Hydrophilic Molecularly Imprinted Chitosan Based on Deep Eutectic Solvents for the Enrichment of Gallic Acid in Red Ginseng Tea
Abstract
:1. Introduction
2. Experimental
2.1. Chemical and Reagents
2.2. Instrument
2.3. Preparation of the Materials
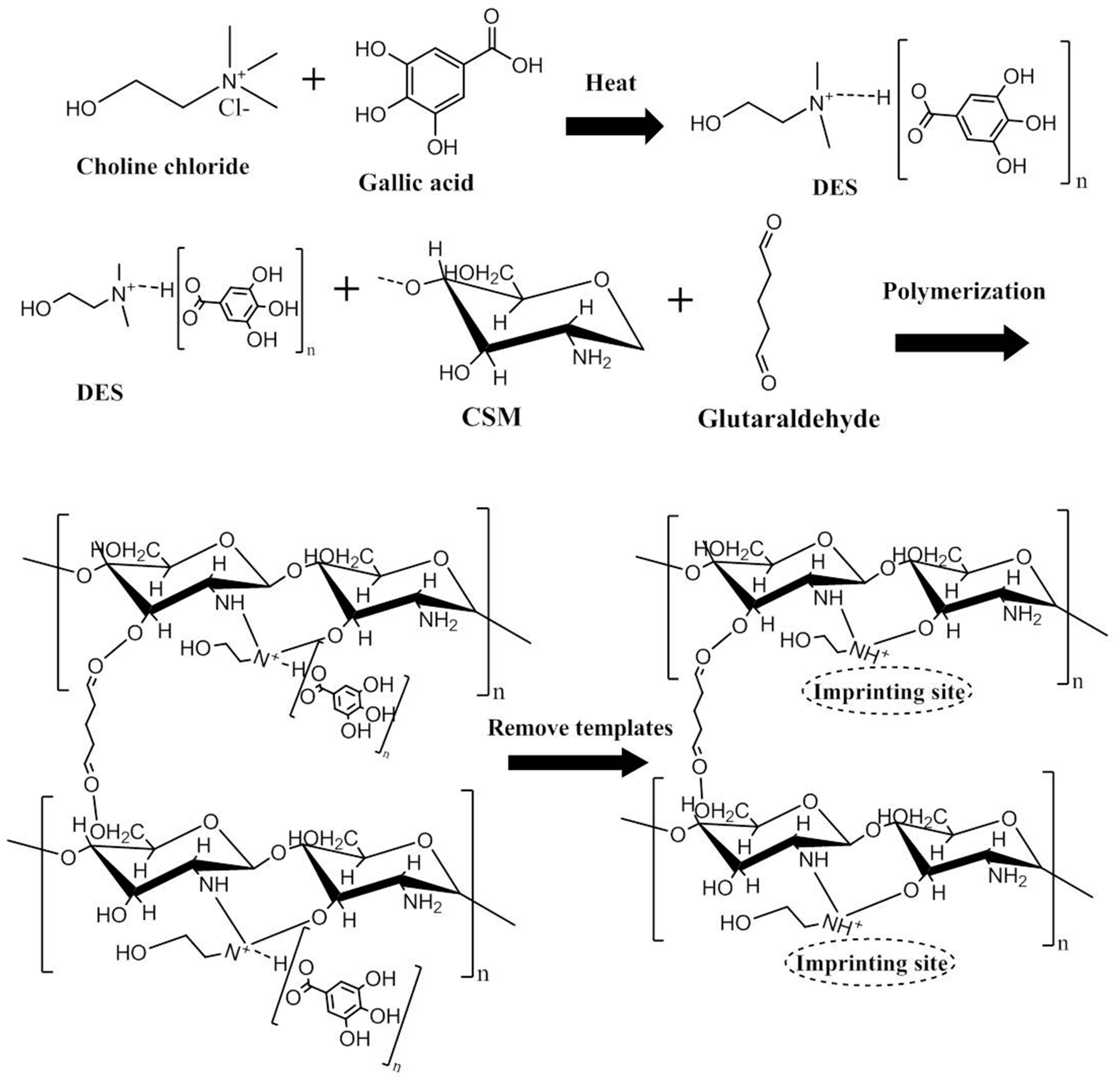
2.3.1. Synthesis of Hydrophilic DESs
2.3.2. Preparation of HMICS and NICS
2.3.3. Adsorption Properties of HMICS and NICS
2.3.4. Selectivity and Reusability Experiments
2.3.5. Optimization of the SPME Conditions
2.3.6. SPME Procedure with Real Samples Using the RSM
3. Results and Discussion
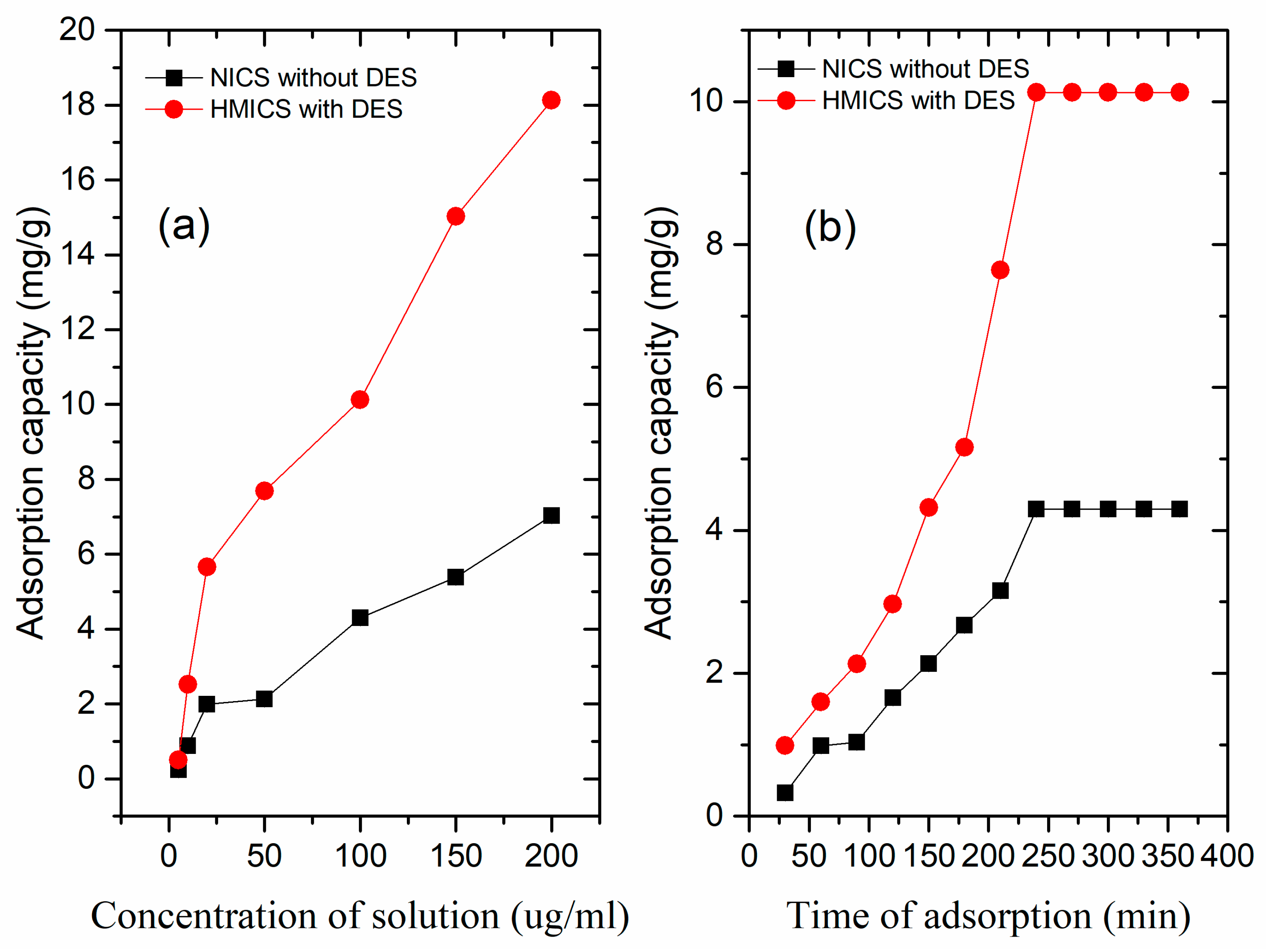
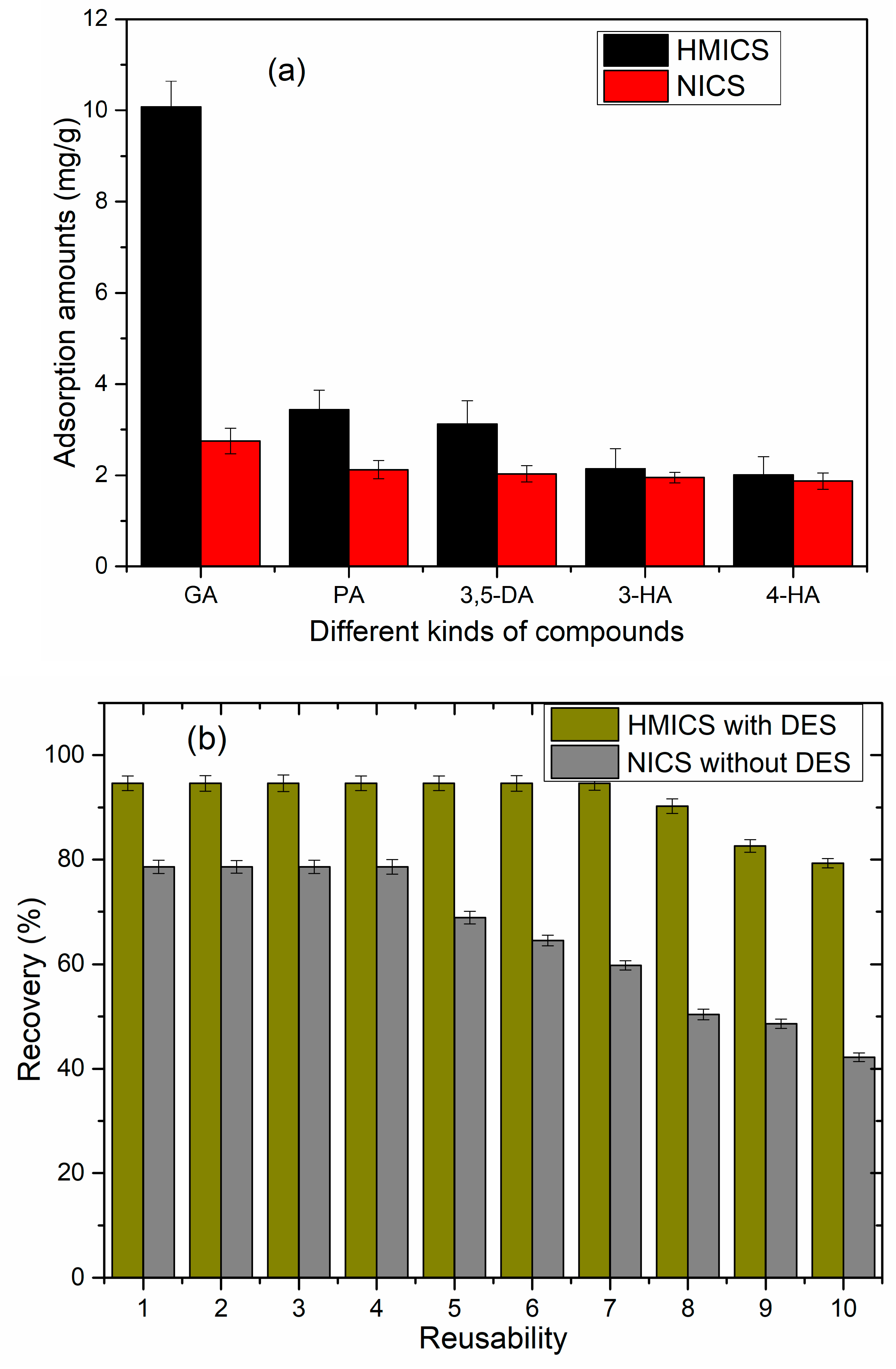
3.1. Adsorption Properties
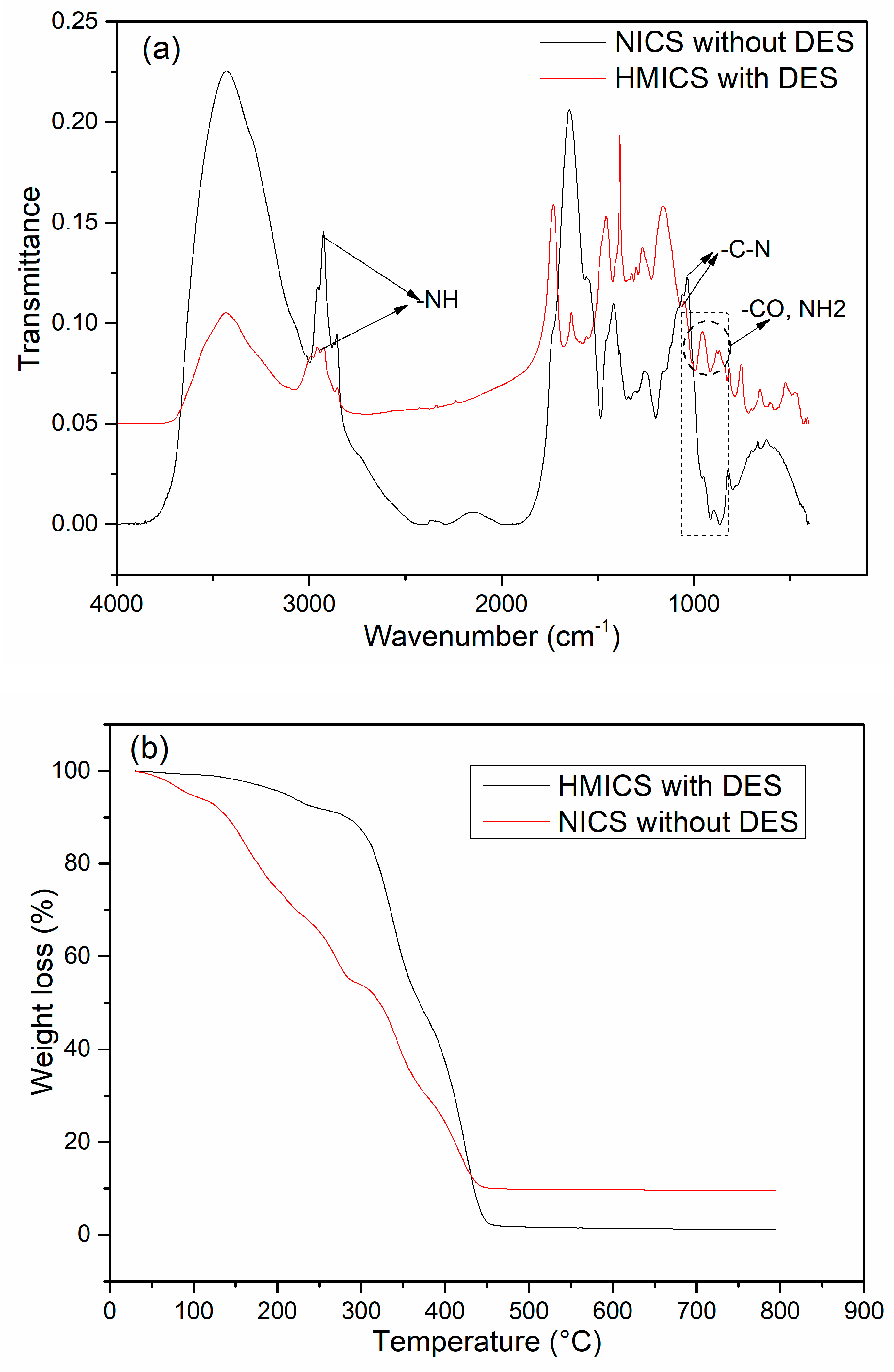
3.2. Characterization
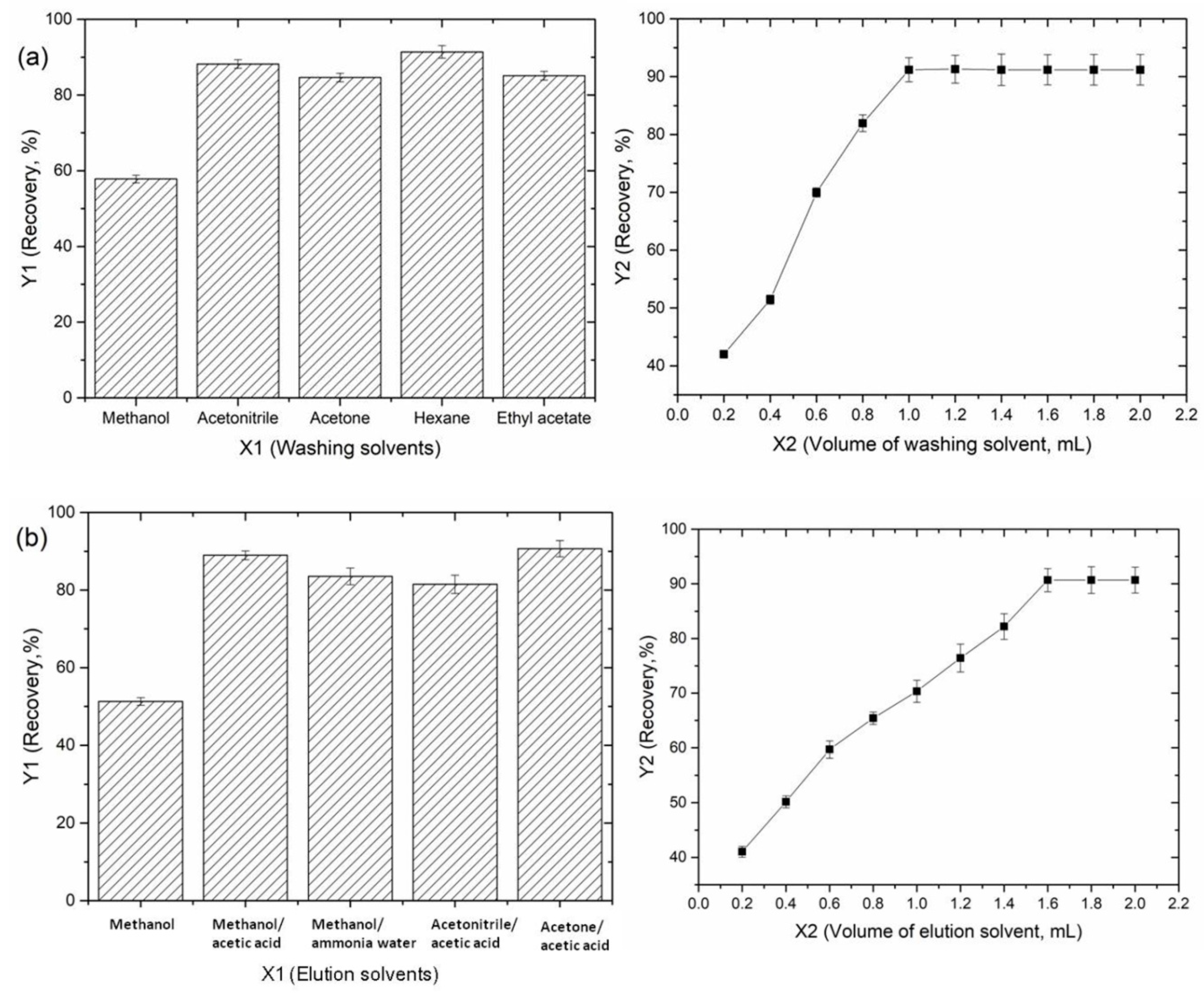
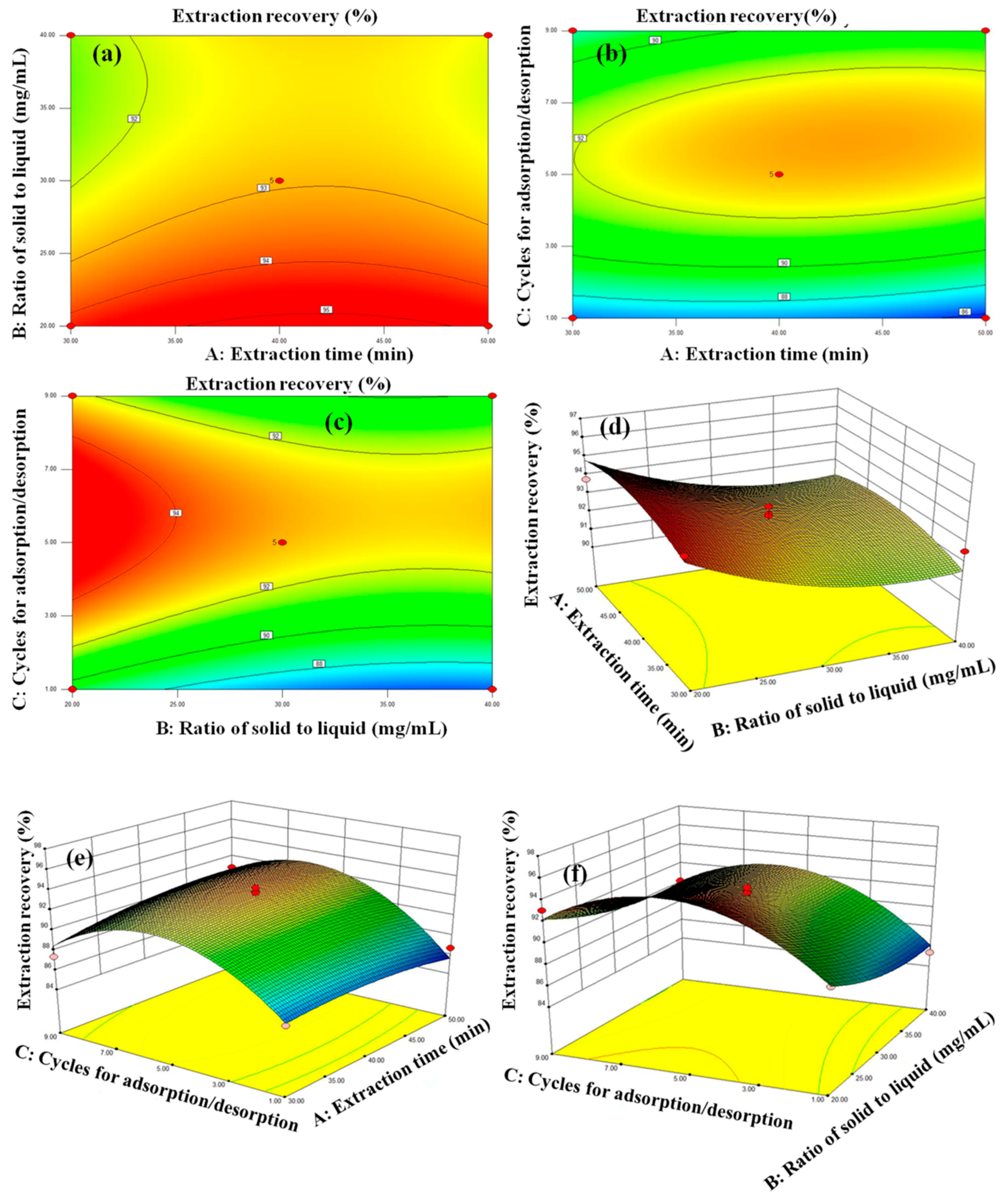
3.3. Optimization of the Extraction Conditions
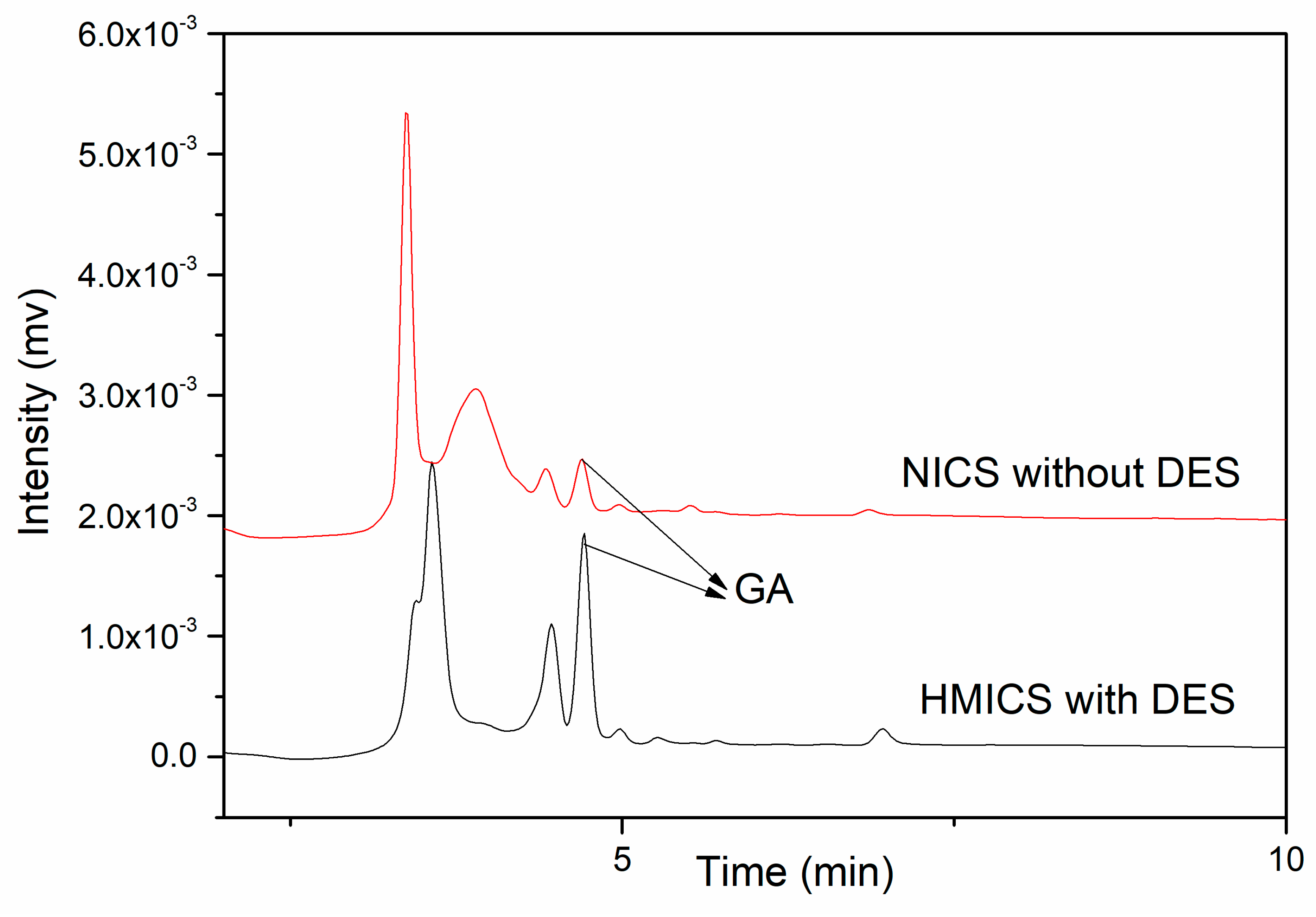
3.4. Selectivity and Reusability of HMICS and NICS
3.5. RSM Model Fitting and Statistical Analysis
