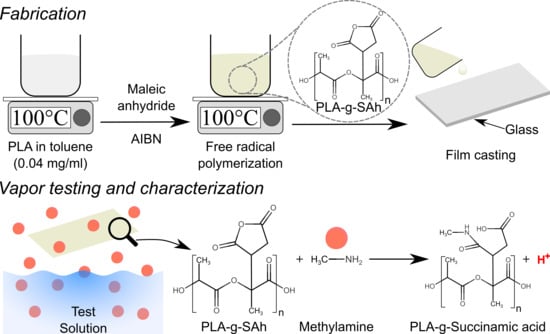
Amine Responsive Poly(lactic acid) (PLA) and Succinic Anhydride (SAh) Graft-Polymer: Synthesis and Characterization
Abstract
:1. Introduction
2. Experimental Methods
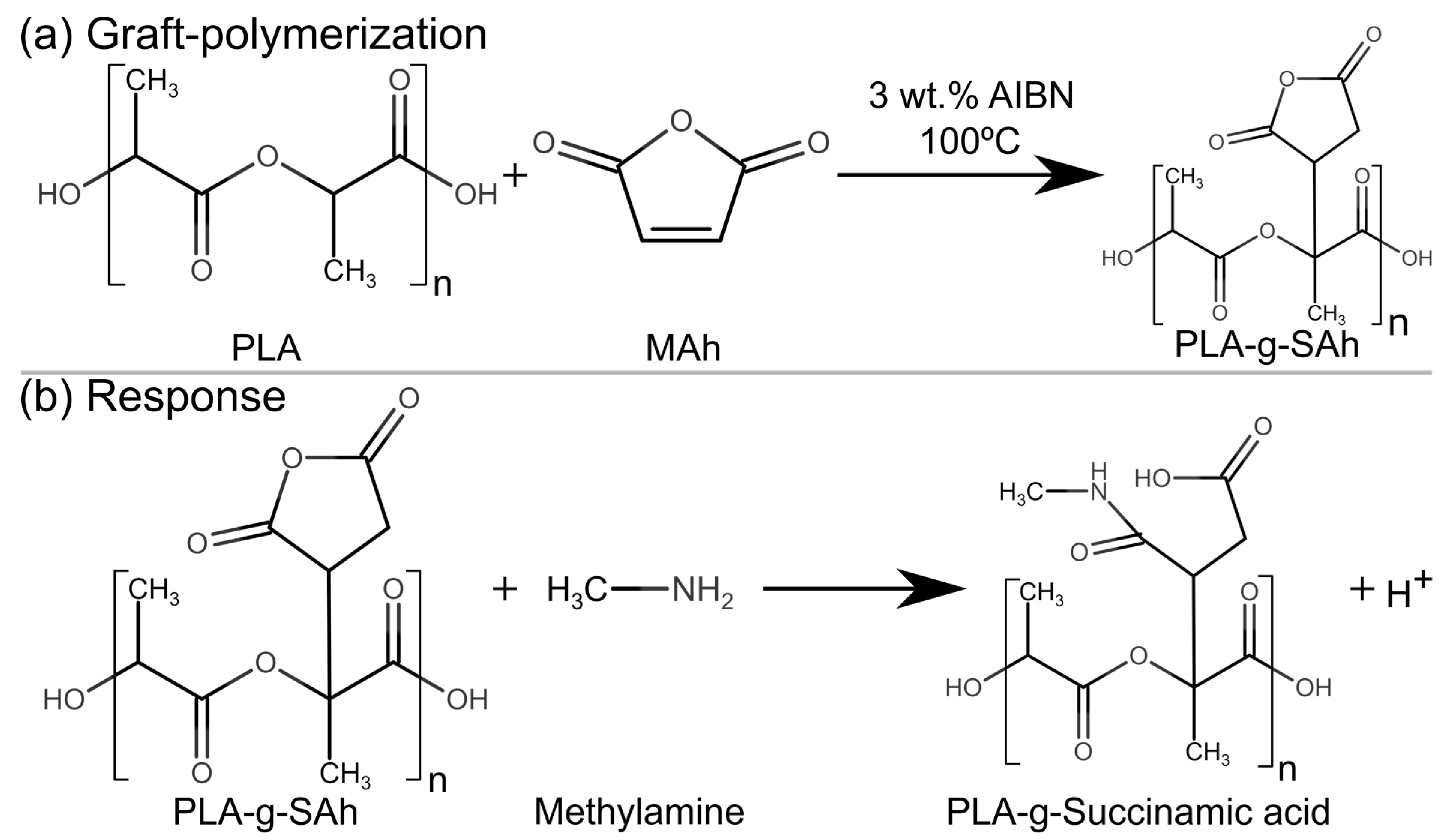
2.1. Polymer Synthesis and Sample Preparation
2.2. Gel Permeation Chromatography (GPC)
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. Thermal Characterization
2.5. Hydrogen Nuclear Magnetic Resonance (1H-NMR)
2.6. Amine Response
2.7. Statistical Analysis
3. Results and Discussion
3.1. Molecular Weight
3.2. Polymer Structure
3.3. Thermal Properties and Degree of Grafting
3.4. Amine Response: Polymer Structure
3.5. Amine Response: Thermal Properties
4. Comparison with Literature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anbukarasu, P.; Sauvageau, D.; Elias, A.L. Time-Temperature Indicator Based on Enzymatic Degradation of Dye-Loaded Polyhydroxybutyrate. Biotechnol. J. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Poyatos-Racionero, E.; Ros-Lis, J.V.; Vivancos, J.L.; Martínez-Máñez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Banimuslem, H.; Hassan, A.; Basova, T.; Esenpınar, A.A.; Tuncel, S.; Durmuş, M.; Ahsen, V. Dye-modified carbon nanotubes for the optical detection of amines vapours. Sens. Actuators B Chem. 2015, 207, 224–234. [Google Scholar] [CrossRef]
- Rodríguez-Méndez, M.L.; Gay, M.; Apetrei, C.; De Saja, J.A. Biogenic amines and fish freshness assessment using a multisensor system based on voltammetric electrodes. Comparison between CPE and screen-printed electrodes. Electrochim. Acta 2009, 54, 7033–7041. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Conte-Júnior, C.A.; Canto, A.C.; Monteiro, M.L.G.; Costa-Lima, B.; da Cruz, A.G.; Franco, R.M. Biogenic amines as bacterial quality indicators in different poultry meat species. LWT Food Sci. Technol. 2015, 60, 15–21. [Google Scholar] [CrossRef]
- Lopera-Valle, A.; Elias, A. Low-resistance silver microparticle-HEMA-PEGDA composites for humidity sensing. Smart Mater. Struct. 2018, 27, 1–16. [Google Scholar] [CrossRef]
- Silbande, A.; Adenet, S.; Chopin, C.; Cornet, J.; Smith-Ravin, J.; Rochefort, K.; Leroi, F. Effect of vacuum and modified atmosphere packaging on the microbiological, chemical and sensory properties of tropical red drum (Sciaenops ocellatus) fillets stored at 4 °C. Int. J. Food Microbiol. 2018, 266, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Mattarozzi, M.; Lambertini, F.; Suman, M.; Careri, M. Liquid chromatography–full scan-high resolution mass spectrometry-based method towards the comprehensive analysis of migration of primary aromatic amines from food packaging. J. Chromatogr. A 2013, 1320, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Casella, I.G.; Gatta, M.; Desimoni, E. Determination of histamine by high-pH anion-exchange chromatography with electrochemical detection. Food Chem. 2001, 73, 367–372. [Google Scholar] [CrossRef]
