Fluorescent and Mechanical Properties of Silicon Quantum Dots Modified Sodium Alginate-Carboxymethylcellulose Sodium Nanocomposite Bio-Polymer Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SiQDs
2.3. Fabrication of SiQDs/SA-CMC Nanocomposites
2.4. Characterization Techniques
3. Results and Discussion
3.1. PL Analysis of SiQDs Under Different Reaction Conditions
3.2. Morphology Analysis of SiQDs
3.3. XRD and FTIR Analysis of SiQDs
3.4. XPS Analysis of SiQDs
3.5. Fluorescence Stability Analysis of SiQDs
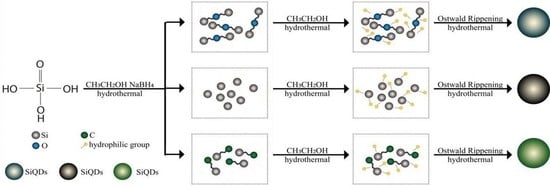
3.6. Formation Mechanism of SiQDs
3.7. PL Analysis of SiQDs/SA-CMC Nanocomposites
3.8. Mechanical Properties Analysis of SiQDs/SA-CMC Nanocomposites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ji, Z.; Dervishi, E.; Doorn, S.K.; Sykora, M. Size-Dependent Electronic Properties of Uniform Ensembles of Strongly Confined Graphene Quantum Dots. J. Phys. Chem. Lett. 2019, 10, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Liu, Y.; Li, H.; Fan, J. Quantum confinement luminescence of trigonal cesium lead bromide quantum dots. Appl. Surf. Sci. 2019, 466, 119–125. [Google Scholar] [CrossRef]
- Cuevas, A.; Campos, B.B.; Romero, R.; Algarra, M.; Vazquez, M.I.; Benavente, J. Eco-friendly modification of a regenerated cellulose based film by silicon, carbon and N-doped carbon quantum dots. Carbohydr. Polym. 2019, 206, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ji, X.; He, Y. Water-Dispersible Fluorescent Silicon Nanoparticles and their Optical Applications. Adv. Mater. 2016, 28, 10567–10574. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Khan, W.U.; Wang, Y. Solid-State Carbon Dots with Efficient Cyan Emission towards White Light-Emitting Diodes. Chem. Asian J. 2019, 14, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Kwak, G.Y.; Kim, J.M.; Jang, C.W.; Choi, S.-H.; Kim, K.J. Remarkable enhancement of stability in high-efficiency Si-quantum-dot heterojunction solar cells by employing bis(trifluoromethanesulfonyl)-amide as a dopant for graphene transparent conductive electrodes. J. Alloys Compd. 2019, 773, 913–918. [Google Scholar] [CrossRef]
- Gu, W.; Liu, X.; Pi, X.; Dai, X.; Zhao, S.; Yao, L.; Li, D.; Jin, Y.; Xu, M.; Yang, D.; et al. Silicon-Quantum-Dot Light-Emitting Diodes With Interlayer-Enhanced Hole Transport. IEEE Photonics J. 2017, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Sun, Y.; Xie, Y.; Wu, S.; Zhang, X.; Zhuang, J.; Hu, C.; Lei, B.; Liu, Y. Synthesis of Silicon Quantum Dots with Highly Efficient Full-Band UV Absorption and Their Applications in Antiyellowing and Resistance of Photodegradation. ACS Appl. Mater. Interfaces 2019, 11, 6634–6643. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.L.; Cai, S.J.; Li, S.; He, X.W.; Li, W.Y.; Li, Y.H.; Zhang, Y.K. One-Pot Microwave Synthesis of Water-Dispersible, High Fluorescence Silicon Nanoparticles and Their Imaging Applications in Vitro and in Vivo. Anal. Chem. 2016, 88, 11631–11638. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, G.; Zeng, W.; Lv, B.; Yi, Y. Highly sensitive and selective “off-on” fluorescent sensing platform for ClO(-) in water based on silicon quantum dots coupled with nanosilver. Anal. Bioanal. Chem. 2019, 411, 1561–1568. [Google Scholar] [CrossRef]
- Abdelhameed, M.; Martir, D.R.; Chen, S.; Xu, W.Z.; Oyeneye, O.O.; Chakrabarti, S.; Zysman-Colman, E.; Charpentier, P.A. Tuning the Optical Properties of Silicon Quantum Dots via Surface Functionalization with Conjugated Aromatic Fluorophores. Sci. Rep. 2018, 8, 3050. [Google Scholar] [CrossRef] [PubMed]
