Study of High Performance Sulfonated Polyether Ether Ketone Composite Electrolyte Membranes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
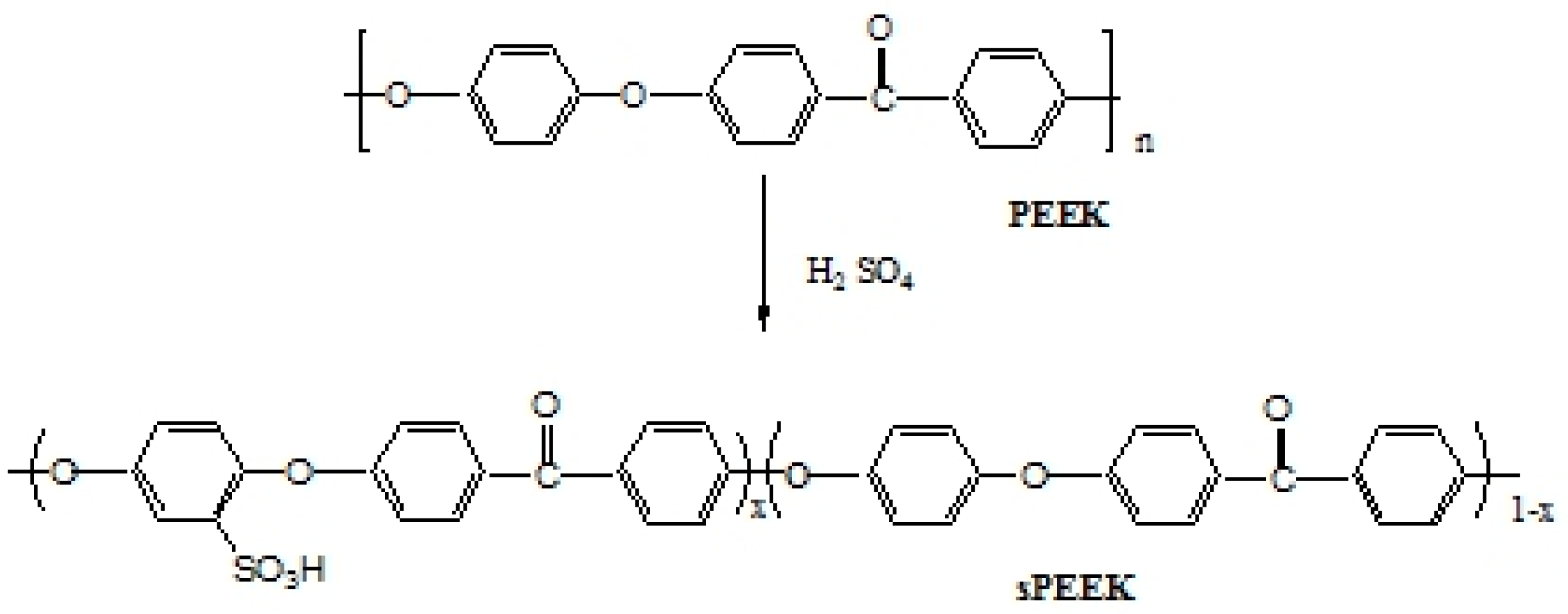
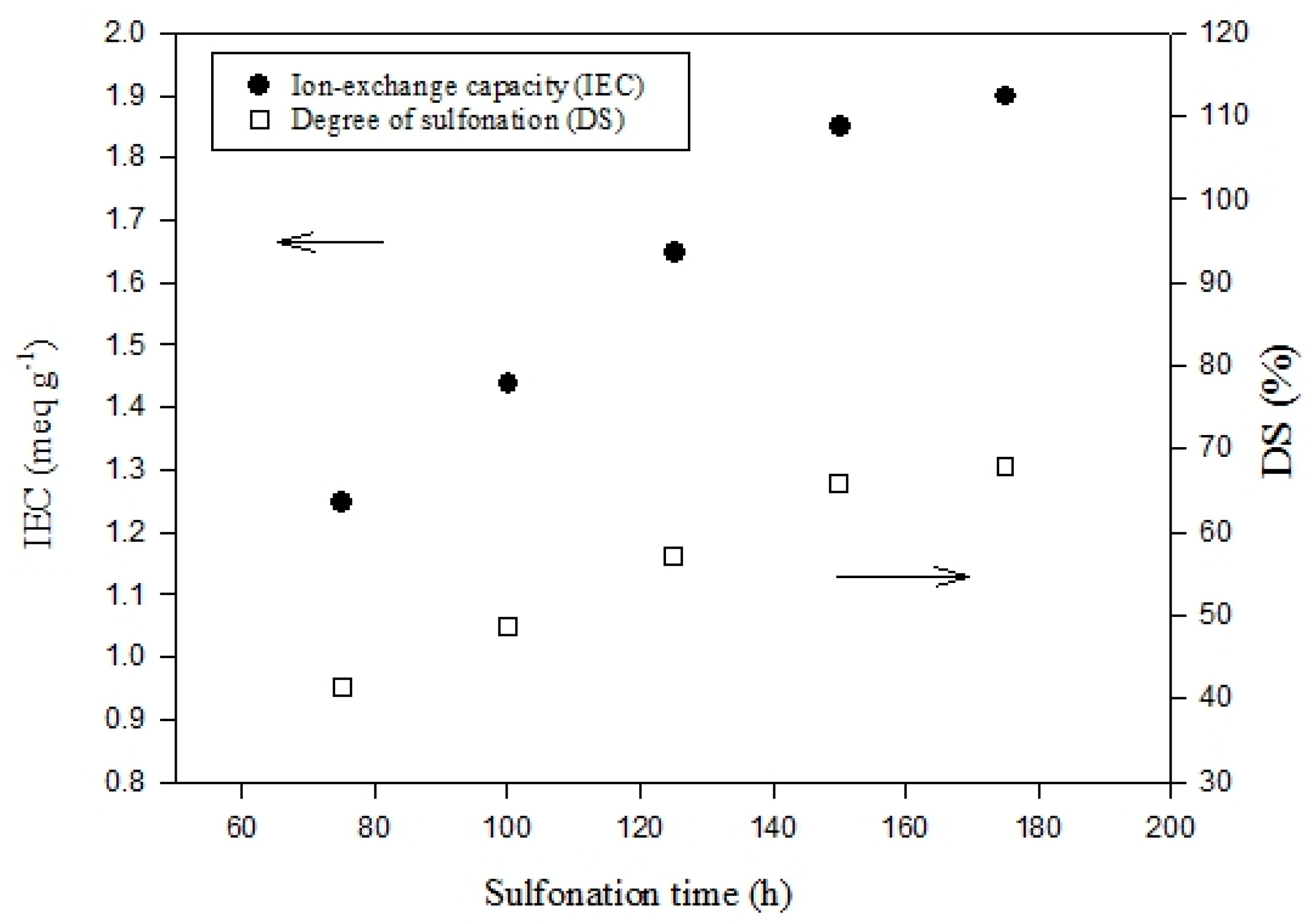
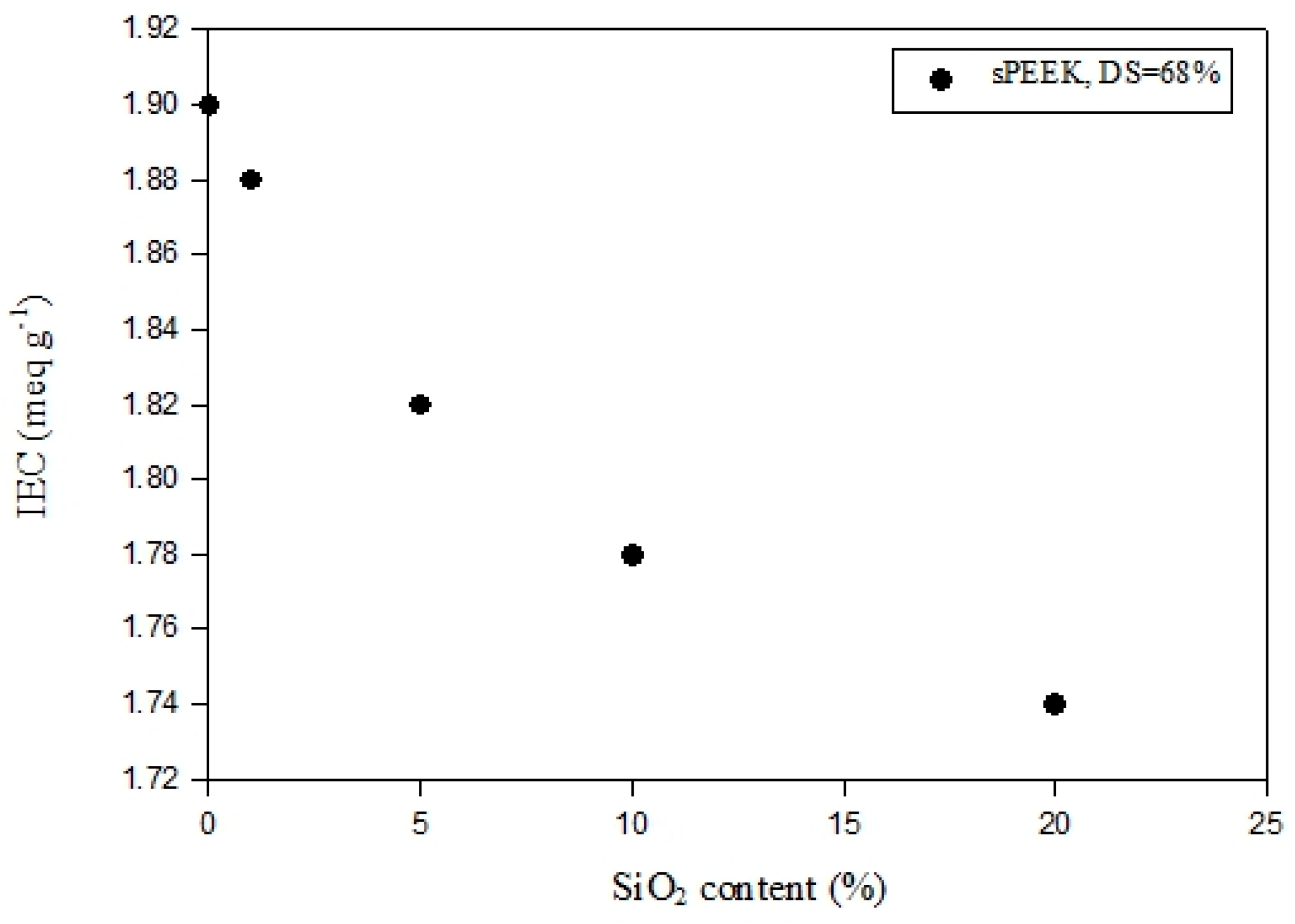
3.1. IEC and DS Analysis
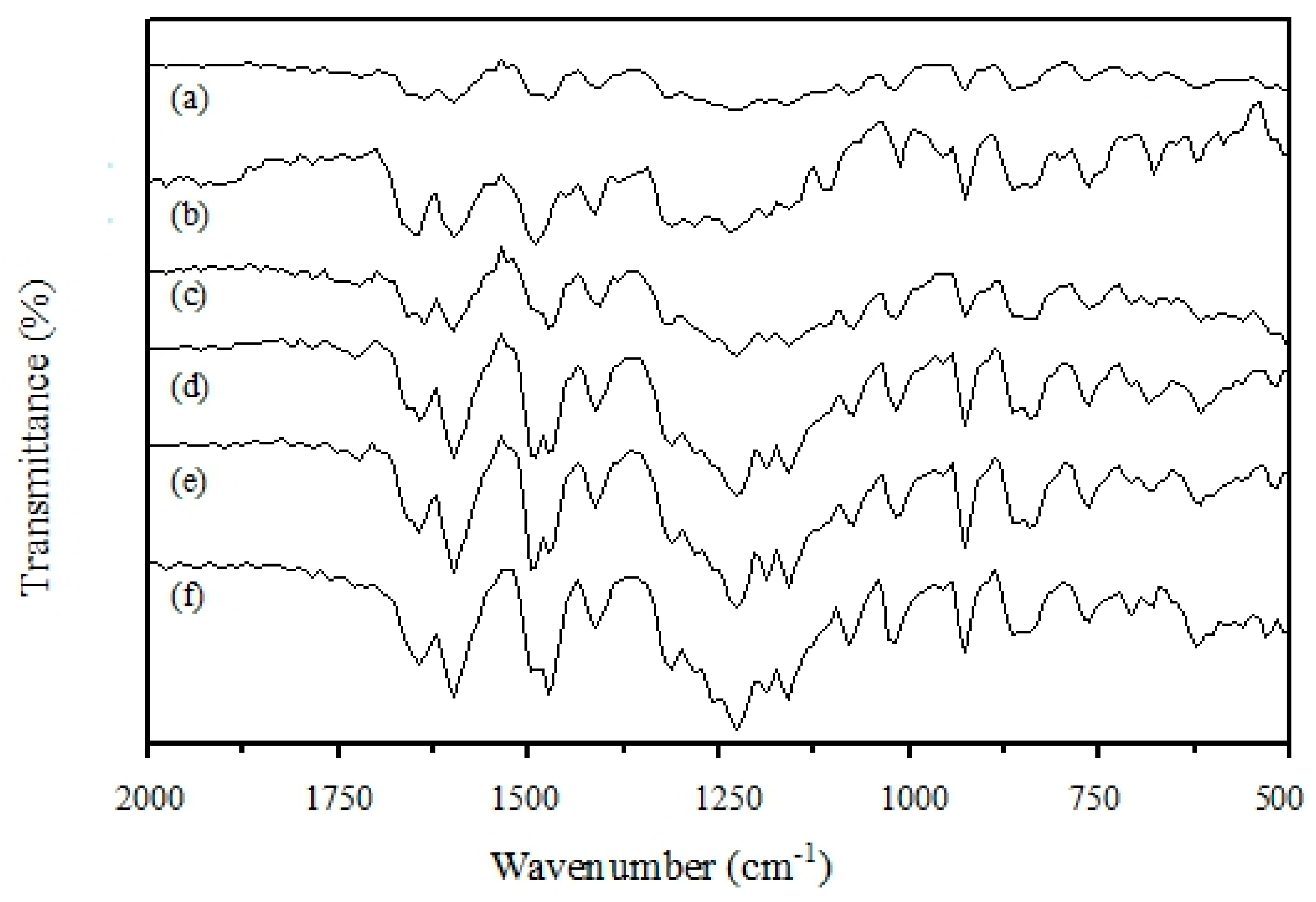
3.2. IR Analysis
3.3. Water Absorption and Ionic Conductivity
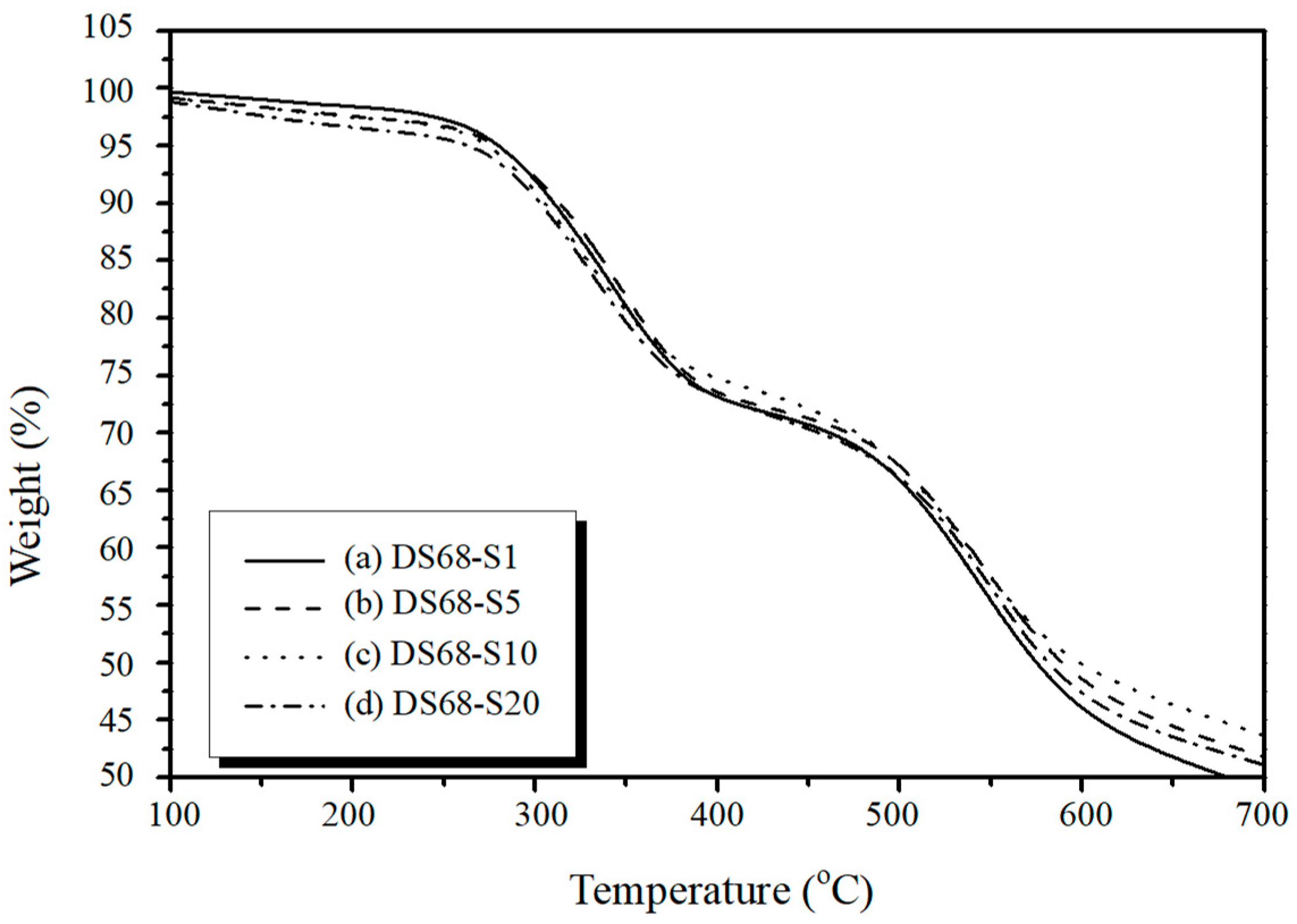
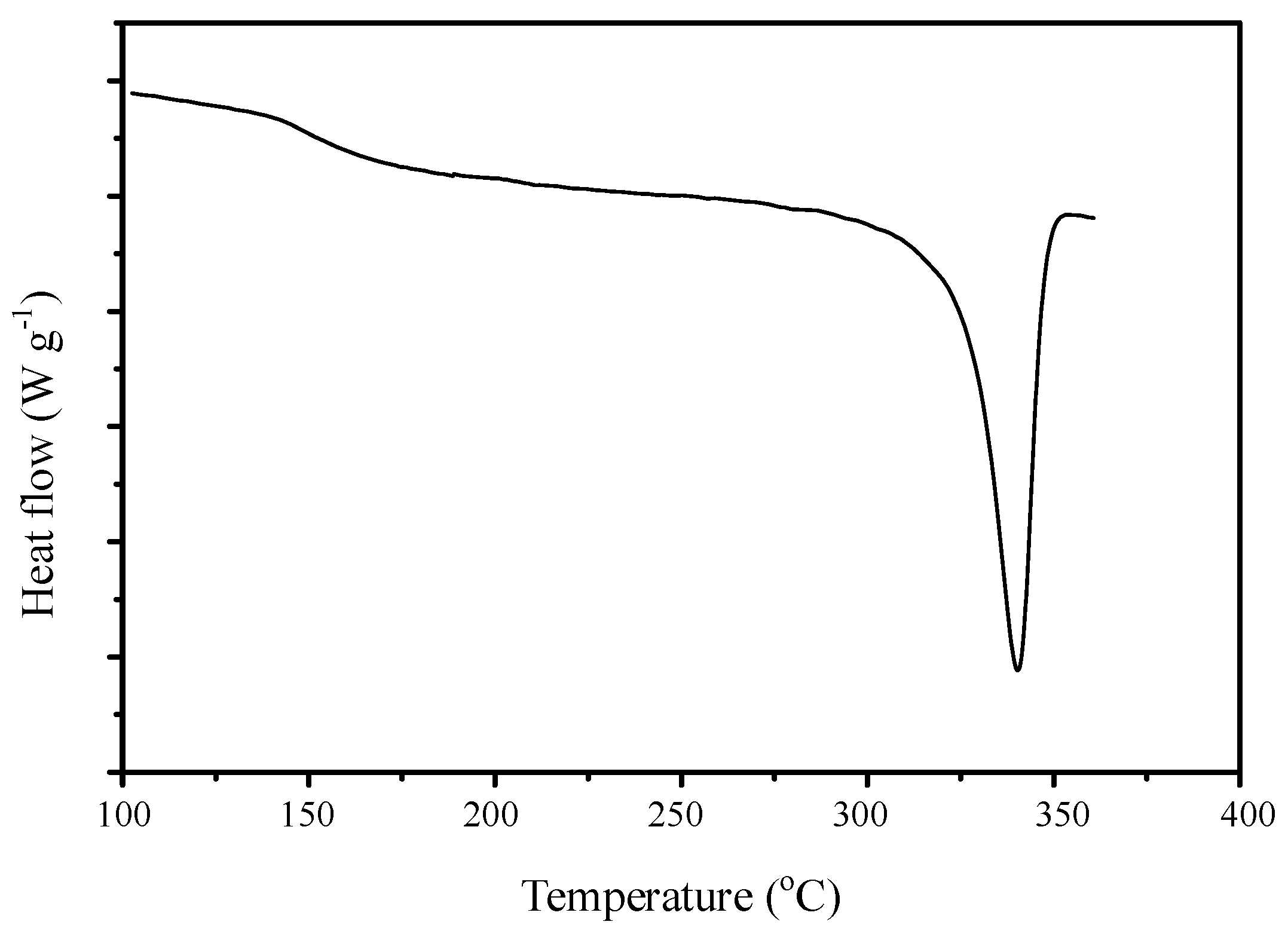
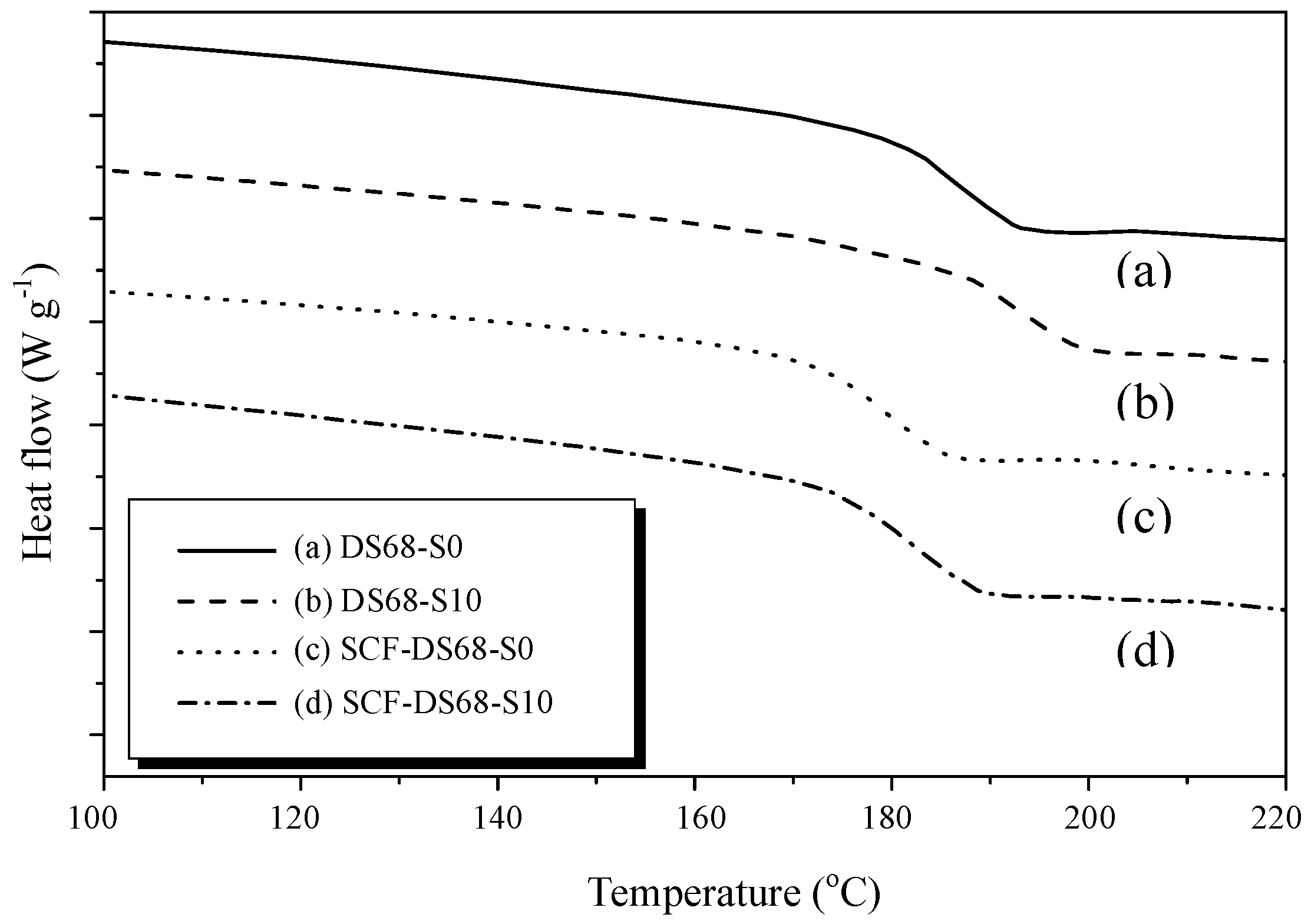
3.4. DSC and TGA Thermal Analysis
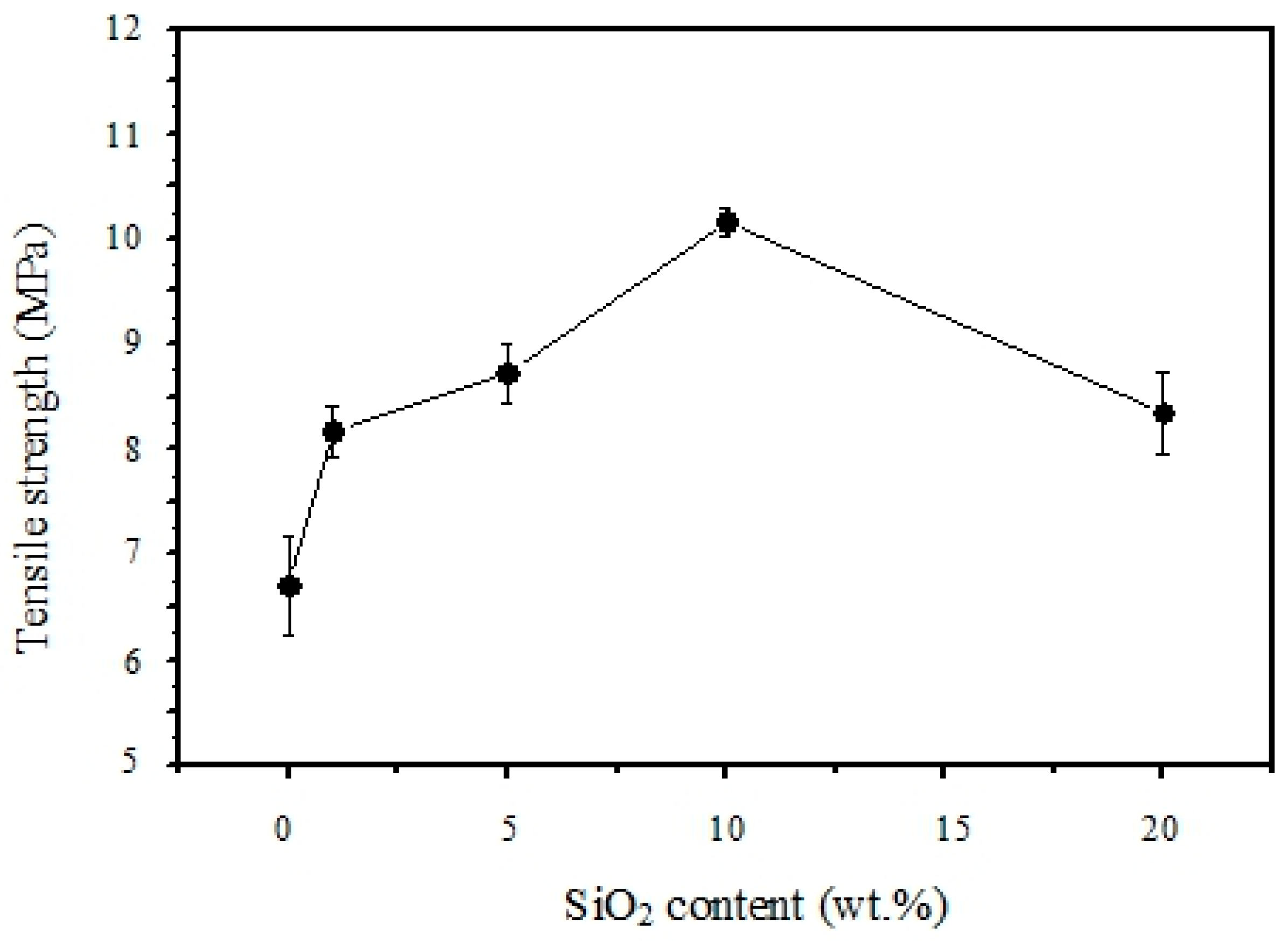
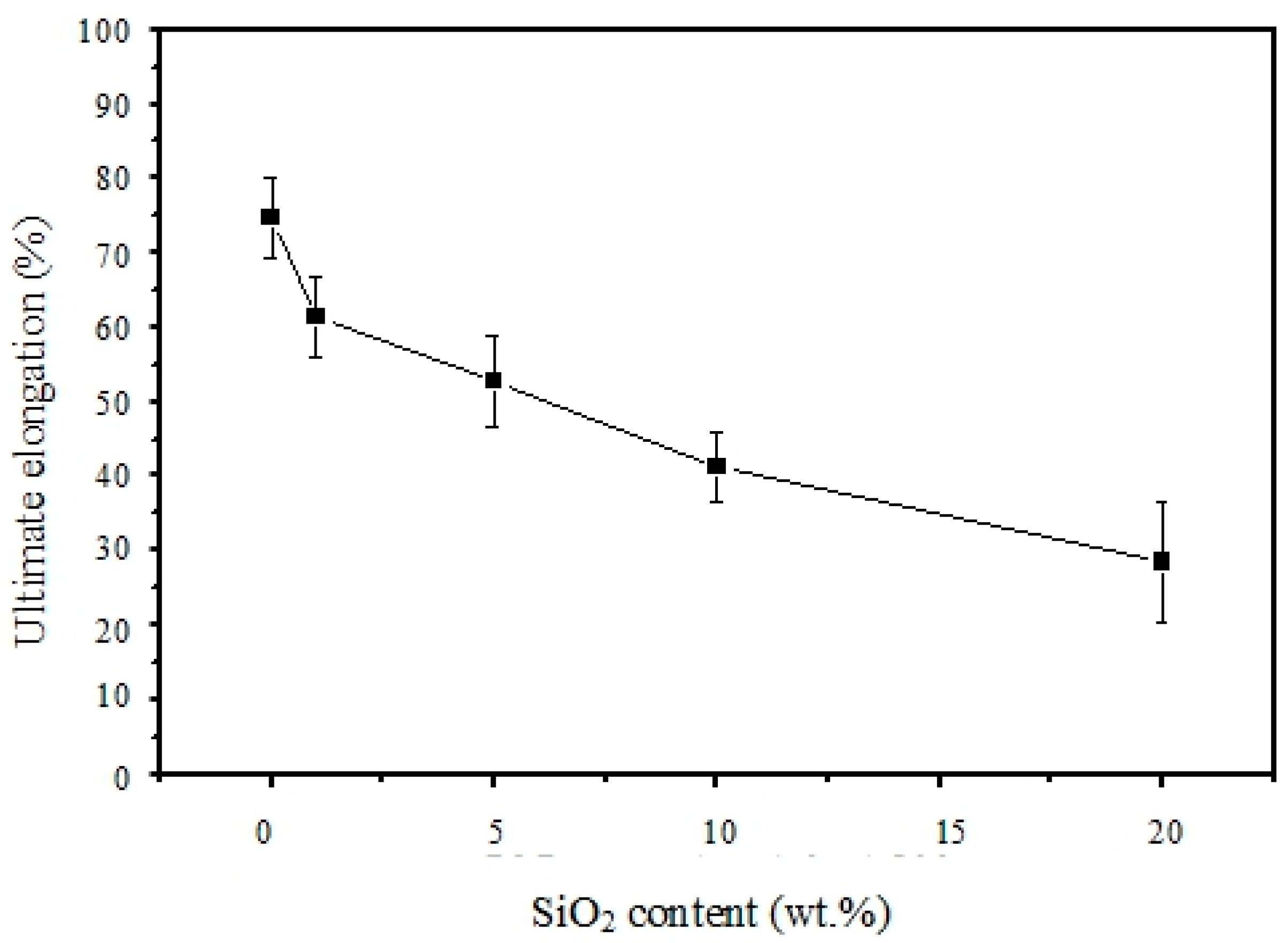
3.5. Mechanical Properties
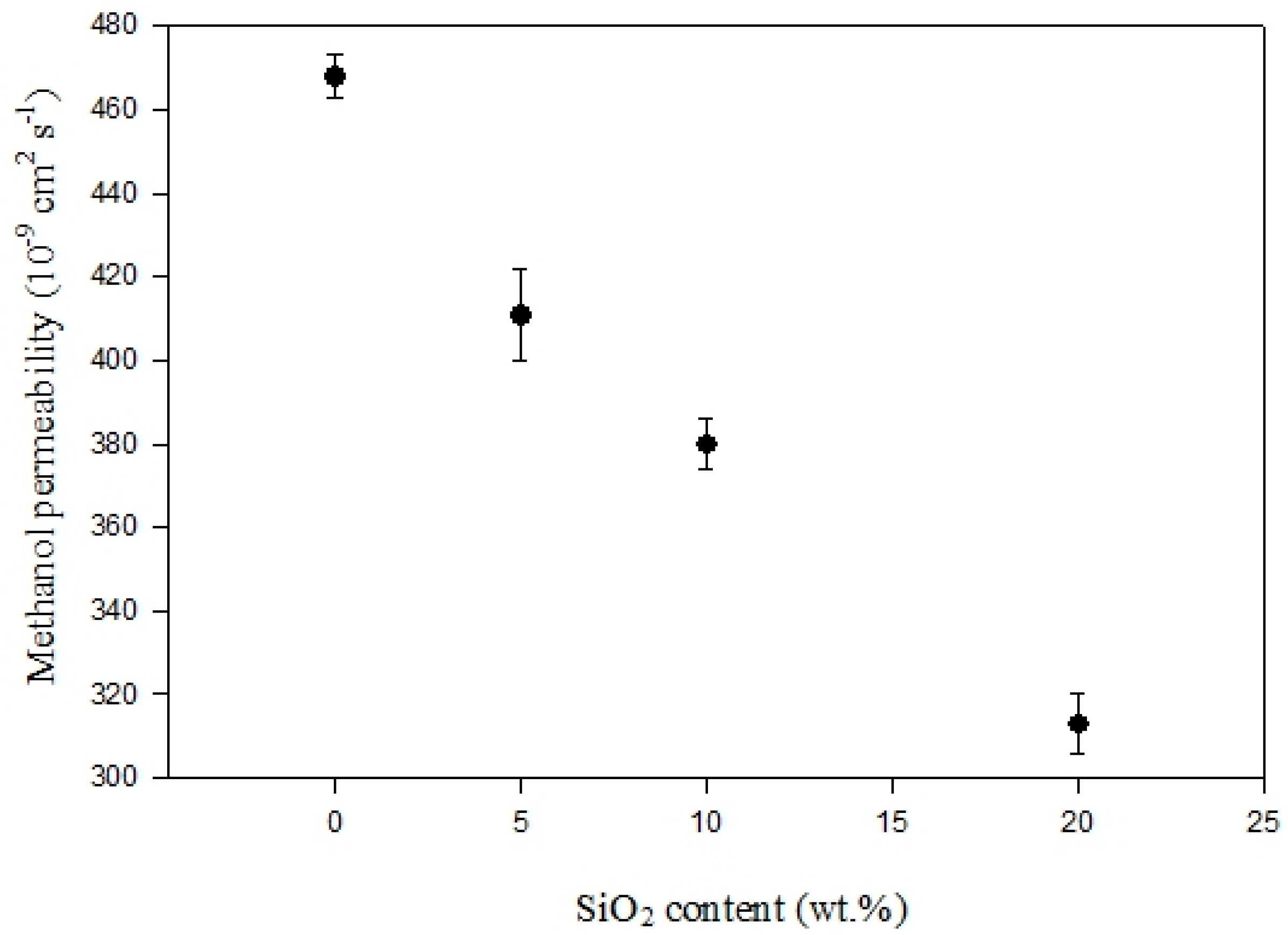
3.6. Methanol Permeability
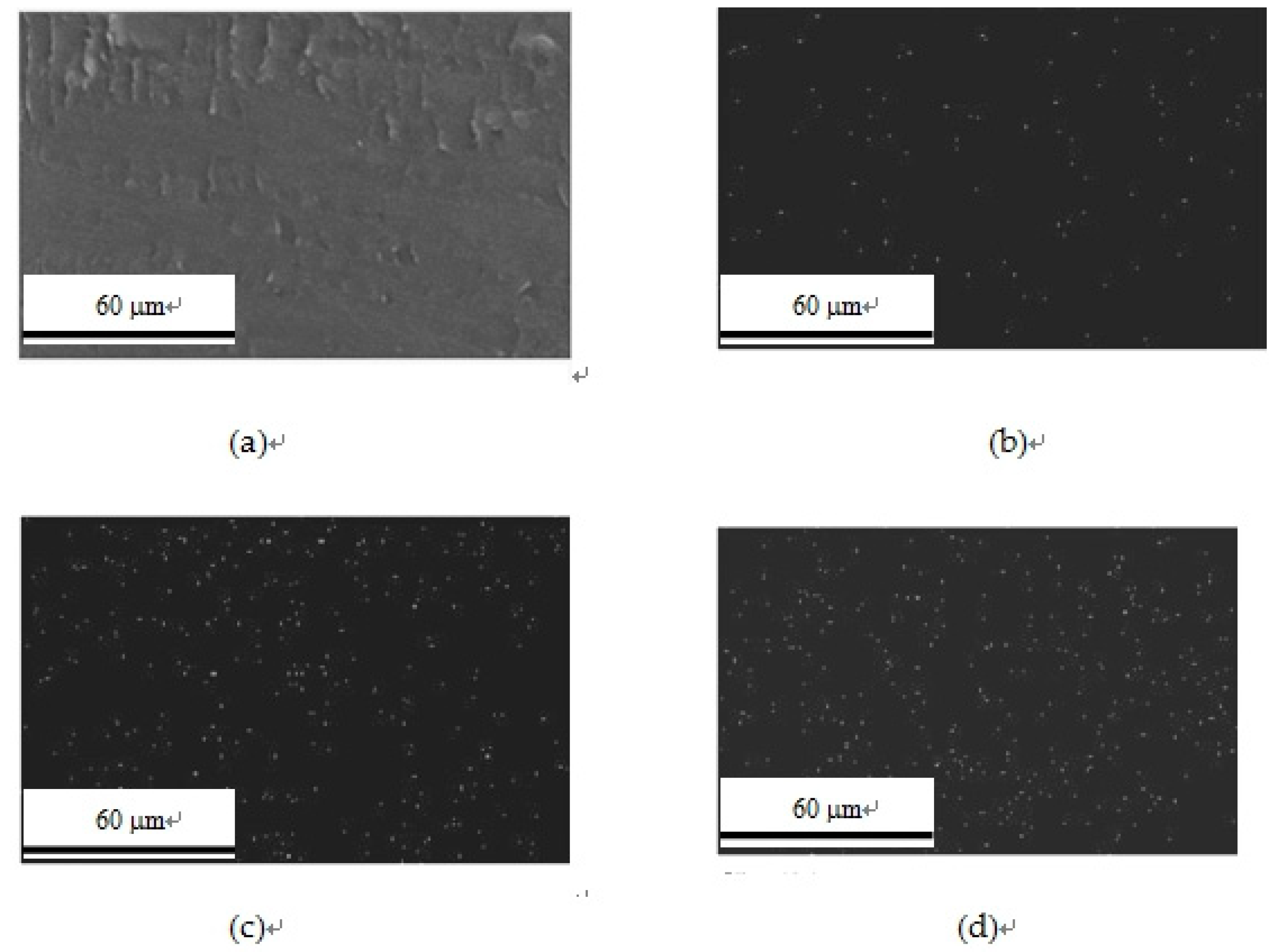
3.7. EDS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jithunsa, M.; Tashiro, K.; Nunes, S.P.; Chirachanchai, S. Poly(acrylic acid-co-4-vinylimidazole)/Sulfonated poly(ether ether ketone) blend membranes: A role of polymer chain with proton acceptor and donor for enhancing proton transfer in anhydrous system. Int. J. Hydrog. Energy 2011, 36, 10384–10391. [Google Scholar] [CrossRef]