- Kang, J.; Hussain, A.T.; Catt, M.; Trenell, M.; Haggett, B.; Yu, E.H. Electrochemical detection of non-esterified fatty acid by layer-by-layer assembled enzyme electrodes. Sens. Actuators B Chem. 2014, 190, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Tanguy, N.R.; Fiddes, L.K.; Yan, N. Enhanced Radio Frequency Biosensor for Food Quality Detection Using Functionalized Carbon Nanofillers. ACS Appl. Mater. Interfaces 2015, 7, 11939–11947. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, X.; Wu, Y.; Chen, Z.; He, C. Lead phthalocyanine modified carbon nanotubes with enhanced NH3sensing performance. Sens. Actuators B Chem. 2012, 171–172, 398–404. [Google Scholar] [CrossRef]
- Hassan, A.; Basova, T.; Yuksel, F.; Gümüş, G.; Gürek, A.G.; Ahsen, V. Study of the interaction between simazine and metal-substituted phthalocyanines using spectral methods. Sens. Actuators B Chem. 2012, 175, 73–77. [Google Scholar] [CrossRef]
- Jin, Y.-J.; Kwak, G. Detection of biogenic amines using a nitrated conjugated polymer. Sens. Actuators B Chem. 2018, 271, 183–188. [Google Scholar] [CrossRef]
- Hu, G.H.; Lindt, J.T. Amidification of poly(styrene-co-maleic anhydride) with amines in tetrahydrofuran solution: A kinetic study. Polym. Bull. 1992, 29, 357–363. [Google Scholar] [CrossRef]
- Silberberg, M. Chemestry, 5th ed.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Johnson, D.W. New Applications for Poly(ethylene-alt-maleic anhydride). Ph.D. Thesis, University of Durham, Durham, UK, 2010. [Google Scholar]
- Samyn, P.; Deconinck, M.; Schoukens, G.; Stanssens, D.; Vonck, L.; Van den Abbeele, H. Synthesis and characterization of imidized poly(styrene-maleic anhydride) nanoparticles in stable aqueous dispersion. Polym. Adv. Technol. 2012, 23, 311–325. [Google Scholar] [CrossRef]
- Donati, I.; Gamini, A.; Vetere, A.; Campa, C.; Paoletti, S. Synthesis, Characterization, and Preliminary Biological Study of Glycoconjugates of Poly(styrene-co-maleic acid). Biomacromolecules 2002, 3, 805–812. [Google Scholar] [CrossRef]
- Cécile, C.; Hsieh, Y.-L. Hydrophilic polystyrene/maleic anhydride ultrafine fibrous membranes. J. Appl. Polym. Sci. 2010, 115, 723–730. [Google Scholar] [CrossRef]
- Su, S.; Du, F.-S.; Li, Z.-C. Synthesis and pH-dependent hydrolysis profiles of mono- and dialkyl substituted maleamic acids. Org. Biomol. Chem. 2017, 15, 8384–8392. [Google Scholar] [CrossRef]
- Kumar, A.; Bhardwaj, N.K.; Singh, S.P. Sizing performance of alkenyl succinic anhydride (ASA) emulsion stabilized by polyvinylamine macromolecules. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 132–139. [Google Scholar] [CrossRef]
