polymers 2010, 2, 649–663. [Google Scholar] [CrossRef]
- Bès, A.; Koo, M.; Phan, T.L.; Lacoste, A.; Pelletier, J. Oxygen plasma etching of hydrocarbon-like polymers: Part I Modeling. Plasma Process. Polym. 2018, 15, 1800038. [Google Scholar] [CrossRef]

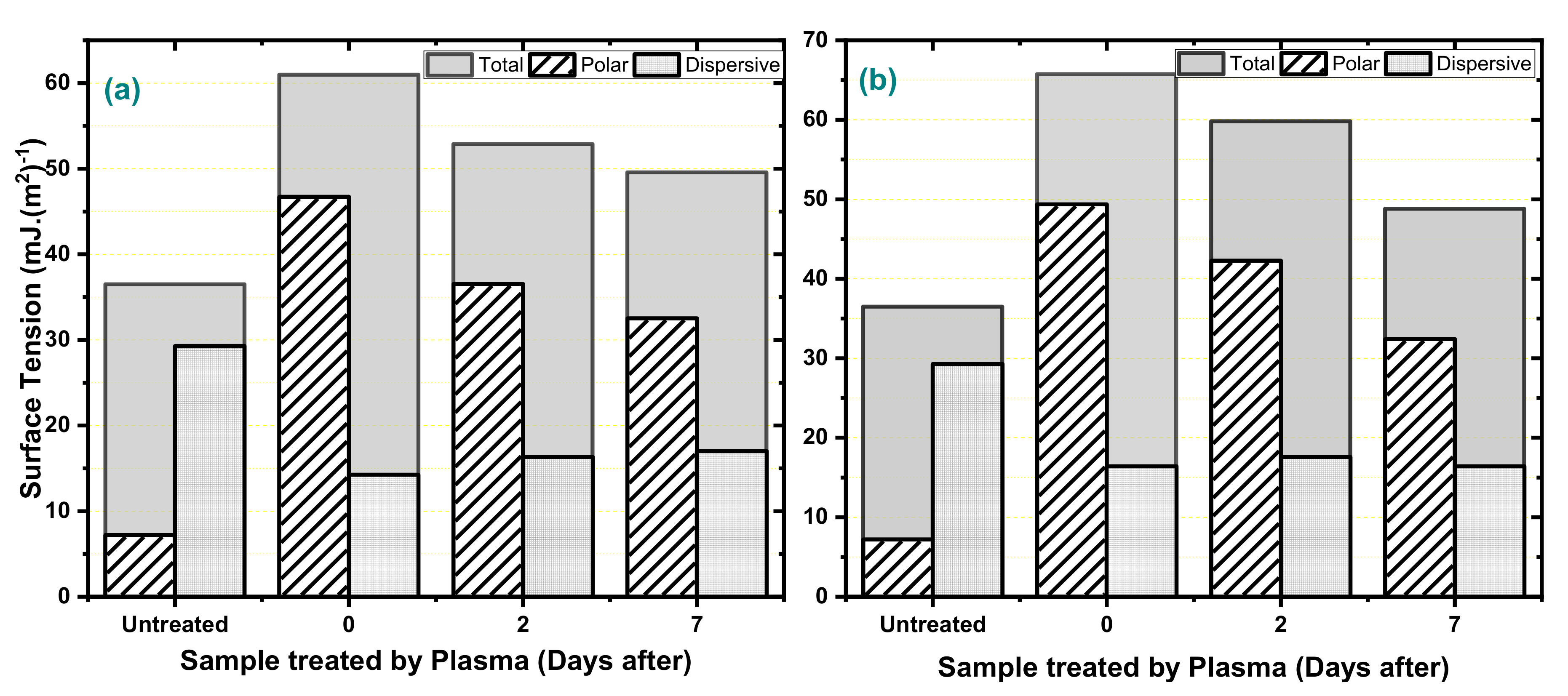
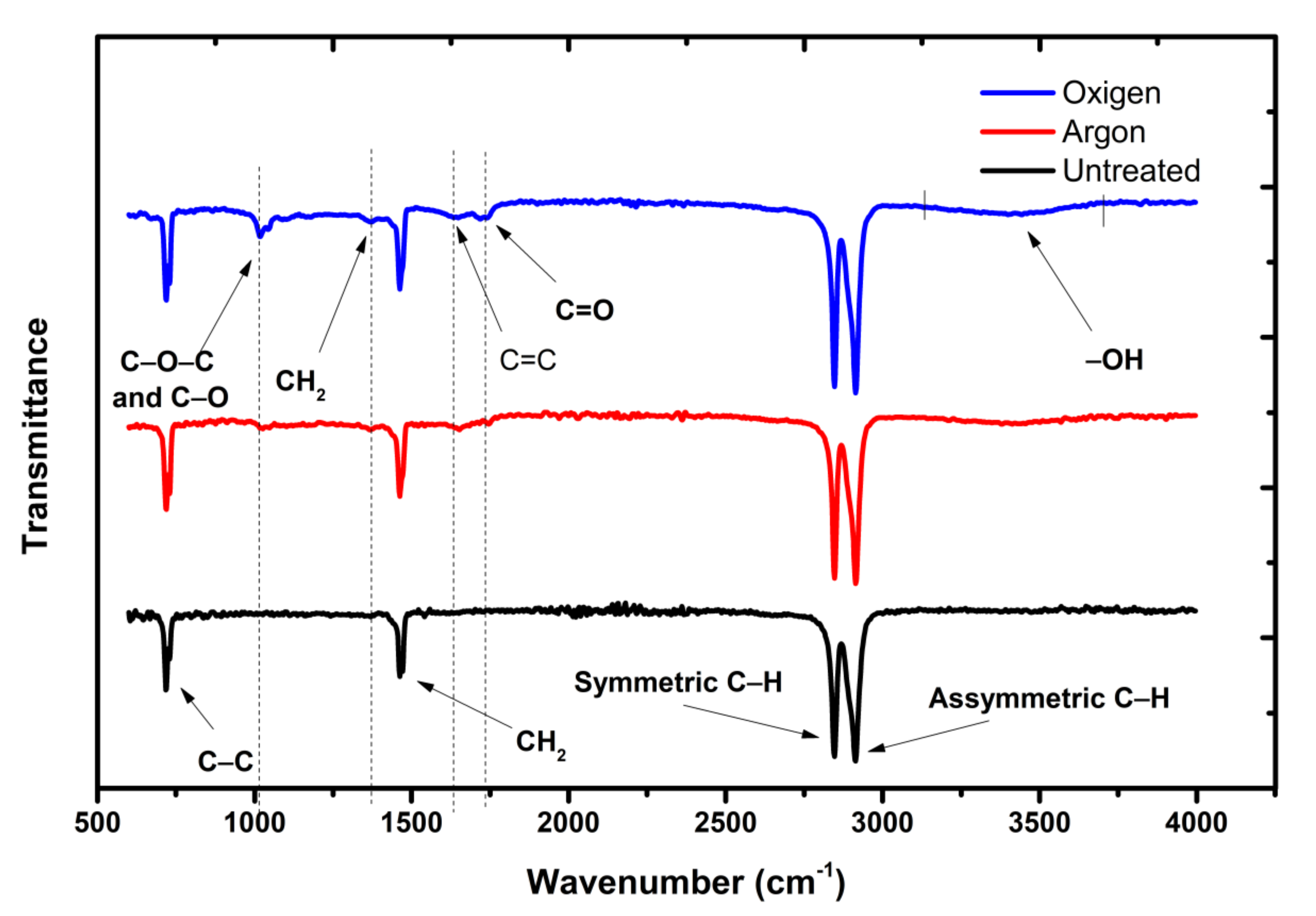
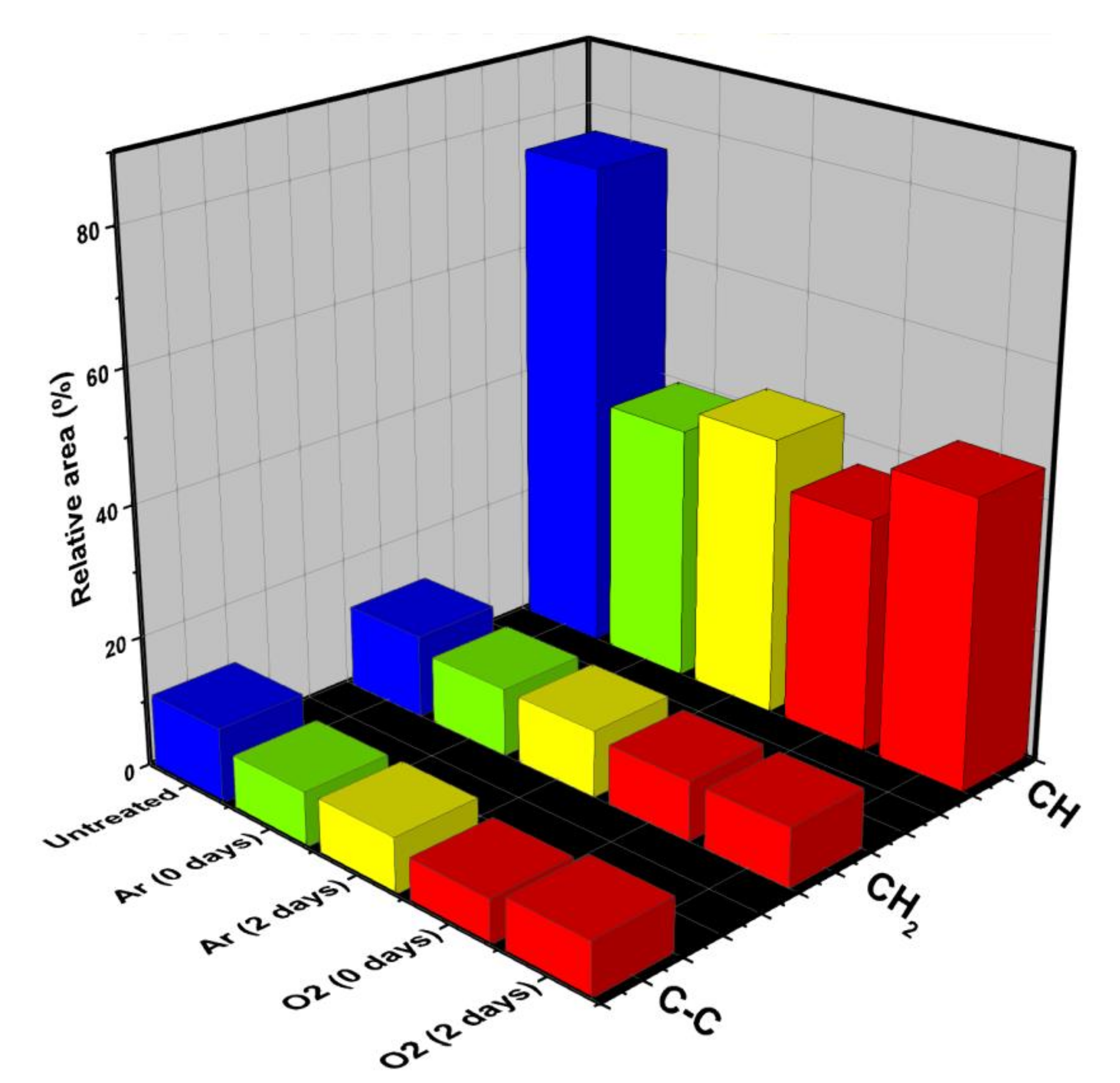
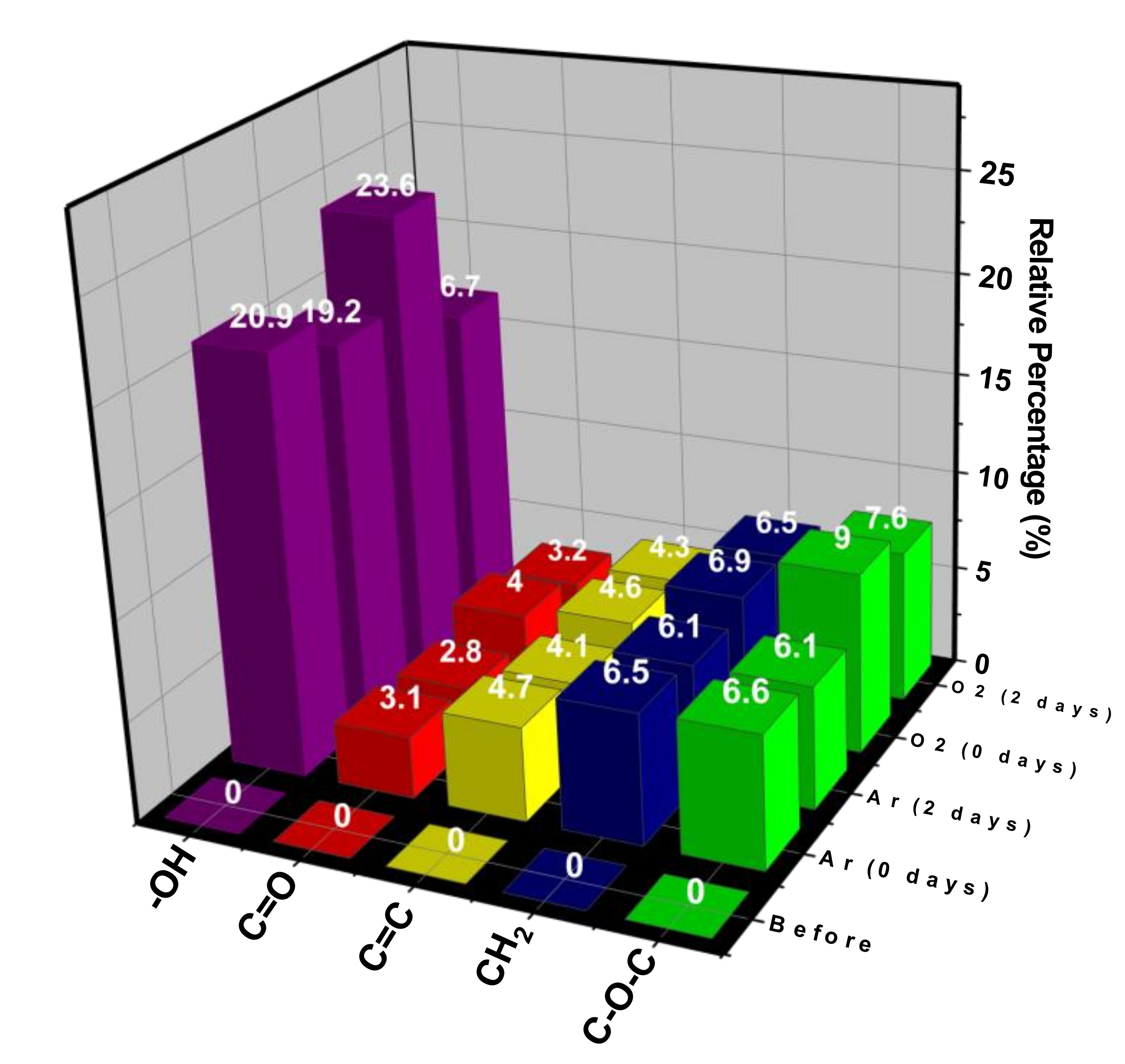

| Applied Voltage (kV) | 20 |
|---|---|
| Frequency (Hz) | 820 |
| Distance between the Electrodes (mm) | 1.7 |
| Time treatment (s) | 600 |
