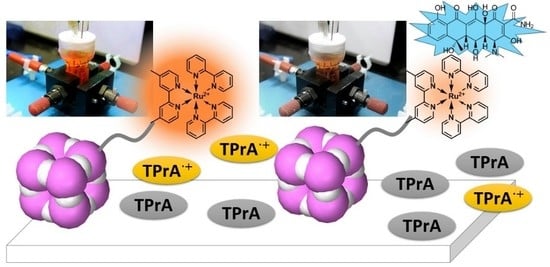
Oxygen-Resistant Electrochemiluminescence System with Polyhedral Oligomeric Silsesquioxane
Abstract
:1. Introduction
2. Results and Discussion
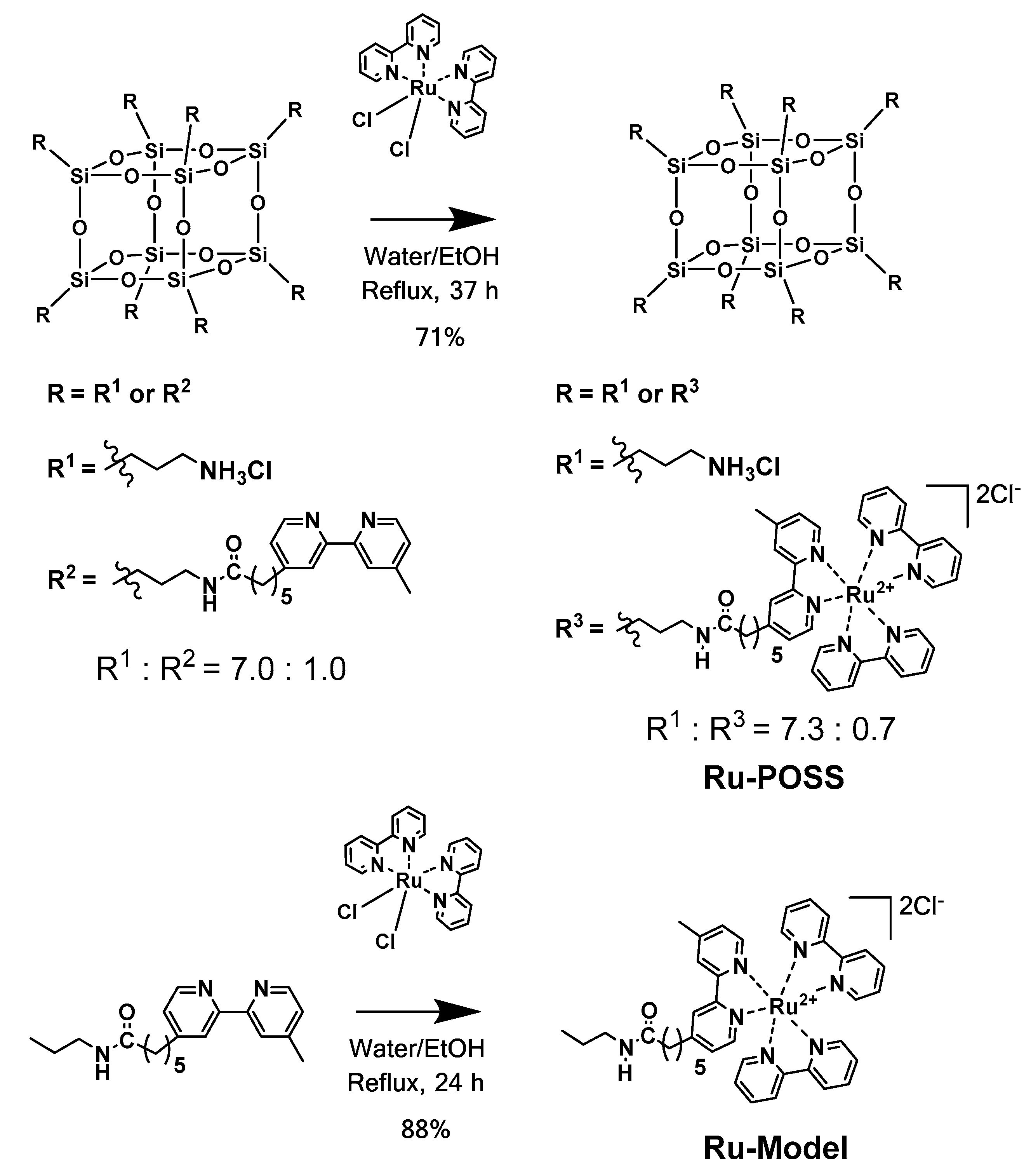
2.1. Characteristics of Ru-POSS
2.2. ECL Properties of Ru(II)-Containing Materials
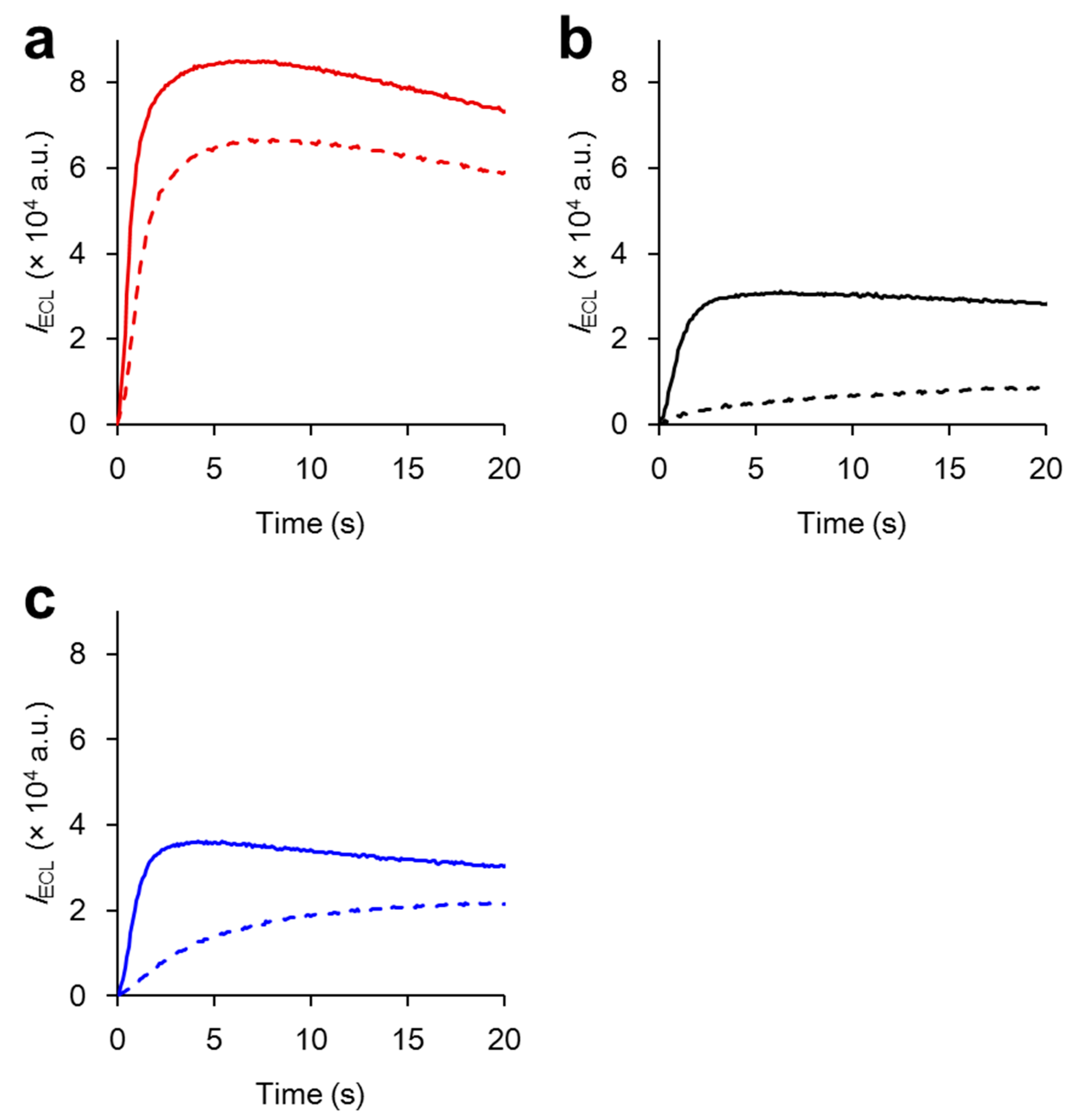
2.3. Resistance to Oxygen Quenching
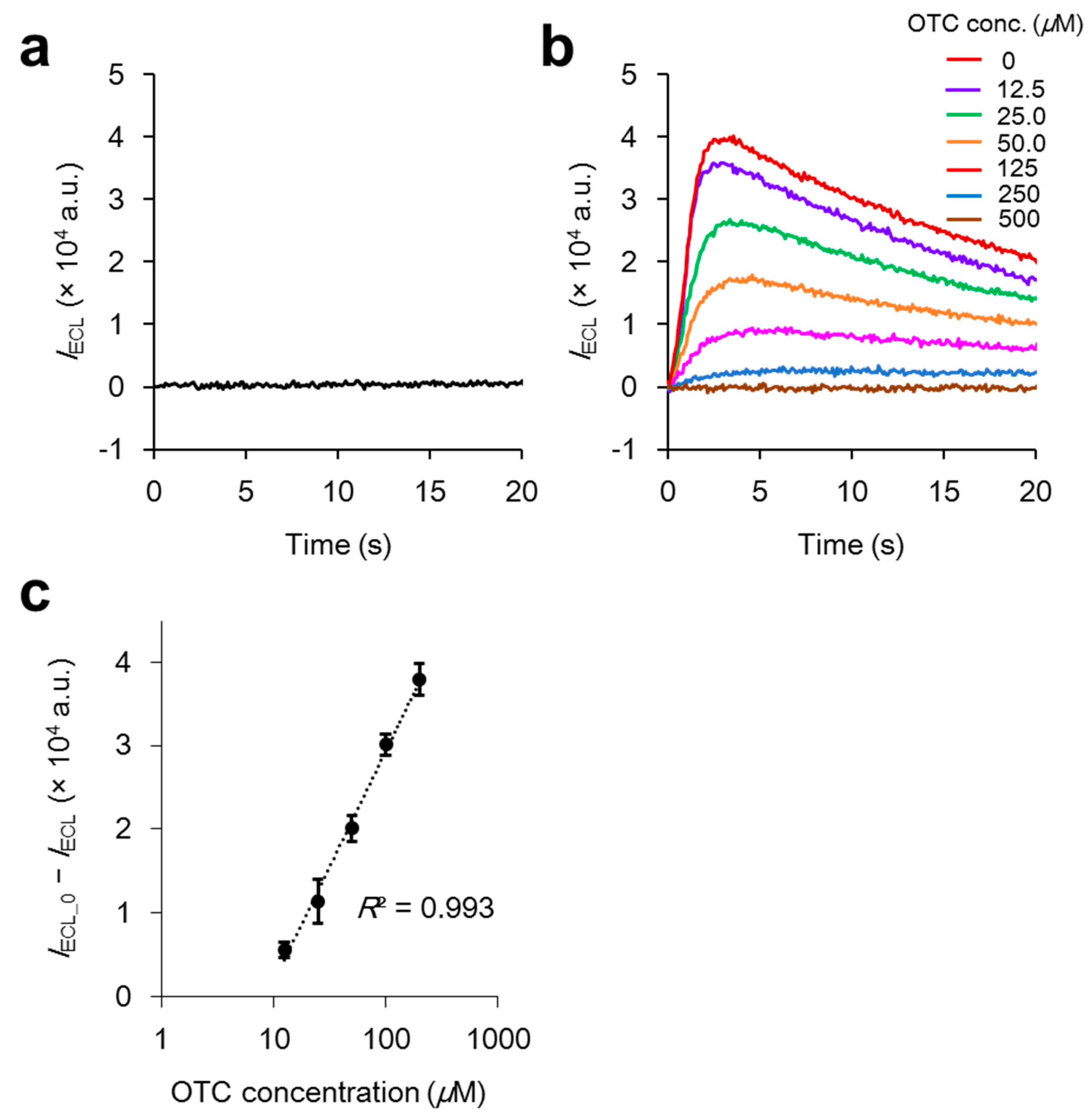
2.4. Analysis of Antibiotics without Deoxygenation
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2008, 104, 3003–3036. [Google Scholar] [CrossRef]
- Miao, W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Fähnrich, K.A.; Pravda, M.; Guilbault, G.G. Recent applications of electrogenerated chemiluminescence in chemical analysis. Talanta 2001, 54, 531–559. [Google Scholar] [CrossRef]
- Gorman, B.A.; Francis, P.S.; Barnett, N.W. Tris(2,2′-bipyridyl)ruthenium(II) chemiluminescence. Analyst 2006, 131, 616–639. [Google Scholar] [CrossRef]
- Qi, H.; Peng, Y.; Gao, Q.; Zhang, C. Applications of nanomaterials in electrogenerated chemiluminescence biosensors. Sensors 2009, 9, 674–695. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Zhu, J.J. Recent Advances in Electrochemiluminescence Analysis. Anal. Chem. 2017, 89, 358–371. [Google Scholar] [CrossRef]
- Demas, J.N.; Diemente, D.; Harris, E.W. Oxygen quenching of charge-transfer excited states of ruthenium(II) complexes. Evidence for singlet oxygen production. J. Am. Chem. Soc. 1973, 95, 6864–6865. [Google Scholar] [CrossRef]