- Salewska, N.; Milewska, M.J. Efficient Method for the Synthesis of Functionalized Basic Maleimides. J. Heterocycl. Chem. 2014, 51, 999–1003. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, J.; Ren, D.; Song, Z.; Jin, F.; Huo, Z. Efficient Synthesis of Succinimide from Succinic Anhydride in Water over Unsupported Nanoporous Nickel Material. ChemistrySelect 2018, 3, 724–728. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerlin, B.S. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 2019, 89, 61–75. [Google Scholar] [CrossRef]
- Liu, H.Y.; Cao, K.; Huang, Y.; Yao, Z.; Li, B.G.; Hu, G.H. Kinetics and simulation of the imidization of poly(styrene-co-maleic anhydride) with amines. J. Appl. Polym. Sci. 2006, 100, 2744–2749. [Google Scholar] [CrossRef]
- Kesim, H.; Rzaev, Z.M.O.; Dinçer, S.; Pişkin, E. Functional bioengineering copolymers. II. Synthesis and characterization of amphiphilic poly(N-isopropyl acrylamide-co-maleic anhydride) and its macrobranched derivatives. Polymer 2003, 44, 2897–2909. [Google Scholar] [CrossRef]
- Díaz, A.; del Valle, L.; Franco, L.; Sarasua, J.R.; Estrany, F.; Puiggalí, J. Anhydric maleic functionalization and polyethylene glycol grafting of lactide-co-trimethylene carbonate copolymers. Mater. Sci. Eng. C 2014, 42, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Lopera-Valle, A.; Caputo, J.V.; Leão, R.; Sauvageau, D.; Luz, S.M.; Elias, A. Influence of Epoxidized Canola Oil (eCO) and Cellulose Nanocrystals (CNCs) on the Mechanical and Thermal Properties of Polyhydroxybutyrate (PHB)—Poly(lactic acid) (PLA) Blends. Polymers 2019, 11, 933. [Google Scholar] [CrossRef]
- Issaadi, K.; Habi, A.; Grohens, Y.; Pillin, I. Maleic anhydride-grafted poly(lactic acid) as a compatibilizer in poly(lactic acid)/graphene oxide nanocomposites. Polym. Bull. 2016, 73, 2057–2071. [Google Scholar] [CrossRef]
- Hwang, S.W.; Lee, S.B.; Lee, C.K.; Lee, J.Y.; Shim, J.K.; Selke, S.E.; Auras, R. Grafting of maleic anhydride on poly(L-lactic acid). Effects on physical and mechanical properties. Polym. Test. 2012, 31, 333–344. [Google Scholar] [CrossRef]
- Ernzen, J.R.; Bondan, F.; Luvison, C.; Henrique Wanke, C.; De Nardi Martins, J.; Fiorio, R.; Bianchi, O. Structure and properties relationship of melt reacted polyamide 6/malenized soybean oil. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Maharana, T.; Pattanaik, S.; Routaray, A.; Nath, N.; Sutar, A.K. Synthesis and characterization of poly(lactic acid) based graft copolymers. React. Funct. Polym. 2015, 93, 47–67. [Google Scholar] [CrossRef]