| Mean Energy Calculated During the Plasma Treatments | Bonds that May Be Influenced by Plasma Treatment | Connection Energies (eV) | |
|---|---|---|---|
| Argon | Oxygen | ||
| 16.31 (eV) | 15.04 (eV) | C–H | 4.29 |
| C–C | 3.60 | ||
| C–O | 3.64 | ||
| C=O | 7.37 | ||
| C=C | 6.33 | ||
| Liquid | Surface Tensions (mJ/m2) | Contact Angle (Degrees) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| γl | γlp | γld | Untreated | Argon | Oxygen | |||||
| 0 Days | 2 Days | 7 Days | 0 Days | 2 Days | 7 Days | |||||
| Water | 72.8 | 51.0 | 21.8 | 79.17 | 28.52 | 36.94 | 48.66 | 33.34 | 44.12 | 49.02 |
| Dimethyl-formamide | 37.3 | 4.9 | 32.4 | 31.72 | 0 | 0 | 0 | 0 | 0 | 15.49 |
| Glycerol | 63.4 | 26.2 | 37.2 | 53.17 | 9.91 | 24.75 | 52.75 | 37.63 | 45.34 | 46.63 |
| Untreated | Argon | Oxygen | |
|---|---|---|---|
| Ra (nm) | 14.084 | 32.314 | 16.659 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, J.F.d.M.; Alves de Souza, I.; Feitor, M.C.; Targino, T.G.; Diniz, G.F.; Libório, M.S.; Sousa, R.R.M.; Costa, T.H.d.C. Study of High-Density Polyethylene (HDPE) Kinetics Modification Treated by Dielectric Barrier Discharge (DBD) Plasma. Polymers 2020, 12, 2422. https://doi.org/10.3390/polym12102422
Neto JFdM, Alves de Souza I, Feitor MC, Targino TG, Diniz GF, Libório MS, Sousa RRM, Costa THdC. Study of High-Density Polyethylene (HDPE) Kinetics Modification Treated by Dielectric Barrier Discharge (DBD) Plasma. Polymers. 2020; 12(10):2422. https://doi.org/10.3390/polym12102422
Chicago/Turabian StyleNeto, João Freire de Medeiros, Ivan Alves de Souza, Michelle Cequeira Feitor, Talita Galvão Targino, Gutembergy Ferreira Diniz, Maxwell Santana Libório, Rômulo Ribeiro Magalhães Sousa, and Thercio Henrique de Carvalho Costa. 2020. "Study of High-Density Polyethylene (HDPE) Kinetics Modification Treated by Dielectric Barrier Discharge (DBD) Plasma" Polymers 12, no. 10: 2422. https://doi.org/10.3390/polym12102422