- Lin, C.T.; Sutin, N. An investigation of the micellar phase of sodium dodecyl sulfate in aqueous sodium chloride solutions using quasielastic light scattering spectroscopy. J. Phys. Chem. 1976, 80, 97–105. [Google Scholar] [CrossRef]
- O’Keeffe, G.; McDonagh, C.M.; O’Kelly, B.; Vos, J.G.; Keyes, E.T.; McGilp, J.F.; MacCraith, B.D. ibre optic oxygen sensor based on fluorescence quenching of evanescent-wave excited ruthenium complexes in sol–gel derived porous coatings. Analyst 1993, 118, 385–388. [Google Scholar]
- Fiaccabrino, G.C.; Koudelka-Hep, M.; Hsueh, Y.T.; Collins, S.D.; Smith, R.L. Electrochemiluminescence of Tris(2,2′-bipyridine)ruthenium in Water at Carbon Microelectrodes. Anal. Chem. 1998, 70, 4157–4161. [Google Scholar] [CrossRef]
- Tomita, I.N.; Bulhões, L.O.S. Electrogenerated chemiluminescence determination of cefadroxil antibiotic. Anal. Chim. Acta 2001, 442, 201–206. [Google Scholar] [CrossRef]
- Zheng, H.; Zu, Y. Emission of Tris(2,2′-bipyridine)ruthenium(II) by Coreactant Electrogenerated Chemiluminescence: From O2-Insensitive to Highly O2-Sensitive. J. Phys. Chem. B 2005, 109, 12049–12053. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Chujo, Y.; Tanaka, K. New Polymeric Materials Based on Element-Blocks. Bull. Chem. Soc. Jpn. 2015, 88, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Gon, M.; Tanaka, K.; Chujo, Y. Recent Progress in the Development of Advanced Element-Block Materials. Polym. J. 2018, 50, 109–126. [Google Scholar] [CrossRef]
- Gon, M.; Kato, K.; Tanaka, K.; Chujo, Y. Elastic and mechanofluorochromic hybrid films with POSS-capped polyurethane and polyfluorene. Mater. Chem. Front. 2019, 3, 1174–1180. [Google Scholar] [CrossRef]
