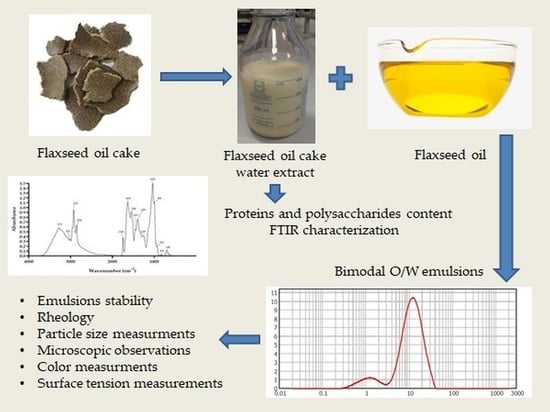
Characterization of Flaxseed Oil Bimodal Emulsions Prepared with Flaxseed Oil Cake Extract Applied as a Natural Emulsifying Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Flaxseed Oil Cake Extract (FOCE), Flaxseed Protein (FP) and Flaxseed Gum (FG) Extraction
2.3. FOCE Characterization
2.4. Determination of Water-Holding and Oil-Binding Capacities of FOCE, FG and FP
2.5. Emulsion Preparation and Emulsifying Properties Measurements
2.6. Determination of Emulsion with FOCE, FP, FG and Tween 80 Particle Size Distribution and SPAN Index
2.7. Optical Microscope Observations of Emulsions with FOCE, FP, FG and Tween 80
2.8. Color Determination of Model Emulsions
2.9. Rheological Measurements and Interfacial Tension
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Proximate Composition of FOCE
3.2. Water-Holding and Oil-Binding Capacities of FOCE, FP, FG, and Interfacial Tension
3.3. Emulsion Stability
3.4. Droplet Size and SPAN Index
3.5. Emulsions Rheological Characteristics and Interfacial Tension
3.6. Emulsion Color
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oluwole, A.J.O.; Ikhu-Omoregbe, D.I.; Jideani, V.A. Physicochemical Properties of African Catfish Mucus and Its Effect on the Stability of Soya Milk Emulsions. Appl. Sci. 2020, 10, 916. [Google Scholar] [CrossRef] [Green Version]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The effect of native and denaturated flaxseed meal extract on physiochemical properties of low fat mayonnaises. J. Food Meas. Charact. 2020, 14, 1135–1145. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Ereifej, K.; Gammoh, S.; Kubow, S.; Tawalbeh, D. Preparation of mayonnaise from extracted plant protein isolates of chickpea, broad bean and lupin flour: Chemical, physiochemical, nutritional and therapeutic properties. J. Food Sci. Technol. 2017, 54, 1395–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, S.; Bhattacharyya, D.K.; Goswami, R.; Bhowal, J. Emulsions stabilized by soy protein nanoparticles as potential functional non-dairy yogurts. J. Sci. Food Agric. 2019, 99, 5808–5818. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht Nasrabadi, M.; Goli, S.A.H.; Sedaghat Doost, A.; Roman, B.; Dewettinck, K.; Stevens, C.V.; Van der Meeren, P. Plant based Pickering stabilization of emulsions using soluble flaxseed protein and mucilage nano-assemblies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 170–182. [Google Scholar] [CrossRef]
- Aiking, H. Future protein supply. Trends Food Sci. Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A. Preparation and characterization of novel flaxseed oil cake yogurt-like plant milk fortified with inulin. J. Food Nutr. Res. 2020, 59, 61–70. [Google Scholar]
- Moure, A.; Sineiro, J.; Domínguez, H.; Parajó, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- Pag, A.I.; Radu, D.G.; Drǎgǎnescu, D.; Popa, M.I.; Sîrghie, C. Flaxseed cake—A sustainable source of antioxidant and antibacterial extracts. Cellul. Chem. Technol. 2014, 48, 265–273. [Google Scholar]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Anjum, F.M.; Butt, M.S.; Siddiq, M.; Sheikh, M.A. Reduction of Cyanogenic Compounds in Flaxseed (Linum usitatissimum L.) Meal Using Thermal Treatment. Int. J. Food Prop. 2013, 16, 1809–1818. [Google Scholar] [CrossRef] [Green Version]