- Nsanzamahoro, S.; Mutuyimana, F.P.; Han, Y.; Ma, S.; Na, M.; Liu, J.; Ma, Y.; Ren, C.; Chen, H.; Chen, X. Highly selective and sensitive detection of catechol by one step synthesized highly fluorescent and water-soluble silicon nanoparticles. Sens. Actuators, B. 2019, 281, 849–856. [Google Scholar] [CrossRef]
- Ma, S.D.; Chen, Y.L.; Feng, J.; Liu, J.J.; Zuo, X.W.; Chen, X.G. One-Step Synthesis of Water-Dispersible and Biocompatible Silicon Nanoparticles for Selective Heparin Sensing and Cell Imaging. Anal. Chem. 2016, 88, 10474–10481. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Liu, X.; Zhou, B.; Zhan, Z.; Cartwright, A.N.; Swihart, M.T. A Solution-Processed UV-Sensitive Photodiode Produced Using a New Silicon Nanocrystal Ink. Adv. Funct. Mater. 2014, 24, 6016–6022. [Google Scholar] [CrossRef]
- He, Y.; Zhong, Y.; Peng, F.; Wei, X.; Su, Y.; Lu, Y.; Su, S.; Gu, W.; Liao, L.; Lee, S.T. One-pot microwave synthesis of water-dispersible, ultraphoto- and pH-stable, and highly fluorescent silicon quantum dots. J. Am. Chem. Soc. 2011, 133, 14192–14195. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Sun, W.; Wang, L.; Chen, C.; Liao, K.; Wang, W.; Jia, J.; Hatton, B.D.; Casillas, G.; Kurylowicz, M.; et al. Non-wettable, oxidation-stable, brightly luminescent, perfluorodecyl-capped silicon nanocrystal film. J. Am. Chem. Soc. 2014, 136, 15849–15852. [Google Scholar] [CrossRef]
- Stanca, L.; Petrache, S.N.; Serban, A.I.; Staicu, A.C.; Sima, C.; Munteanu, M.C.; Zărnesc, O.; Dinu, D.; Dinischiotu, A. Interaction of silicon-based quantum dots with gibel carp liver: Oxidative and structural modifications. Nanoscale Res. Lett. 2013, 8, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Lian, M.; Xiong, J. A new water-soluble imidazole based silicon quantum dot was prepared and used for fluorescence detection of trace copper in fruits and vegetables. J. Chin. Anal. Chem. 2016, 44, 367. [Google Scholar]
- Anderson, I.E.; Shircliff, R.A.; Macauley, C. Silanization of Low-Temperature-Plasma Synthesized Silicon Quantum Dots for Production of a Tunable, Stable, Colloidal Solution. J. Phys. Chem. C. 2012, 116, 3979. [Google Scholar] [CrossRef]
- Bley, R.A.; Kauzlarich, S.M. A low-temperature solution phase route for the synthesis of silicon nanoclusters. J. Am. Chem. Soc. 1996, 118, 12461. [Google Scholar] [CrossRef]
- Heintz, A.S.; Fink, M.J.; Mitchell, B.S. Mechanochemical synthesis of blue luminescent alkyl/alkenyl-passivated silicon nanoparticles. Adv. Mater. 2007, 19, 3984. [Google Scholar] [CrossRef]
- Xu, X.; Ma, S.; Xiao, X.; Hu, Y.; Zhao, D. The preparation of high-quality water-soluble silicon quantum dots and their application in the detection of formaldehyde. RSC Adv. 2016, 6, 98899–98907. [Google Scholar] [CrossRef]
- Wu, J.; Dai, J.; Shao, Y.; Sun, Y. One-step synthesis of fluorescent silicon quantum dots (Si-QDs) and their application for cell imaging. RSC Adv. 2015, 5, 83581–83587. [Google Scholar] [CrossRef]