- Wu, G.; Lin, S.; Yang, C. High performance composite solid polymer electrolyte systems for electrochemical cells. J. Power Sources 2013, 244, 287–293. [Google Scholar] [CrossRef]
- Fujiwara, N.; Yao, M.; Siroma, Z.; Senoh, H.; Ioroi, T.; Yasuda, K. Reversible air electrodes integrated with an anion-exchange membrane for secondary air batteries. J. Power Sources 2011, 196, 808–813. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Chen, D.; Ye, Z. Sulfonated Binaphthyl-Containing Poly(arylene ether ketone)s with Rigid Backbone and Excellent Film-Forming Capability for Proton Exchange Membranes. Polymers 2018, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Iulianelli, A.; Clarizia, G.; Gugliuzza, A.; Ebrasu, D.; Bevilacqua, A.; Trotta, F.; Basile, A. Sulfonation of PEEK-WC polymer via chloro-sulfonic acid for potential PEM fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 12688–12695. [Google Scholar] [CrossRef]
- Lee, K.; Chu, J.; Kim, A.; Yoo, D. Facile fabrication and characterization of improved proton conducting sulfonated poly(arylene biphenylether sulfone) blocks containing fluorinated hydrophobic units as proton exchange membrane fuel cell applications. Polymers 2018, 10, 1367. [Google Scholar] [CrossRef]
