Design and Optimization of Flexible Polypyrrole/Bacterial Cellulose Conductive Nanocomposites Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PPy/BC Composite by in Situ Chemical Polymerization
2.3. Box-Behnken Design
2.4. Characterization and Electrical Measurements
3. Results and Discussion
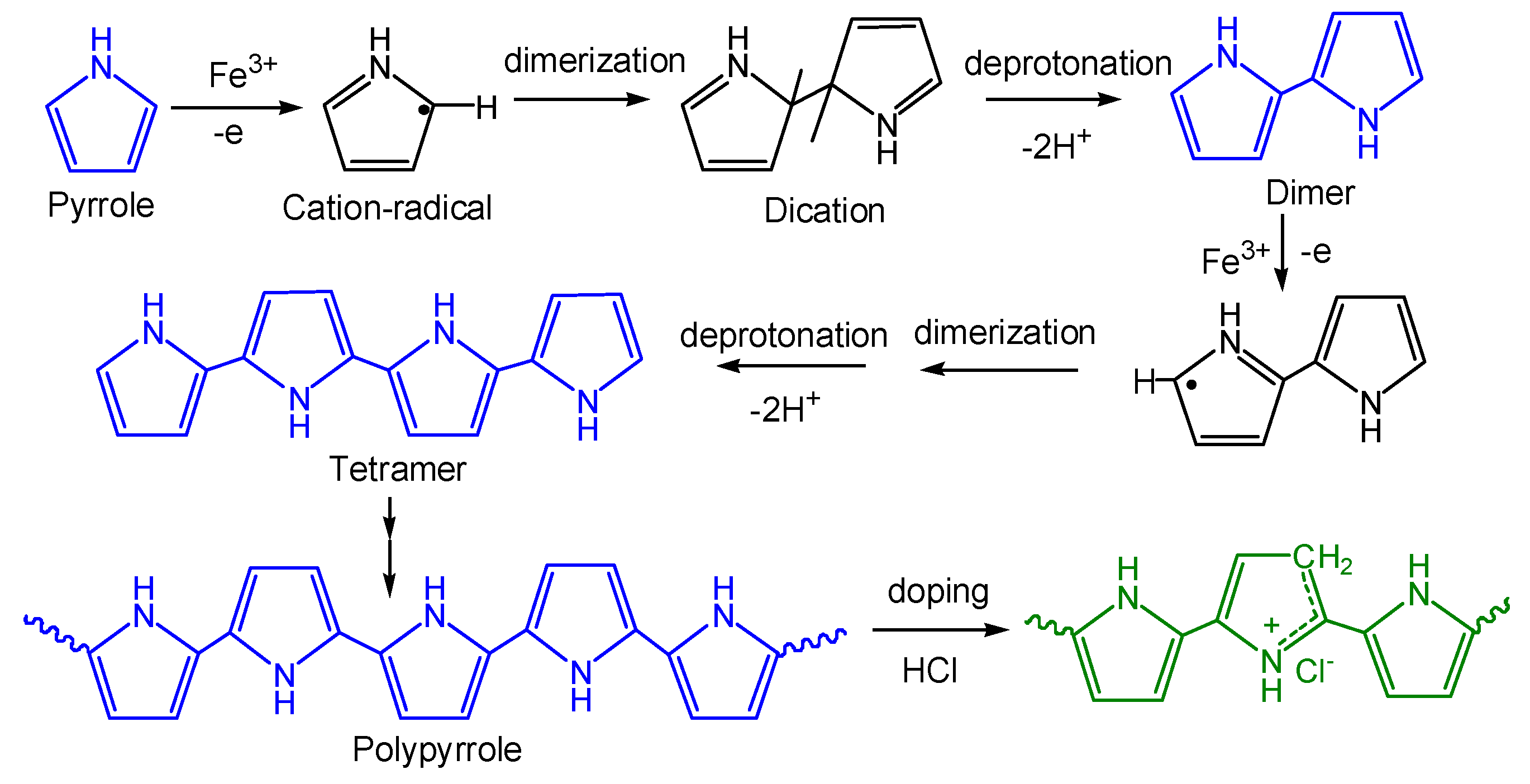