- Gon, M.; Sato, K.; Kato, K.; Tanaka, K.; Chujo, Y. Preparation of Bright-Emissive Hybrid Materials Based on Light-Harvesting POSS Having Radially Integrated Luminophores and Commodity π-Conjugated Polymers. Mater. Chem. Front. 2019, 3, 314–320. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, K.; Chujo, Y. Optical, Electrical and Thermal Properties of Organic−Inorganic Hybrids with Conjugated Polymers Based on POSS Having Heterogeneous Substituents. Polymers 2019, 11, 44. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, K.; Chujo, Y. Fluoroalkyl POSS with Dual Functional Groups as a Molecular Filler for Lowering Refractive Indices and Improving Thermomechanical Properties of PMMA. Polymers 2018, 10, 1332. [Google Scholar] [CrossRef]
- Narikiyo, H.; Gon, M.; Tanaka, K.; Chujo, Y. Control of Intramolecular Excimer Emission in Luminophore-Integrated Ionic POSSs Possessing Flexible Side-Chains. Mater. Chem. Front. 2018, 2, 1449–1455. [Google Scholar] [CrossRef]
- Narikiyo, H.; Kakuta, T.; Matsuyama, H.; Gon, M.; Tanaka, K.; Chujo, Y. Development of the Optical Sensor for Discriminating Isomers of Fatty Acids Based on Emissive Network Polymers Composed of Polyhedral Oligomeric Silsesquioxane. Bioorg. Med. Chem. 2017, 25, 3431–3436. [Google Scholar] [CrossRef]
- Kakuta, T.; Jeon, J.-H.; Tanaka, K.; Chujo, Y. Development of Highly-Sensitive Detection System in 19F NMR for Bioactive Compounds Based on the Assembly of Paramagnetic Complexes with Fluorinated Cubic Silsesquioxanes. Bioorg. Med. Chem. 2017, 25, 1389–1393. [Google Scholar] [CrossRef]