- Luo, H.; Vaivars, G.; Mathe, M. Double cross-linked polyetheretherketone proton exchange membrane for fuel cell. Int. J. Hydrog. Energy 2012, 37, 6148–6152. [Google Scholar] [CrossRef]
- Mahendiravarman, E.; Sangeetha, D. Increased microbial fuel cell performance using quaternized poly ether ether ketone anionic membrane electrolyte for electricity generation. Int. J. Hydrog. Energy 2013, 38, 2471–2479. [Google Scholar] [CrossRef]
- Reisert, M.; Aphale, A.; Singh, P. Solid Oxide Electrochemical Systems: Material Degradation Processes and Novel Mitigation Approaches. Materials 2018, 11, 2169. [Google Scholar] [CrossRef]
- Hoffmann, V.; Jung, D.; Zimmermann, J.; Rodriguez Correa, C.; Elleuch, A.; Halouani, K.; Kruse, A. Conductive carbon materials from the hydrothermal carbonization of vineyard residues for the application in electrochemical double-layer capacitors (EDLCs) and direct carbon fuel cells (DCFCs). Materials 2019, 12, 1703. [Google Scholar] [CrossRef]
- Sasikumar, G.; Ihm, J.; Ryu, H. Optimum Nafion content in PEM fuel cell electrodes. Electrochim. Acta 2004, 50, 601–605. [Google Scholar] [CrossRef]
- Lokkiluoto, A.; Gasik, M.M. Modeling and experimental assessment of Nafion membrane properties used in SO2 depolarized water electrolysis for hydrogen production. Int. J. Hydrog. Energy 2013, 38, 10–19. [Google Scholar] [CrossRef]
- Silva, V.S.; Schirmer, J.; Reissner, R.; Ruffmann, B.; Silva, H.; Gallego, Y.A.; Mendes, A.; Madeira, L.M.; Nunes, S.P. Proton electrolyte membrane properties and direct methanol fuel cell performance: II. Fuel cell performance and membrane properties effects. J. Power Sources 2005, 140, 41–49. [Google Scholar] [CrossRef]
- Di Vona, M.L.; D’Epifanio, A.; Marani, D.; Trombetta, M.; Traversa, E.; Licoccia, S. SPEEK/PPSU-based organic–inorganic membranes: Proton conducting electrolytes in anhydrous and wet environments. J. Membr. Sci. 2006, 279, 186–191. [Google Scholar] [CrossRef]
- Gohil, G.; Nagarale, R.; Binsu, V.; Shahi, V.K.; Nagarale, R. Preparation and characterization of monovalent cation selective sulfonated poly(ether ether ketone) and poly(ether sulfone) composite membranes. J. Colloid Interface Sci. 2006, 298, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, X.; Ma, Y.; Huang, Y.; Liu, X. Constructing Continuous Proton-Conducting Highways within Sulfonated Poly(Arylene Ether Nitrile) Composite Membrane by Incorporating Amino-Sulfo-Bifunctionalized GO. Polymers 2018, 10, 1005. [Google Scholar] [CrossRef]
- Lakshmi, R.T.S.M.; Choudhary, V.; Varma, I.K. Sulphonated poly(ether ether ketone): Synthesis and characterisation. J. Mater. Sci. 2005, 40, 629–636. [Google Scholar] [CrossRef]
- Iulianelli, A.; Basile, A. Sulfonated PEEK-based polymers in PEMFC and DMFC applications: A review. Int. J. Hydrog. Energy 2012, 37, 15241–15255. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Park, C.J.; Yoo, D.J. Alleviating the Mechanical and Thermal Degradations of Highly Sulfonated Poly(Ether Ether Ketone) Blocks via Copolymerization with Hydrophobic Unit for Intermediate Humidity Fuel Cells. Polymers 2018, 10, 1346. [Google Scholar] [CrossRef]
- Kim, A.R.; Yoo, D.J. A Comparative Study on Physiochemical, Thermomechanical, and Electrochemical Properties of Sulfonated Poly(Ether Ether Ketone) Block Copolymer Membranes with and without Fe3O4 Nanoparticles. Polymers 2019, 11, 536. [Google Scholar] [CrossRef]