- Oomah, B.D.; Mazza, G. Optimization of a spray drying process for flaxseed gum. Int. J. Food Sci. Technol. 2001, 36, 135–143. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Barczewski, M.; Mysiukiewicz, O.; Kloziński, A. Complex modification effect of linseed cake as an agricultural waste filler used in high density polyethylene composites. Iran. Polym. J. Engl. Ed. 2018, 27, 677–688. [Google Scholar] [CrossRef] [Green Version]
- Sielicka, M.; Małecka, M. Enhancement of Oxidative Stability of Flaxseed Oil Through Flaxseed, Evening Primrose and Black Cumin Cake Extracts. J. Food Process. Preserv. 2017, 41, e13070. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms 2020, 8, 1266. [Google Scholar] [CrossRef]
- Sanmartin, C.; Taglieri, I.; Venturi, F.; Macaluso, M.; Zinnai, A.; Tavarini, S.; Botto, A.; Serra, A.; Conte, G.; Flamini, G.; et al. Flaxseed Cake as a Tool for the Improvement of Nutraceutical and Sensorial Features of Sourdough Bread. Foods 2020, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Mysiukiewicz, O.; Barczewski, M.; Szulc, J. The influence of poly(Vinyl alcohol) on oil release behavior of polylactide-based composites filled with linseed cake. J. Renew. Mater. 2020, 8, 347–363. [Google Scholar] [CrossRef]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Singh, A.K.; Arora, S.; Lal, D.; Sabikhi, L. Development of stable flaxseed oil emulsions as a potential delivery system of ω-3 fatty acids. J. Food Sci. Technol. 2015, 52, 4256–4265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitez, L.O.; Castagnini, J.M.; Añón, M.C.; Salgado, P.R. Development of oil-in-water emulsions based on rice bran oil and soybean meal as the basis of food products able to be included in ketogenic diets. LWT—Food Sci. Technol. 2020, 118, 108809. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Liu, C.; Chen, J.; Zhang, R.; Zhang, Z.; Dai, T.; McClements, D.J. Potential physicochemical basis of Mediterranean diet effect: Ability of emulsified olive oil to increase carotenoid bioaccessibility in raw and cooked tomatoes. Food Res. Int. 2016, 89, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Querol, N.; Barreneche, C.; Cabeza, L.F. Storage stability of bimodal emulsions vs. monomodal emulsions. Appl. Sci. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, C.; Liang, C.; Liu, C.; Li, J. Study of Bimodal Drop Size Distributions of Emulsion. J. Dispers. Sci. Technol. 2014, 35, 397–402. [Google Scholar] [CrossRef]
- Dybowska, B.E. Whey protein-stabilized emulsion properties in relation to thermal modification of the continuous phase. J. Food Eng. 2011, 104, 81–88. [Google Scholar] [CrossRef]
- Karefyllakis, D.; Octaviana, H.; van der Goot, A.J.; Nikiforidis, C.V. The emulsifying performance of mildly derived mixtures from sunflower seeds. Food Hydrocoll. 2019, 88, 75–85. [Google Scholar] [CrossRef]