3.6. Method Validation and Real Sample Analysis
3.7. Comparison with other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.J.; Li, B.Q.; Yue, H.J.; Zheng, Y.S. Highly selective and efficient imprinted polymers based on carboxyl-functionalized magnetic nanoparticles for the extraction of gallic acid from pomegranate rind. J. Sep. Sci. 2018, 41, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Khouzani, O.; Sharifi, A.; Jafari, A. A Systematic Review of the Potential Gallic Acid Effective in Liver Oxidative Stress in Rats. J. Med. Res. 2018, 4, 5–12. [Google Scholar]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [PubMed]
- Dludla, P.; Nkambule, B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I.S. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.S.L.; de Souza Pereira, A.; Speziali, M.G. Bifunctionalized chitosan: A versatile adsorbent for removal of Cu (II) and Cr (VI) from aqueous solution. Carbohydrate Polym. 2018, 201, 218–227. [Google Scholar] [CrossRef]
- Wang, B.J.; Bai, Z.S.; Jiang, H.R.; Prinsen, P.; Luque, R.; Zhao, S.L.; Xuan, J. Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: Characteristics, modeling and application. J. Hazard. Mater. 2019, 364, 192–205. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Zhang, Y.S.; Lu, A.G.; Li, X.R.; Liu, L.Z.; Qin, G.M.; Fang, Z.L.; Zhang, J.L.; Liu, Y.X. A novel design and synthesis of multifunctional magnetic chitosan microsphere based on phase change materials. Mater. Lett. 2019, 237, 185–187. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, N.; Xu, Y.; Li, Z.L.; Yan, C.R.; Mei, K.; Ding, M.L.; Ding, S.C.; Guan, P.; Qian, L.W.; et al. Molecularly Imprinted Materials for Selective Biological Recognition. Macromol. Rapid Comm. 2019, 1900096, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Row, K.H. Ternary deep eutectic solvent magnetic molecularly imprinted polymers for the dispersive magnetic solid-phase microextraction of green tea. J. Sep. Sci. 2018, 41, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Long, R.Q.; Tong, C.Y.; Li, T.; Liu, Y.G.; Shi, S.Y. Shell