- Alvarez, A.; Guzman, C.; Carbone, A.; Sacca, A.; Gatto, I.; Passalaqua, E.; Nava, R.; Ornelas, R.; Ledesma-Garcia, J.; Arriaga, L.G. Influence of silica morphology in composite Nafionmembranes properties. Int. J. Hydrog. Energy 2011, 36, 14725–14733. [Google Scholar] [CrossRef]
- Zhang, Z.; Handa, Y.P. CO2-Assisted Melting of Semicrystalline Polymers. Macromolecules 1997, 30, 8505–8507. [Google Scholar] [CrossRef]
- Tominaga, Y.; Hirahara, S.; Asai, S.; Sumita, M. Ionic conductivity studies of poly(ethylene oxide)–lithium salt electrolytes in high-pressure carbon dioxide. Polymers 2005, 46, 8113–8118. [Google Scholar] [CrossRef]
- Tominaga, Y.; Izumi, Y.; Kwak, G.-H.; Asai, S.; Sumita, M. Effect of Supercritical Carbon Dioxide Processing on Ionic Association and Conduction in a Crystalline Poly(ethylene oxide)−LiCF3SO3Complex. Macromolecules 2003, 36, 8766–8772. [Google Scholar] [CrossRef]
- Kwak, G.-H.; Tominaga, Y.; Asai, S.; Sumita, M. AC complex impedance measurement of comb-like type polyether electrolytes under high-pressure carbon dioxide. Electrochim. Acta 2003, 48, 4069–4075. [Google Scholar] [CrossRef]