- Östbring, K.; Tullberg, C.; Burri, S.; Malmqvist, E.; Rayner, M. Protein recovery from rapeseed press cake: Varietal and processing condition effects on yield, emulsifying capacity and antioxidant activity of the protein rich extract. Foods 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Xu, X.; Cheng, H.; Li, J.; Guang, C.; Liang, L. Comparison of whey protein particles and emulsions for the encapsulation and protection of α-tocopherol. J. Food Eng. 2019, 247, 56–63. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.B.; Lee, J.S. Rheological behavior of bimodal distribution emulsions on flow adoptability. Biomicrofluidics 2019, 13, 14109. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, L.-J.; Li, S.-J.; Adhikari, B. Effects of drying methods on the functional properties of flaxseed gum powders. Carbohydr. Polym. 2010, 81, 128–133. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Adhikari, B. Microencapsulation of flaxseed oil in flaxseed protein and flaxseed gum complex coacervates. Food Res. Int. 2016, 86, 1–8. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Goa, J. A Micro Biuret Method for Protein Determination Determination of Total Protein in Cerebrospinal Fluid. Scand. J. Clin. Lab. Investig. 1953, 5, 218–222. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Gong, K.-J.; Shi, A.-M.; Liu, H.-Z.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016, 170, 33–40. [Google Scholar] [CrossRef]
- Cameron, D.R.; Weber, M.E.; Idziak, E.S.; Neufeld, R.J.; Cooper, D.G. Determination of Interfacial Areas in Emulsions Using Turbidimetric and Droplet Size Data: Correction of the Formula for Emulsifying Activity Index. J. Agric. Food Chem. 1991, 39, 655–659. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.M.; Guo, S.D. Rheological, texture and sensory properties of low-fat mayonnaise with different fat mimetics. LWT—Food Sci. Technol. 2007, 40, 946–954. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Mahdi, S.; Sarabandi, K.; Mohammadi, M. Colloids and Surfaces B: Biointerfaces In fluence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [CrossRef]
- Cui, W.; Mazza, G.; Biliaderis, C.G. Chemical Structure, Molecular Size Distributions, and Rheological Properties of Flaxseed Gum. J. Agric. Food Chem. 1994, 42, 1891–1895. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Geerts, M.E.J.; Nikiforidis, C.V.; van der Goot, A.J.; van der Padt, A. Protein nativity explains emulsifying properties of aqueous extracted protein components from yellow pea. Food Struct. 2017, 14, 104–111. [Google Scholar] [CrossRef]
- Pham, L.B.; Wang, B.; Zisu, B.; Adhikari, B. Complexation between flaxseed protein isolate and phenolic compounds: Effects on interfacial, emulsifying and antioxidant properties of emulsions. Food Hydrocoll. 2019, 94, 20–29. [Google Scholar] [CrossRef]
- Basiri, S.; Haidary, N.; Shekarforoush, S.S.; Niakousari, M. Flaxseed mucilage: A natural stabilizer in stirred yogurt. Carbohydr. Polym. 2018, 187, 59–65. [Google Scholar] [CrossRef]
- Sun, J.; Liu, W.Y.; Feng, M.Q.; Xu, X.L.; Zhou, G.H. Characterization of olive oil emulsions stabilized by flaxseed gum. J. Food Eng. 2019, 247, 74–79. [Google Scholar] [CrossRef]
- Oomah, B.D.; Kenaschuk, E.O.; Cui, W.; Mazza, G. Variation in the Composition of Water-Soluble Polysaccharides in Flaxseed. J. Agric. Food Chem. 1995, 43, 1484–1488. [Google Scholar] [CrossRef]
- Singh, K.K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A potential source of food, feed and fiber. Crit. Rev. Food Sci. Nutr. 2011, 51, 210–222. [Google Scholar] [CrossRef]
- Hu, Y.; Shim, Y.Y.; Reaney, M.J.T. Flaxseed Gum Solution Functional Properties. Foods 2020, 9, 681. [Google Scholar] [CrossRef]