- Dayyoub, T.; Maksimkin, A.; Kaloshkin, S.; Kolesnikov, E.; Chukov, D.; Dyachkova, T.Y.; Gutnik, I. The Structure and Mechanical Properties of the UHMWPE Films Modified by the Mixture of Graphene Nanoplates with Polyaniline. Polymers 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, P.; Gniadek, M.; Echegoyen, L.; Plonska-Brzezinska, M. Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors. Polymers 2018, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, A.; Huang, K.; Xiang, C.; Marti, A.A.; Tour, J.M. Luminescent Polymer Composite Films Containing Coal-Derived Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2015, 7, 26063–26068. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, B.; Ji, Y.; Wang, L. Luminescent and UV-Shielding ZnO Quantum Dots/Carboxymethylcellulose Sodium Nanocomposite Polymer Films. Polymers 2019, 10, 1112. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, L. Sodium alginate/carboxymethyl cellulose films containing pyrogallic acid: Physical and antibacterial properties. J. Sci. Food Agric. 2017, 97, 1295–1301. [Google Scholar] [CrossRef]
- Sathesh, V.; Chen, J.-K.; Chang, C.-J.; Aimi, J.; Chen, Z.-C.; Hsu, Y.-C.; Huang, Y.-S.; Huang, C.-F. Synthesis of Poly(ε-caprolactone)-Based Miktoarm Star Copolymers through ROP, SA ATRC, and ATRP. Polymers 2018, 10, 858. [Google Scholar] [CrossRef]
- Wu, Y.S.; Zhang, H.; Pan, A.Z.; Wang, Q.; Zhang, Y.F.; Zhou, G.J.; He, L. White-Light-Emitting Melamine-Formaldehyde Microspheres through Polymer-Mediated Aggregation and Encapsulation of Graphene Quantum Dots. Adv. Sci. 2019, 6, 1801432. [Google Scholar] [CrossRef]
- Lv, Z.-Y.; Mei, L.-P.; Chen, W.-Y.; Feng, J.-J.; Chen, J.-Y.; Wang, A.-J. Shaped-controlled electrosynthesis of gold nanodendrites for highly selective and sensitive SERS detection of formaldehyde. Sens. Actuators B 2014, 201, 92–99. [Google Scholar] [CrossRef]
- Rodio, M.; Brescia, R.; Diaspro, A.; Intartaglia, R. Direct surface modification of ligand-free silicon quantum dots prepared by femtosecond laser ablation in deionized water. J. Colloid Interface Sci. 2016, 465, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.L.; Peng, F.; Bao, F.; Wang, S.Y.; Ji, X.Y.; Yang, L.; Su, Y.Y.; Lee, S.T.; He, Y. Large-Scale Aqueous Synthesis of Fluorescent and Biocompatible Silicon Nanoparticles and Their Use as Highly Photostable Biological Probes. J. Am. Chem. Soc. 2013, 135, 8350–8356. [Google Scholar] [CrossRef] [PubMed]
- Olenych, I.B.; Aksimentyeva, O.I.; Tsizh, B.R.; Horbenko, Y.Y. Transport and Relaxation of Charge in Organic-Inorganic Nanocomposites. Acta Phys. Pol. A 2018, 133, 851–855. [Google Scholar] [CrossRef]
- Zou, J.P.; Le Rendu, P.; Musa, I.; Yang, S.H.; Dan, Y.; That, C.T.; Nguyen, T.P. Investigation of the optical properties of polyfluorene/ZnO nanocomposites. Thin Solid Films 2011, 519, 3997–4003. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Zhang, H.; Zhang, C.; Quan, Z.; Huang, M.; Wang, L. Fluorescent and Mechanical Properties of Silicon Quantum Dots Modified Sodium Alginate-Carboxymethylcellulose Sodium Nanocomposite Bio-Polymer Films. Polymers 2019, 11, 1476. https://doi.org/10.3390/polym11091476
Ji Y, Zhang H, Zhang C, Quan Z, Huang M, Wang L. Fluorescent and Mechanical Properties of Silicon Quantum Dots Modified Sodium Alginate-Carboxymethylcellulose Sodium Nanocomposite Bio-Polymer Films. Polymers. 2019; 11(9):1476. https://doi.org/10.3390/polym11091476
Chicago/Turabian StyleJi, Yali, Huimin Zhang, Canfu Zhang, Zhiyi Quan, Min Huang, and Lili Wang. 2019. "Fluorescent and Mechanical Properties of Silicon Quantum Dots Modified Sodium Alginate-Carboxymethylcellulose Sodium Nanocomposite Bio-Polymer Films" Polymers 11, no. 9: 1476. https://doi.org/10.3390/polym11091476