- Tanaka, K.; Okada, H.; Ohashi, W.; Jeon, J.-H.; Inafuku, K.; Chujo, Y. Hypoxic Conditions-Selective Upconversion via Triplet-Triplet Annihilation Based on POSS-Core Dendrimer Complexes. Bioorg. Med. Chem. 2013, 21, 2678–2681. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Tanaka, K.; Chujo, Y. Light-Driven Artificial Enzymes for Selective Oxidation of Guanosine Triphosphate Using Water-Soluble POSS Network Polymers. Org. Biomol. Chem. 2014, 12, 6500–6506. [Google Scholar] [CrossRef]
- McClanahan, S.F.; Dallinger, R.F.; Holler, F.J.; Kincaid, J.R. Mixed-ligand poly(pyridine) complexes of ruthenium(II). Resonance Raman spectroscopic evidence for selective population of ligand-localized 3MLCT excited states. J. Am. Chem. Soc. 1985, 107, 4853–4860. [Google Scholar] [CrossRef]
- Juris, A.; Balzani, V.; Belser, P.; VON Zelewsky, V.A. Characterization of the Excited State Properties of Some New Photosensitizers of the Ruthenium (Polypyridine) Family. Helv. Chim. Acta 1981, 64, 2175–2182. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yun, Y.J.; Kim, D.Y.; Han, S.H. Formation of a Self-Assembled Monolayer of Diaminododecane and a Heteropolyacid Monolayer on the ITO Surface. Langmuir 1999, 15, 4690–4692. [Google Scholar] [CrossRef]
- Kim, K.; Song, H.; Park, J.T.; Kwak, J. C60 Self-Assembled Monolayer Using Diamine as a Prelayer. Chem. Lett. 2000, 29, 958–959. [Google Scholar] [CrossRef]
- Bermudez, V.M.; Berry, A.D.; Kim, H.; Piqué, A. Functionalization of Indium Tin Oxide. Langmuir 2006, 22, 11113–11125. [Google Scholar] [CrossRef]