- Fedeniuk, R.W.; Biliaderis, C.G. Composition and physicochemical properties of linseed (Linum usitatissimum L.) mucilage. J. Agric. Food Chem. 1994, 42, 240–247. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Sawada, K.; Moritaka, S.; Misaki, M.; Toda, J.; Wada, T.; Ishii, K. Whipping and Emulsifying Properties of Soybean Products. Agric. Biol. Chem. 1972, 36, 719–727. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Wang, L.J.; Özkan, N. Effect of concentrated flaxseed protein on the stability and rheological properties of soybean oil-in-water emulsions. J. Food Eng. 2010, 96, 555–561. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Ramsey, S.R. Effect of Gums on Low-Fat Meat Batters. J. Food Sci. 1986, 51, 33–36. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Goli, S.A.H.; Sedaghat Doost, A.; Dewettinck, K.; Van der Meeren, P. Bioparticles of flaxseed protein and mucilage enhance the physical and oxidative stability of flaxseed oil emulsions as a potential natural alternative for synthetic surfactants. Colloids Surf. B Biointerfaces 2019, 184, 110489. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Kumar, G.S.; Chon, B.H.; Sangwai, J.S. Thermal stability of oil-in-water Pickering emulsion in the presence of nanoparticle, surfactant, and polymer. J. Ind. Eng. Chem. 2015, 22, 324–334. [Google Scholar] [CrossRef]
- Ding, H.H.; Cui, S.W.; Goff, H.D.; Wang, Q.; Chen, J.; Han, N.F. Soluble polysaccharides from flaxseed kernel as a new source of dietary fibres: Extraction and physicochemical characterization. Food Res. Int. 2014, 56, 166–173. [Google Scholar] [CrossRef]
- Romero, A.; Beaumal, V.; David-Briand, E.; Cordobés, F.; Guerrero, A.; Anton, M. Interfacial and oil/water emulsions characterization of potato protein isolates. J. Agric. Food Chem. 2011, 59, 9466–9474. [Google Scholar] [CrossRef]
- Kuhn, R.K.; Guimarães Drummond Silva, F.; Maria Netto, F.; Lopes da Cunha, R. Assessing the potential of flaxseed protein as an emulsifier combined with whey protein isolate. Food Res. Int. 2014, 58, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Krause, J.-P.; Schultz, M.; Dudek, S. Effect of extraction conditions on composition, surface activity and rheological properties of protein isolates from flaxseed (Linum usitativissimum L). J. Sci. Food Agric. 2002, 82, 970–976. [Google Scholar] [CrossRef]
- Drozłowska, E.; Weronis, M.; Bartkowiak, A. The influence of thermal hydrolysis process on emulsifying properties of potato protein isolate. J. Food Sci. Technol. 2020, 57, 1131–1137. [Google Scholar] [CrossRef]
- Taylor, P. Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar] [CrossRef]
- Henry, J.V.L.; Fryer, P.J.; Frith, W.J.; Norton, I.T. Emulsification mechanism and storage instabilities of hydrocarbon-in-water sub-micron emulsions stabilised with Tweens (20 and 80), Brij 96v and sucrose monoesters. J. Colloid Interface Sci. 2009, 338, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kanafusa, S.; Chu, B.-S.; Nakajima, M. Factors affecting droplet size of sodium caseinate-stabilized O/W emulsions containing β-carotene. Eur. J. Lipid Sci. Technol. 2007, 109, 1038–1041. [Google Scholar] [CrossRef] [Green Version]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of canola and flaxseed protein isolates produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2991–2998. [Google Scholar] [CrossRef]