- Detyothin, S.; Selke, S.E.M.; Narayan, R.; Rubino, M.; Auras, R.A. Effects of molecular weight and grafted maleic anhydride of functionalized polylactic acid used in reactive compatibilized binary and ternary blends of polylactic acid and thermoplastic cassava starch. J. Appl. Polym. Sci. 2015, 132, 1–15. [Google Scholar] [CrossRef]
- Detyothin, S.; Selke, S.E.M.; Narayan, R.; Rubino, M.; Auras, R. Reactive functionalization of poly(lactic acid), PLA: Effects of the reactive modifier, initiator and processing conditions on the final grafted maleic anhydride content and molecular weight of PLA. Polym. Degrad. Stab. 2013, 98, 2697–2708. [Google Scholar] [CrossRef]
- Ma, P.; Jiang, L.; Ye, T.; Dong, W.; Chen, M. Melt free-radical grafting of maleic anhydride onto biodegradable poly(lactic acid) by using styrene as a comonomer. Polymers 2014, 6, 1528–1543. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Xie, X.; Xu, M.; Song, Y. Styrene-assisted maleic anhydride grafted poly(lactic acid) as an effective compatibilizer for wood flour/poly(lactic acid) bio-composites. Polymers 2017, 9, 623. [Google Scholar] [CrossRef]
- Csikós, Á.; Faludi, G.; Domján, A.; Renner, K.; Móczó, J.; Pukánszky, B. Modification of interfacial adhesion with a functionalized polymer in PLA/wood composites. Eur. Polym. J. 2015, 68, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Birnin-yauri, A.U.; Ibrahim, N.A.; Zainuddin, N.; Abdan, K.; Then, Y.Y.; Chieng, B.W. Effect of maleic anhydride-modified poly(lactic acid) on the properties of its hybrid fiber biocomposites. Polymers 2017, 9, 165. [Google Scholar] [CrossRef]
- Dhar, P.; Tarafder, D.; Kumar, A.; Katiyar, V. Thermally recyclable polylactic acid/cellulose nanocrystal films through reactive extrusion process. Polymer 2016, 87, 268–282. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A.; Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Thermal Properties of Poly(lactic Acid). In Polylactic Acid, 1st ed.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 127–128. [Google Scholar]
- Sütekin, S.D.; Atıcı, A.B.; Güven, O.; Hoffman, A.S. Controlling of free radical copolymerization of styrene and maleic anhydride via RAFT process for the preparation of acetaminophen drug conjugates. Radiat. Phys. Chem. 2018, 148, 5–12. [Google Scholar] [CrossRef]
- Murillo, E.A.; López, B.L. Effect of the maleic anhydride content on the structural, thermal, rheological and film properties of the n-butyl methacrylate–maleic anhydride copolymers. Prog. Org. Coat. 2015, 78, 96–102. [Google Scholar] [CrossRef]
- Boztuǧ, A.; Basan, S. Syntheses and characterization of maleic anhydride-styrene-allyl propionate terpolymer ester derivatives. J. Appl. Polym. Sci. 2003, 89, 296–299. [Google Scholar] [CrossRef]
- Kurmaz, S.V.; Poshchupkin, V.P.; Kochneva, I.S. IR Spectroscopy for Studying the Conversion of Double Bonds in the Radical Copolymerisation of Oligodimethacrylates with Styrene. Int. Polym. Sci. Technol. 2003, 30, 43–48. [Google Scholar] [CrossRef]