- Bearinger, J.P.; Vörös, J.; Hubbell, J.A.; Textor, M. Electrochemical optical waveguide lightmode spectroscopy (EC-OWLS): A pilot study using evanescent-field optical sensing under voltage control to monitor polycationic polymer adsorption onto indium tin oxide (ITO)-coated waveguide chips. Biotechnol. Bioeng. 2003, 82, 465–473. [Google Scholar] [CrossRef]
- Yang, H.J.; Sugiura, Y.; Ishizaki, I.; Sanada, N.; Ikegami, K.; Zaima, N.; Shrivas, K.; Setou, M. Imaging of lipids in cultured mammalian neurons by matrix assisted laser/desorption ionization and secondary ion mass spectrometry. Surf. Interface Anal. 2010, 42, 1606–1611. [Google Scholar] [CrossRef]
- Choi, Y.; Yagati, A.K.; Cho, S. Electrochemical Characterization of Poly-l-Lysine Coating on Indium Tin Oxide Electrode for Enhancing Cell Adhesion. J. Nanosci. Nanotechnol. 2015, 15, 7881–7885. [Google Scholar] [CrossRef]
- Koller, K.B.; Hawkridge, F.M. Temperature and electrolyte effects on the electron-transfer reactions of cytochrome c. J. Am. Chem. Soc. 1985, 107, 7412–7417. [Google Scholar] [CrossRef]
- Valenti, G.; Zangheri, M.; Sansaloni, S.E.; Mirasoli, M.; Penicaud, A.; Roda, A.; Paolucci, F. Transparent Carbon Nanotube Network for Efficient Electrochemiluminescence Devices. Chem. Eur. J. 2015, 21, 12640–12645. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Modification of Indium Tin Oxide with Dendrimer-Encapsulated Nanoparticles To Provide Enhanced Stable Electrochemiluminescence of Ru(bpy)32+/Tripropylamine While Preserving Optical Transparency of Indium Tin Oxide for Sensitive Electrochemiluminescence-Based Analyses. Anal. Chem. 2014, 86, 1654–1660. [Google Scholar]