- Iulianelli, A.; Gatto, I.; Trotta, F.; Biasizzo, M.; Passalacqua, E.; Carbone, A.; Bevilacqua, A.; Clarizia, G.; Gugliuzza, A.; Basile, A. Electrochemical characterization of sulfonated PEEK-WC membranes for PEM fuel cells. Int. J. Hydrog. Energy 2013, 38, 551–557. [Google Scholar] [CrossRef]
- Martos, A.M.; Biasizzo, M.; Trotta, F.; Del Río, C.; Várez, A.; Levenfeld, B. Synthesis and characterization of sulfonated PEEK-WC-PES copolymers for fuel cell proton exchange membrane application. Eur. Polym. J. 2017, 93, 390–402. [Google Scholar] [CrossRef]
- Xue, S.; Yin, G. Methanol permeability in sulfonated poly(etheretherketone) membranes: A comparison with Nafion membranes. Eur. Polym. J. 2006, 42, 776–785. [Google Scholar] [CrossRef]


| Sample | Membrane Properties | |||
|---|---|---|---|---|
| Sulfonation Time (h) | DS (%) | Absorption (%) | Ionic Conductivity (× 10−3 S cm−1) | |
| DS41 | 72 | 41.0 | 18.2 | 0.05 |
| DS49 | 96 | 48.7 | 24.9 | 0.26 |
| DS57 | 120 | 57.2 | 32.0 | 1.29 |
| DS66 | 144 | 65.8 | 36.1 | 2.62 |
| DS68 | 175 | 68.0 | 45.3 | 4.68 |
| Sample | Properties | ||||
|---|---|---|---|---|---|