- Schlebrowski, T.; Beucher, L.; Bazzi, H.; Hahn, B.; Wehner, S.; Fischer, C.B. Prediction of a-C:H layer failure on industrial relevant biopolymer polylactide acide (PLA) foils based on the sp2/sp3 ratio. Surf. Coat. Technol. 2019, 368, 79–87. [Google Scholar] [CrossRef]
- Tomasella, E.; Thomas, L.; Dubois, M.; Meunier, C. Structural and mechanical properties of a-C:H thin films grown by RF-PECVD. Diam. Relat. Mater. 2004, 13, 1618–1624. [Google Scholar] [CrossRef]
- González-López, M.E.; Robledo-Ortíz, J.R.; Manríquez-González, R.; Silva-Guzmán, J.A.; Pérez-Fonseca, A.A. Polylactic acid functionalization with maleic anhydride and its use as coupling agent in natural fiber biocomposites: A review. Compos. Interfaces 2018, 25, 515–538. [Google Scholar] [CrossRef]
- Piemonte, V.; Gironi, F. Kinetics of Hydrolytic Degradation of PLA. J. Polym. Environ. 2013, 21, 313–318. [Google Scholar] [CrossRef]
- Fiddes, L.K.; Chang, J.; Yan, N. Electrochemical detection of biogenic amines during food spoilage using an integrated sensing RFID tag. Sens. Actuators B Chem. 2014, 202, 1298–1304. [Google Scholar] [CrossRef]
- Ellis, D.I.; Broadhurst, D.; Goodacre, R. Rapid and quantitative detection of the microbial spoilage of beef by Fourier transform infrared spectroscopy and machine learning. Anal. Chim. Acta 2004, 514, 193–201. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Qu, X.; Xiao, J.; Yang, X.; Xu, Z. Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1, 2,4-triazole-5-thione to malachite by ToF-SIMS, XPS, FTIR, contact angle, zeta potential and micro-flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 34–42. [Google Scholar] [CrossRef]
- Losito, I.; De Giglio, E.; Cioffi, N.; Malitesta, C. Spectroscopic investigation on polymer films obtained by oxidation of o-phenylenediamine on platinum electrodes at different pHs. J. Mater. Chem. 2001, 11, 1812–1817. [Google Scholar] [CrossRef]
- Barrio, J.; Grafmüller, A.; Tzadikov, J.; Shalom, M. Halogen-hydrogen bonds: A general synthetic approach for highly photoactive carbon nitride with tunable properties. Appl. Catal. B Environ. 2018, 237, 681–688. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, Z.; Zhang, H.; Liu, S.; Cheng, S.Z.D. Crystal transitions of Nylon 11 under drawing and annealing. Polymer 2001, 42, 5543–5547. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Sugai, N.; Asai, S.; Tezuka, Y.; Yamamoto, T. Photoinduced topological transformation of cyclized polylactides for switching the properties of homocrystals and stereocomplexes. Polym. Chem. 2015, 6, 3591–3600. [Google Scholar] [CrossRef] [Green Version]