- Pan, S.; Liu, J.; Hill, C.M. Observation of Local Redox Events at Individual Au Nanoparticles Using Electrogenerated Chemiluminescence Microscopy. J. Phys. Chem. C 2015, 119, 27095–27103. [Google Scholar] [CrossRef]
- Su, B.; Xu, L.; Yang, Q.; Zhou, Z.; Guo, W. Two orders-of-magnitude enhancement in the electrochemiluminescence of Ru(bpy)32+ by vertically ordered silica mesochannels. Anal. Chim. Acta 2015, 886, 48–55. [Google Scholar]
- Miller, C.J.; McCord, P.; Bard, A.J. Study of Langmuir monolayers of ruthenium complexes and their aggregation by electrogenerated chemiluminescence. Langmuir 1991, 7, 2781–2787. [Google Scholar] [CrossRef]
- Leland, J.K.; Powell, M.J. Electrogenerated Chemiluminescence: An Oxidative-Reduction Type ECL Reaction Sequence Using Tripropyl Amine. J. Electrochem. Soc. 1990, 137, 3127–3131. [Google Scholar] [CrossRef]
- Miao, W.; Choi, J.P.; Bard, A.J. Electrogenerated Chemiluminescence 69: The Tris(2,2′-bipyridine)ruthenium(II), (Ru(bpy)32+)/Tri-n-propylamine (TPrA) System Revisited−A New Route Involving TPrA•+ Cation Radicals. J. Am. Chem. Soc. 2002, 124, 14478–14485. [Google Scholar] [CrossRef]
- Zu, Y.; Li, F. Characterization of the low-oxidation-potential electrogenerated chemiluminescence of tris(2,2′-bipyridine)ruthenium(II) with tri-n-propylamine as coreactant. Anal. Chim. Acta 2005, 550, 47–52. [Google Scholar] [CrossRef]