thickness controlled hydrophilic magnetic molecularly imprinted resins for high-efficient extraction of benzoic acids in aqueous samples. Talanta 2019, 194, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.Y.; Ding, J.; He, Z.Y.; Li, J.Y.; Liang, Z.H.; Li, C.Y.; Li, Y.; Chen, Y.H.; Ding, L. Preparation of magnetic superhydrophilic molecularly imprinted composite resin based on multi-walled carbon nanotubes to detect triazines in environmental water. Chem. Eng. J. 2018, 334, 2293–2302. [Google Scholar] [CrossRef]
- Abbasi Ghaeni, F.; Karimi, G.; Mohsenzadeh, M.S.; Nazarzadeh, M.; Motamedshariaty, V.S.; Mohajeri, S.A. Preparation of dual-template molecularly imprinted nanoparticles for organophosphate pesticides and their application as selective sorbents for water treatment. Sep. Sci. Technol. 2018, 53, 2517–2526. [Google Scholar] [CrossRef]
- Li, G.; Row, K.H. Recent applications of molecularly imprinted polymers (MIPs) on micro-extraction techniques. Sep. Purif. Rev. 2018, 47, 1–18. [Google Scholar] [CrossRef]
- Yuan, Y.N.; Yang, C.L.; Lv, T.W.; Qiao, F.X.; Zhou, Y.; Yan, H.Y. Green synthesis of hydrophilic protein-imprinted resin with specific recognition of bovine serum albumin in aqueous matrix. Anal. Chim. Acta 2018, 1033, 213–220. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Cao, H.W.; Huang, Q.W.; Liu, X.Y.; Zhang, H.X. Isolation of transferrin by imprinted nanoparticles with magnetic deep eutectic solvents as monomer. Anal. Bioanal. Chem. 2018, 410, 6237–6245. [Google Scholar] [CrossRef]
- Li, G.; Row, K.H. Selective extraction of 3,4-dihydroxybenzoic acid in Ilex chinensis Sims by meticulous mini-solid-phase microextraction using ternary deep eutectic solvent-based molecularly imprinted polymers. Anal. Bioanal. Chem. 2018, 410, 7849–7858. [Google Scholar] [CrossRef]
- Wang, R.; Li, W.; Chen, Z. Solid phase microextraction with poly (deep eutectic solvent) monolithic column online coupled to HPLC for determination of non-steroidal anti-inflammatory drugs. Anal. Chim. Acta 2018, 1018, 111–118. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.P. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.J.; Wang, Y.Z.; Wei, X.X.; Chen, J.; Xu, P.L.; Zhou, Y.G. Preparation of magnetic molecularly imprinted polymers based on a deep eutectic solvent as the functional monomer for specific recognition of lysozyme. Microchim. Acta 2018, 185, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.J.; Liu, X.L.; Li, L.T.; Tang, B.K.; Row, K.H. Ternary choline chloride/caffeic acid/ethylene glycol deep eutectic solvent as both a monomer and template in a molecularly imprinted polymer. J. Sep. Sci. 2017, 40, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Li, Q.; Mao, Q.; He, G.H. Cross-linked chitosan microspheres: An efficient and eco-friendly adsorbent for iodide removal from waste water. Carbohyd. Polym. 2019, 209, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Scoma, A.; Cirillo, G.; Bertin, L.; Fava, F.; Picci, N. Selective extraction and purification of gallic acid from actual site olive mill wastewaters by means of molecularly imprinted microparticles. Chem. Eng. J. 2012, 198, 529–535. [Google Scholar] [CrossRef]
- Pardeshi, S.; Dhodapkar, R.; Kumar, A. Molecularly imprinted microspheres and nanoparticles prepared using precipitation polymerisation method for selective extraction of gallic acid from Emblica officinalis. Food Chem. 2014, 146, 385–393. [Google Scholar] [CrossRef] [PubMed]


| Targets | Concentration (μg·mL−1) | Inter-day | Intra-day | ||||
|---|---|---|---|---|---|---|---|
| Limit of Detection (μg·mL−1) | Limit of Quantification (μg·mL−1) | Recovery (%) | Relative Standard Deviation (%, n = 4) | Recovery (%) | Relative Standard Deviation (%, n=4) | ||
| Gallic Acid | 50 | 0.21 | 0.24 | 90.13 | 4.16 | 90.04 | 3.68 |
| 100 | 0.15 | 0.18 | 94.82 | 3.84 | 93.68 | 3.15 | |
| 200 | 0.08 | 0.13 | 102.68 | 2.65 | 100.84 | 3.06 | |
| Materials Types | Function Monomer | Source | Recovery (%) | Reference |
|---|---|---|---|---|
| Molecularly imprinted microparticles | Methacrylic acid | Olive mill wastewaters | 85.0–97.0 | [25] |
| Molecularly imprinted microspheres and nanoparticles | Acrylic acid | Emblica officinalis | 75.0–83.4 | [26] |
| Hydrophilic molecularly imprinted chitosan microsphere | DES | Tea sample | 90.0–102.7 | This research |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Rwo, K.H. Hydrophilic Molecularly Imprinted Chitosan Based on Deep Eutectic Solvents for the Enrichment of Gallic Acid in Red Ginseng Tea. Polymers 2019, 11, 1434. https://doi.org/10.3390/polym11091434
Li G, Rwo KH. Hydrophilic Molecularly Imprinted Chitosan Based on Deep Eutectic Solvents for the Enrichment of Gallic Acid in Red Ginseng Tea. Polymers. 2019; 11(9):1434. https://doi.org/10.3390/polym11091434
Chicago/Turabian StyleLi, Guizhen, and Kyung Ho Rwo. 2019. "Hydrophilic Molecularly Imprinted Chitosan Based on Deep Eutectic Solvents for the Enrichment of Gallic Acid in Red Ginseng Tea" Polymers 11, no. 9: 1434. https://doi.org/10.3390/polym11091434