- Morales Chabrand, R.; Kim, H.-J.; Zhang, C.; Glatz, C.E.; Jung, S. Destabilization of the Emulsion Formed during Aqueous Extraction of Soybean Oil. J. Am. Oil Chem. Soc. 2008, 85, 383–390. [Google Scholar] [CrossRef]
- Sridharan, S.; Meinders, M.B.J.; Bitter, J.H.; Nikiforidis, C.V. Pea flour as stabilizer of oil-in-water emulsions: Protein purification unnecessary. Food Hydrocoll. 2020, 101, 105533. [Google Scholar] [CrossRef]
- Sourdet, S.; Relkin, P.; César, B. Effects of milk protein type and pre-heating on physical stability of whipped and frozen emulsions. Colloids Surf. B Biointerfaces 2003, 31, 55–64. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S. Effects of protein concentration and oil-phase volume fraction on the stability and rheology of menhaden oil-in-water emulsions stabilized by whey protein isolate with xanthan gum. Food Hydrocoll. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- McClements, D.J. Colloidal basis of emulsion color. Curr. Opin. Colloid Interface Sci. 2002, 7, 451–455. [Google Scholar] [CrossRef]
- Kupongsak, S.; Sathitvorapojjana, S. Properties and Storage Stability of O/W Emulsion Replaced with Medium-Chain Fatty Acid Oil. Polish J. Food Nutr. Sci. 2017, 67, 107–115. [Google Scholar] [CrossRef] [Green Version]





| Sample * | γ [mN/m] |
|---|---|
| FOCE/FO | 26.89 ± 0.64 a |
| W/FO | 47.63 ± 0.03 b |
| FG/FO | 35.89 ± 0.54 c |
| FP/FO | 38.71 ± 0.09 d |
| 3% Tween 80/FO | 19.89 ± 0.26 e |
| D4.3 (µm) | ||||
| Sample | FOCE | FG | FP | Tween 80 |
| A | 7.13 ± 0.00 d | 21.58 ± 0.02 e | 26.73 ± 0.10 e | 52.76 ± 0.11 a |
| B | 6.21 ± 0.10 e | 25.06 ± 0.02 d | 57.36 ± 0.97 d | 8.94 ± 0.02 b |
| C | 8.72 ± 0.03 c | 37.30 ± 0.13 c | 122.17 ± 0.05 c | 5.96 ± 0.01 c |
| D | 9.72 ± 0.01 b | 43.36 ± 0.10 b | 125.04 ± 0.72 b | 4.51 ± 0.05 d |
| E | 12.19 ± 0.30 a | 44.99 ± 0.42 a | 205.98 ± 0.30 a | 4.03 ± 0.10 e |
| SPAN (-) | ||||
| A | 1.864 ± 0.00 a | 1.503 ± 0.02 a | 1.451 ± 0.03 d | 1.332 ± 0.10 d |
| B | 1.646 ± 0.10 c | 1.208 ± 0.02 b | 1.362 ± 0.00 b | 1.195 ± 0.05 e |
| C | 1.803 ± 0.01 b | 1.069 ± 0.10 c | 1.154 ± 0.05 a | 1.672 ± 0.03 b |
| D | 1.865 ± 0.03 a | 0.988 ± 0.15 d | 1.154 ± 0.02 a | 1.892 ± 0.06 a |
| E | 1.770 ± 0.30 b | 0.809 ± 0.02 e | 1.354 ± 0.03 c | 1.569 ± 0.00 c |
| τy [Pa] | ||||
| FOCE | FG | FP | Tween 80 | |
| 0.0010 ± 0.001 Af | 0.3456 ± 0.001 Bb | 0.0045 ± 0.001 Cf | - | |
| A | 0.0014 ± 0.003 Ae | 0.0005 ± 0.001 Bf | 0.1436 ± 0.004 Cc | 0.0007 ± 0.001 Bc |
| B | 0.0041 ± 0.001 Ad | 0.0007 ± 0.000 Be | 0.0172 ± 0.005 Ce | 0.0001 ± 0.012 De |
| C | 0.1450 ± 0.000 Ac | 0.0203 ± 0.003 Bd | 0.0958 ± 0.003 Cd | 0.0020 ± 0.006 Dd |
| D | 0.6590 ± 0.001 Aa | 0.3209 ± 0.002 Bc | 0.1471 ± 0.006 Cb | 0.0810 ± 0.001 Da |
| E | 0.3570 ± 0.002 Ab | 0.6987 ± 0.003 Ba | 0.6987 ± 0.003 Ba | 0.0048 ± 0.002 Cb |
| Viscosity [Pa·s] | ||||
| FOCE | FG | FP | Tween 80 | |
| 0.0540 ± 0.005 Af | 0.0007 ± 0.000 Bf | 0.0006 ± 0.000 Cf | - | |
| A | 0.0960 ± 0.000 Ae | 0.0060 ± 0.004 Be | 0.0005 ± 0.003 Ce | 0.0021 ± 0.001 Dd |
| B | 0.1590 ± 0.001 Ad | 0.0095 ± 0.003 Bd | 0.0029 ± 0.001 Cd | 0.0036 ± 0.000 Dc |
| C | 1.1640 ± 0.003 Ac | 0.0272 ± 0.001 Bc | 0.0057 ± 0.000 Cc | 0.0035 ± 0.001 Dc |
| D | 3.9080 ± 0.001 Aa | 0.0594 ± 0.000 Bb | 0.0234 ± 0.000 Ca | 0.0040 ± 0.005 Db |
| E | 3.6320 ± 0.001 Ab | 0.1336 ± 0.002 Ba | 0.0081 ± 0.005 Cb | 0.0065 ± 0.000 Da |