- Wightman, R.M.; Forry, S.P.; Maus, R.; Badocco, D.; Pastore, P. Rate-Determining Step in the Electrogenerated Chemiluminescence from Tertiary Amines with Tris(2,2′-bipyridyl)ruthenium(II). J. Phys. Chem. B 2004, 108, 19119–19125. [Google Scholar] [CrossRef]
- Kanoufi, F.; Zu, Y.; Bard, A.J. Homogeneous Oxidation of Trialkylamines by Metal Complexes and Its Impact on Electrogenerated Chemiluminescence in the Trialkylamine/Ru(bpy)32+ System. J. Phys. Chem. B 2001, 105, 210–216. [Google Scholar] [CrossRef]
- Gross, E.M.; Pastore, P.; Wightman, R.M. High-Frequency Electrochemiluminescent Investigation of the Reaction Pathway between Tris(2,2′-bipyridyl)ruthenium(II) and Tripropylamine Using Carbon Fiber Microelectrodes. J. Phys. Chem. B 2001, 105, 8732–8738. [Google Scholar] [CrossRef]
- Pang, Y.Q.; Cui, H.; Zheng, H.S.; Wan, G.H.; Liu, L.J.; Yu, X.F. Flow injection analysis of tetracyclines using inhibited Ru(bpy)32+/tripropylamine electrochemiluminescence system. Luminescence 2005, 20, 8–15. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, B.; Liu, J.; Chen, K. Determination of p-aminobenzenesulfonic acid based on the electrochemiluminescence quenching of tris(2,2′-bipyridine)-ruthenium (II). Luminescence 2013, 28, 363–367. [Google Scholar] [CrossRef]
- Tagiri-Endo, M.; Suzuki, S.; Nakamura, T.; Hatakeyama, T.; Kawamukai, K. Rapid determination of five antibiotic residues in swine wastewater by online solid-phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 393, 1367–1375. [Google Scholar] [CrossRef]