- He, D.F.; Liu, Y.W.; Lu, Y.; Liu, S.M.; Chen, X.G.; Li, N.; Liu, S.X. An UV equipped box for photoactivation with a fluorescent coordination polymer for recognizing amine gases by “turn-color” in air. Sens. Actuators B Chem. 2017, 247, 238–244. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Stereo-regulated Schiff base siloxane polymer coated QCM sensor for amine vapor detection. Mater. Chem. Phys. 2019, 226, 214–219. [Google Scholar] [CrossRef]
- Khan, A.A.; Hussain, R.; Khan, M.Q. Amines vapour sensing kinetics and electrical properties of synthetic Polyaniline-titanium(IV)sulphosalicylophosphate cation exchange nanocomposite. Environ. Nanotechnol. Monit. Manag. 2017, 8, 187–198. [Google Scholar] [CrossRef]
- Shen, M.L.; Wei, Z.; Xu, L.; Liu, B.; Jiao, H. A mixed matrix Eu-4,4′-biphenyldicarboxylate coordination polymer film as a fluorescence turn-off sensor to aniline vapor. J. Solid State Chem. 2019, 269, 87–93. [Google Scholar] [CrossRef]
- Fiddes, L.K.; Yan, N. RFID tags for wireless electrochemical detection of volatile chemicals. Sens. Actuators B Chem. 2013, 186, 817–823. [Google Scholar] [CrossRef]







| Sample Name | Poly(lactic acid) (PLA) Wt % | Maleic anhydrite (MAh) Wt % | Azobisisobutyronitrile (AIBN) Wt % |
|---|---|---|---|
| Neat PLA | 100 | 0 | 0 |
| PLA-g-SAh10 | 87 | 10 | 3 |
| PLA-g-SAh25 | 72 | 25 | 3 |
| PLA-g-SAh50 | 47 | 50 | 3 |
| PLA-g-SAh75 | 22 | 75 | 3 |
| Sample Name | Molecular number (Mn) kDa | Molecular weight (Mw) kDa | Polydispersity index (PDI) Mw/Mn |
|---|---|---|---|
| Neat PLA | 122 ± 9 | 159 ± 8 | 1.3 ± 0.03 |
| PLA-g-SAh10 | 80 ± 4 | 131 ± 4 | 1.6 ± 0.09 |
| PLA-g-SAh25 | 81 ± 6 | 131 ± 2 | 1.6 ± 0.09 |
| PLA-g-SAh50 | 85 ± 1 | 141 ± 2 | 1.7 ± 0.03 |
| PLA-g-SAh75 | 102 ± 2 | 271 ± 19 | 2.7 ± 0.12 |
| Sample Name | Tg (°C) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|
| Neat PLA | 31 ± 2 | 140 ± 3 | 29.8 ± 0.5 |
| PLA-g-SAh10 | 32 ± 1 | 133 ± 2 | 21.8 ± 0.4 |
| PLA-g-SAh25 | 30 ± 2 | 121 ± 3 | 20.6 ± 0.6 |
| PLA-g-SAh50 | 28 ± 1 | 116 ± 2 | 19.2 ± 0.7 |
| PLA-g-SAh75 | 21 ± 2 | 108 ± 3 | 2 ± 0.8 |
| MAh | - | 53 | 138 |
| Before | After | |||||
|---|---|---|---|---|---|---|
| Sample Name | Tg (°C) | Tm (°C) | ΔHm (J/g) | Tg (°C) | Tm (°C) | ΔHm (J/g) |
| PLA-g-SAh10 | 32 ± 1 | 133 ± 2 | 21.8 ± 0.4 | 31 ± 3 | 142 ± 2 | 24.8 ± 0.4 |
| PLA-g-SAh25 | 30 ± 2 | 121 ± 3 | 20.6 ± 0.6 | 32 ± 2 | 135 ± 1 | 26.9 ± 0.6 |
| PLA-g-SAh50 | 28 ± 1 | 116 ± 2 | 19.2 ± 0.7 | 31 ± 1 | 126 ± 2 | 23.8 ± 0.5 |
| PLA-g-SAh75 | 21 ± 2 | 108 ± 3 | 2 ± 0.8 | 30 ± 3 | 121 ± 1 | 20.6 ± 0.9 |
| Material | Amines | Mechanism | Response | Ref. |
|---|---|---|---|---|