| K [Pa·sn] | ||||
| FOCE | FG | FP | Tween 80 | |
| 0.076 ± 0.001 Af | 0.0001 ± 0.000 Bf | 0.0003 ± 0.001 Ce | - | |
| A | 0.146 ± 0.004 Ae | 0.0071 ± 0.002 Be | 0.0001± 0.003 Cf | 0.0021 ± 0.003 Dd |
| B | 0.211 ± 0.001 Ad | 0.0125 ± 0.001 Bd | 0.0042 ± 0.000 Cd | 0.0053 ± 0.002 Db |
| C | 1.083 ± 0.000 Ac | 0.0471 ± 0.000 Bc | 0.0076 ± 0.008 Cc | 0.0044 ± 0.000 Dc |
| D | 1.799 ± 0.001 Aa | 0.0987 ± 0.006 Bb | 0.0562 ± 0.000 Ca | 0.0011 ± 0.001 Dd |
| E | 1.420 ± 0.001 Ab | 0.2330 ± 0.000 Ba | 0.0116 ± 0.000 Cb | 0.0065 ± 0.003 Da |
| n [-] | ||||
| FOCE | FG | FP | Tween 80 | |
| 0.88 ± 0.001 Aa | 1.00 ± 0.001 Ba | 1.13 ± 0.001 Cb | - | |
| A | 0.82 ± 0.002 Ac | 0.97 ± 0.003 Bb | 1.24 ± 0.001 Ca | 0.99 ± 0.001 Dc |
| B | 0.85 ± 0.001 Ab | 0.94 ± 0.001 Bc | 0.93 ± 0.000 Cd | 0.93 ± 0.001 Ce |
| C | 0.53 ± 0.000 Ad | 0.85 ± 0.000 Bd | 0.94 ± 0.004 Cc | 0.96 ± 0.003 Dd |
| D | 0.43 ± 0.004 Ae | 0.82 ± 0.000 Be | 0.77 ± 0.000 Ce | 1.24 ± 0.001 Da |
| E | 0.86 ± 0.000 Ab | 0.72 ± 0.005 Bf | 0.93 ± 0.000 Cd | 1.00 ± 0.000 Db |
| L | ||||
| FOCE | FG | FP | Tween 80 | |
| 53.34 ± 0.01 Af | 32.60 ± 0.01 Bf | 31.59 ± 0.02 Bf | - | |
| A | 89.53 ± 0.03 Aa | 89.90 ± 0.00 Ba | 79.19 ± 0.51 Bc | 81.95 ± 0.23 Ce |
| B | 89.43 ± 0.01 Ab | 89.42 ± 0.00 Ab | 78.36 ± 0.69 Cb | 90.89 ± 0.01 Cc |
| C | 87.83 ± 0.02 Ac | 81.46 ± 0.01 Bc | 79.24 ± 0.29 Cd | 91.24 ± 0.01 Ca |
| D | 87.52 ± 0.03 Ad | 80.59 ± 0.01 Bd | 80.29 ± 0.22 Cc | 91.18 ± 0.02 Cb |
| E | 83.28 ± 0.02 Ae | 80.08 ± 0.10 Be | 79.19 ± 0.51 Ce | 90.18 ± 0.01 Cd |
| a | ||||
| FOCE | FG | FP | Tween 80 | |
| 2.12 ± 0.00 Aa | −1.67 ± 0.02 Bf | −1.55 ± 0.01 Cf | - | |
| A | 0.50 ± 0.00 Ac | −1.00 ± 0.00 Be | −0.90 ± 0.01 Ce | −0.29 ± 0.04 De |
| B | 0.27 ± 0.01 Af | 0.41 ± 0.00 Bd | 0.33 ± 0.32 Cd | 0.49 ± 0.01 Dc |
| C | 0.53 ± 0.00 Ab | 2.63 ± 0.03 Bb | 2.56 ± 0.11 Cc | 0.74 ± 0.01 Da |
| D | 0.34 ± 0.01 Ae | 2.27 ± 0.01 Bc | 2.81 ± 0.04 Cb | 0.52 ± 0.01 Db |
| E | 0.47 ± 0.00 Ad | 3.97 ± 0.01 Ba | 3.65 ± 0.03 Ca | 0.07 ± 0.01 Dd |
| b | ||||
| FOCE | FG | FP | Tween 80 | |
| 36.01 ± 0.01 Aa | −6.44 ± 0.02 Bf | −6.57 ± 0.01 Cf | - | |
| A | 24.18 ± 0.06 Ae | 32.20 ± 0.17 Be | 32.54 ± 1.03 Ce | 40.24 ± 1.48 Da |
| B | 21.94 ± 0.04 Af | 41.56 ± 0.21 Bd | 42.07 ± 2.50 Cd | 30.74 ± 0.04 Db |
| C | 26.23 ± 0.01 Ac | 52.42 ± 0.42 Bc | 55.46 ± 1.46 Cc | 30.24 ± 0.19 Dc |
| D | 25.20 ± 0.03 Ad | 55.18 ± 0.11 Bb | 58.82 ± 0.51 Cb | 29.49 ± 0.02 Dd |
| E | 26.70 ± 0.01 Ab | 61.50 ± 0.06 Ba | 61.66 ± 0.30 Ca | 28.53 ± 0.03 De |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozłowska, E.; Bartkowiak, A.; Łopusiewicz, Ł. Characterization of Flaxseed Oil Bimodal Emulsions Prepared with Flaxseed Oil Cake Extract Applied as a Natural Emulsifying Agent. Polymers 2020, 12, 2207. https://doi.org/10.3390/polym12102207
Drozłowska E, Bartkowiak A, Łopusiewicz Ł. Characterization of Flaxseed Oil Bimodal Emulsions Prepared with Flaxseed Oil Cake Extract Applied as a Natural Emulsifying Agent. Polymers. 2020; 12(10):2207. https://doi.org/10.3390/polym12102207
Chicago/Turabian StyleDrozłowska, Emilia, Artur Bartkowiak, and Łukasz Łopusiewicz. 2020. "Characterization of Flaxseed Oil Bimodal Emulsions Prepared with Flaxseed Oil Cake Extract Applied as a Natural Emulsifying Agent" Polymers 12, no. 10: 2207. https://doi.org/10.3390/polym12102207