- McCall, J.; Alexander, C.; Richter, M.M. Quenching of Electrogenerated Chemiluminescence by Phenols, Hydroquinones, Catechols, and Benzoquinones. Anal. Chem. 1999, 71, 2523–2527. [Google Scholar] [CrossRef]
- Zheng, H.; Zu, Y. Highly Efficient Quenching of Coreactant Electrogenerated Chemiluminescence by Phenolic Compounds. J. Phys. Chem. B 2005, 109, 16047–16051. [Google Scholar] [CrossRef]
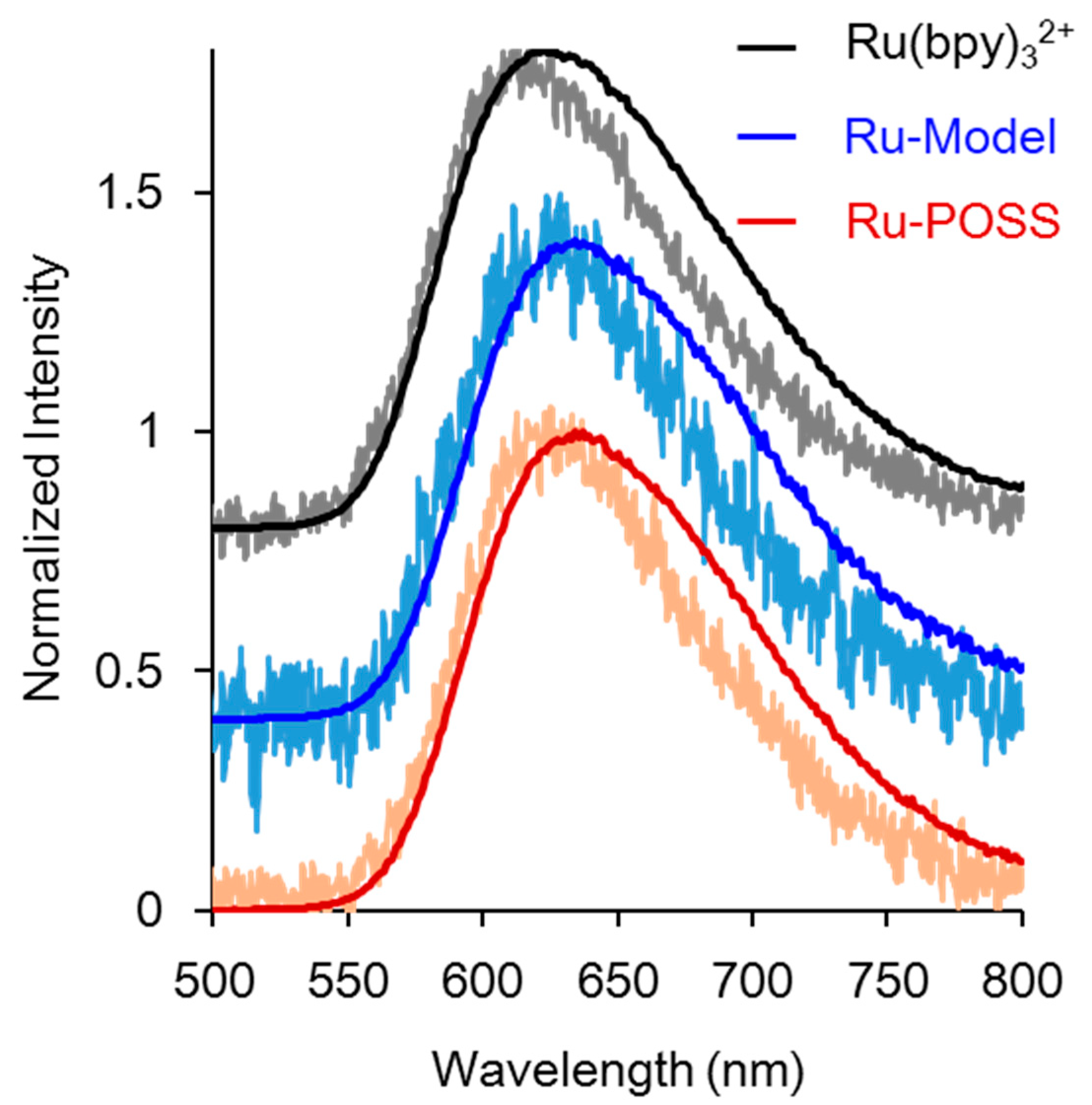

| Compounds | λabs_LC (nm) | λabs_MLCT (nm) | λem_PL (nm) b | ΦPL (%) c | τ (µs) d | λem_ECL (nm) | Intensity (×104 a.u.) e |
|---|---|---|---|---|---|---|---|
| Ru-POSS | 286 | 456 | 634 | 5.6 | 0.44 | 607 | 13.5 |
| Ru-Model | 286 | 456 | 632 | 5.8 | 0.43 | 631 | 9.5 |
| Ru(bpy)32+ | 286 | 454 | 623 | 6.3 | 0.50 | 632 | 8.8 |
| Compounds | IECLMax_O2/IECLMax_Ar (%) | mECLMax_O2/mECLMax_Ar (%) |
|---|---|---|
| Ru-POSS | 78 | 51 |
| Ru(bpy)32+ | 38 | 9 |
| Ru-Model | 61 | 13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, R.; Narikiyo, H.; Gon, M.; Tanaka, K.; Chujo, Y. Oxygen-Resistant Electrochemiluminescence System with Polyhedral Oligomeric Silsesquioxane. Polymers 2019, 11, 1170. https://doi.org/10.3390/polym11071170
Nakamura R, Narikiyo H, Gon M, Tanaka K, Chujo Y. Oxygen-Resistant Electrochemiluminescence System with Polyhedral Oligomeric Silsesquioxane. Polymers. 2019; 11(7):1170. https://doi.org/10.3390/polym11071170
Chicago/Turabian StyleNakamura, Ryota, Hayato Narikiyo, Masayuki Gon, Kazuo Tanaka, and Yoshiki Chujo. 2019. "Oxygen-Resistant Electrochemiluminescence System with Polyhedral Oligomeric Silsesquioxane" Polymers 11, no. 7: 1170. https://doi.org/10.3390/polym11071170