| Nitrated polythiophene (NPTh) | Ethylenediamine, putrescine, cadaverine, spermidine, phenethylamine, histamine | The biogenic amine (BA) easily diffuses into the polymer film and forms charge transfer complexes with NPTh. These NPThδ+-BAδ− complexes lead to the change in color of the film. | A fast change in color from light brown to a highly deep dark brown | [14] |
| Alkaline earth metal–organic coordination polymer | Methylamine, dimethylamine, trimethylamine | Amines combine with unsaturated carboxylic groups in the polymer. The carboxylic group can no longer vibrate, increasing the rigidity and educe the loss of non-radiation energy of the ligand, causing the increase of the fluorescence emission intensity | On/off change in fluorescence with initial and final peaks at 525 nm and 612 nm | [59] |
| Polyaniline-titanium(IV) sulphosalicylophosphate composite | Methylamine, ethylamine | The lone pair of nitrogen of amine interacts with the imine nitrogen of polyaniline, decreasing the intensity of positive charge which decreases the conductivity | Reversible change in resistivity measured with 4-point probe | [61] |
| Schiff base 3(aminopropyl)triethoxysilane (APTES) | Methylamine, ethylamine, diethylamine, triethylamine, tertbutylamine ammonia | Small molecules of amines are trapped in molecular pores introduced by the bulky group of Schiff base attached to polysiloxane. They are stabilized by H-bonds and dipole-dipole interaction | Polymer-coated quartz crystal microbalance (QCM) substrate absorbs the amines, increasing the mass of the film and the mass | [60] |
| EuCl3 with 4,4′-biphenyldicarboxylic acid (H2BPDC) | Methylamine, dimethylamine, trimethylamine | Fluorescence quenching by the amines | Drop in fluorescent emission at 413, 578, 592, 614 (main peak), 650 and 704 nm when excited by UV light of 311 nm | [62] |
| PLA-g-SAh | Methylamine | Amidation of SAh in PLA-g-SAh with methylamine. Lone electron pair of the amine conducts a nucleophilic attack on the C=O π bond of SAh to start a ring opening reaction | Increase in melting point and donation of protons during ring opening of SAh. Potential in color change indicators and electrochemical sensing | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopera-Valle, A.; Elias, A. Amine Responsive Poly(lactic acid) (PLA) and Succinic Anhydride (SAh) Graft-Polymer: Synthesis and Characterization. Polymers 2019, 11, 1466. https://doi.org/10.3390/polym11091466
Lopera-Valle A, Elias A. Amine Responsive Poly(lactic acid) (PLA) and Succinic Anhydride (SAh) Graft-Polymer: Synthesis and Characterization. Polymers. 2019; 11(9):1466. https://doi.org/10.3390/polym11091466
Chicago/Turabian StyleLopera-Valle, Adrián, and Anastasia Elias. 2019. "Amine Responsive Poly(lactic acid) (PLA) and Succinic Anhydride (SAh) Graft-Polymer: Synthesis and Characterization" Polymers 11, no. 9: 1466. https://doi.org/10.3390/polym11091466