| Sulfonation Time (h) | SiO2 Content (%) | Absorption (%) | Ionic Conductivity (× 10−3 S cm−1) | Supercritical CO2 Fluid Treatment | |
| DS68-S0 | 175 | 0 | 45.3 | 4.68 | No |
| DS68-S1 | 175 | 1 | 44.7 | 3.37 | No |
| DS68-S5 | 175 | 5 | 42.0 | 3.18 | No |
| DS68-S10 | 175 | 10 | 52.6 | 6.46 | No |
| DS68-S20 | 175 | 20 | 59.2 | 7.34 | No |
| SCF-DS68-S0 | 175 | 0 | 56.5 | 9.14 | Yes |
| SCF-DS68-S10 | 175 | 10 | 63.1 | 15.47 | Yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Lin, S.-J.; Hsu, I.-C.; Su, J.-Y.; Chen, D.W. Study of High Performance Sulfonated Polyether Ether Ketone Composite Electrolyte Membranes. Polymers 2019, 11, 1177. https://doi.org/10.3390/polym11071177
Wu G, Lin S-J, Hsu I-C, Su J-Y, Chen DW. Study of High Performance Sulfonated Polyether Ether Ketone Composite Electrolyte Membranes. Polymers. 2019; 11(7):1177. https://doi.org/10.3390/polym11071177
Chicago/Turabian StyleWu, Gwomei, Sheng-Jen Lin, I-Chan Hsu, Juin-Yih Su, and Dave W. Chen. 2019. "Study of High Performance Sulfonated Polyether Ether Ketone Composite Electrolyte Membranes" Polymers 11, no. 7: 1177. https://doi.org/10.3390/polym11071177