3.1. Synthesis of PPy/BC Composite
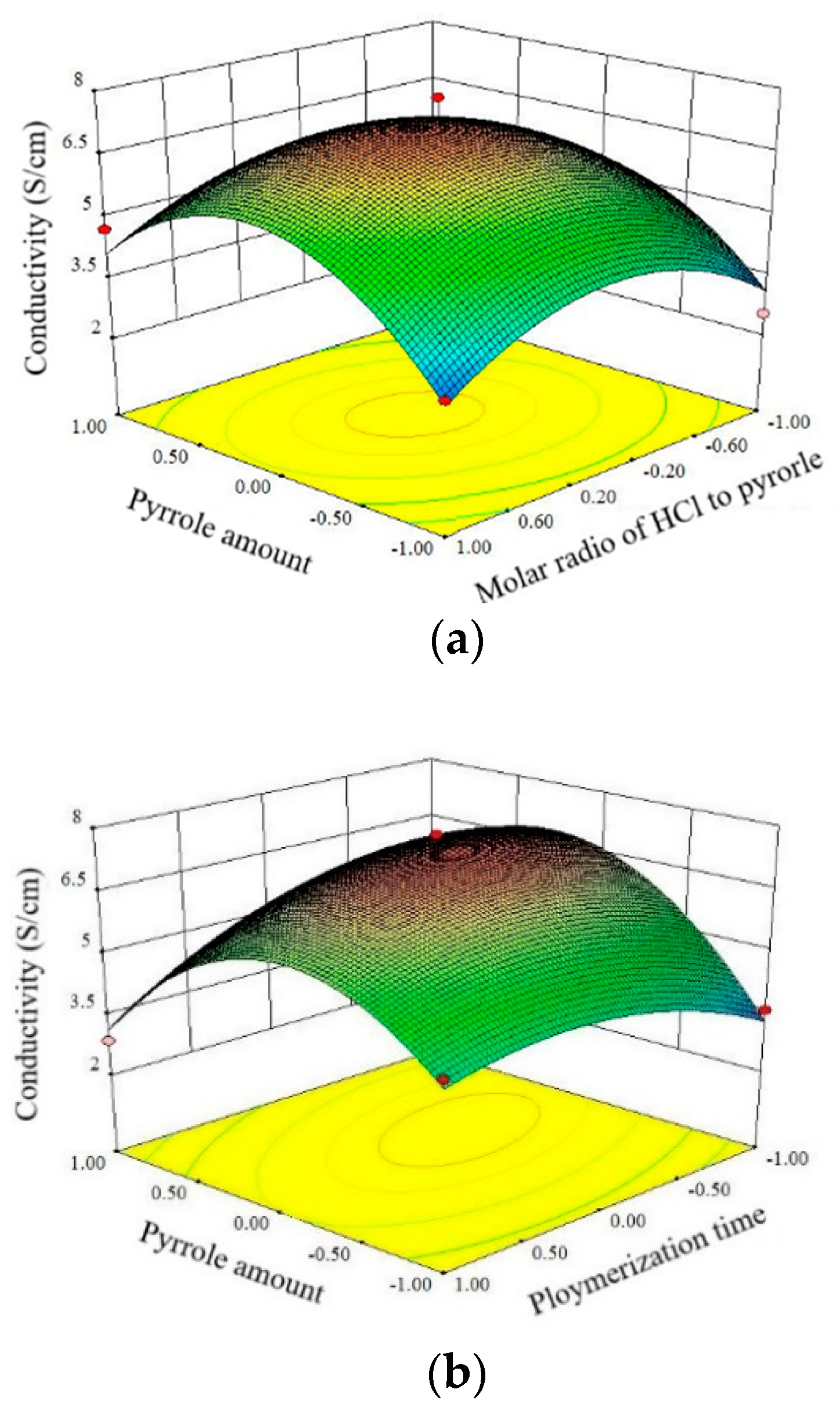
3.2. Response Surface Methodology Analysis
3.2.1. Effect of Pyrrole Amount and Molar Ratio HCl to Pyrrole on Conductivity
3.2.2. Effect of Pyrrole Amount and Polymerization Time on Conductivity
3.2.3. Effect of Molar Ratio of HCl to Pyrrole and Polymerization Time on Conductivity
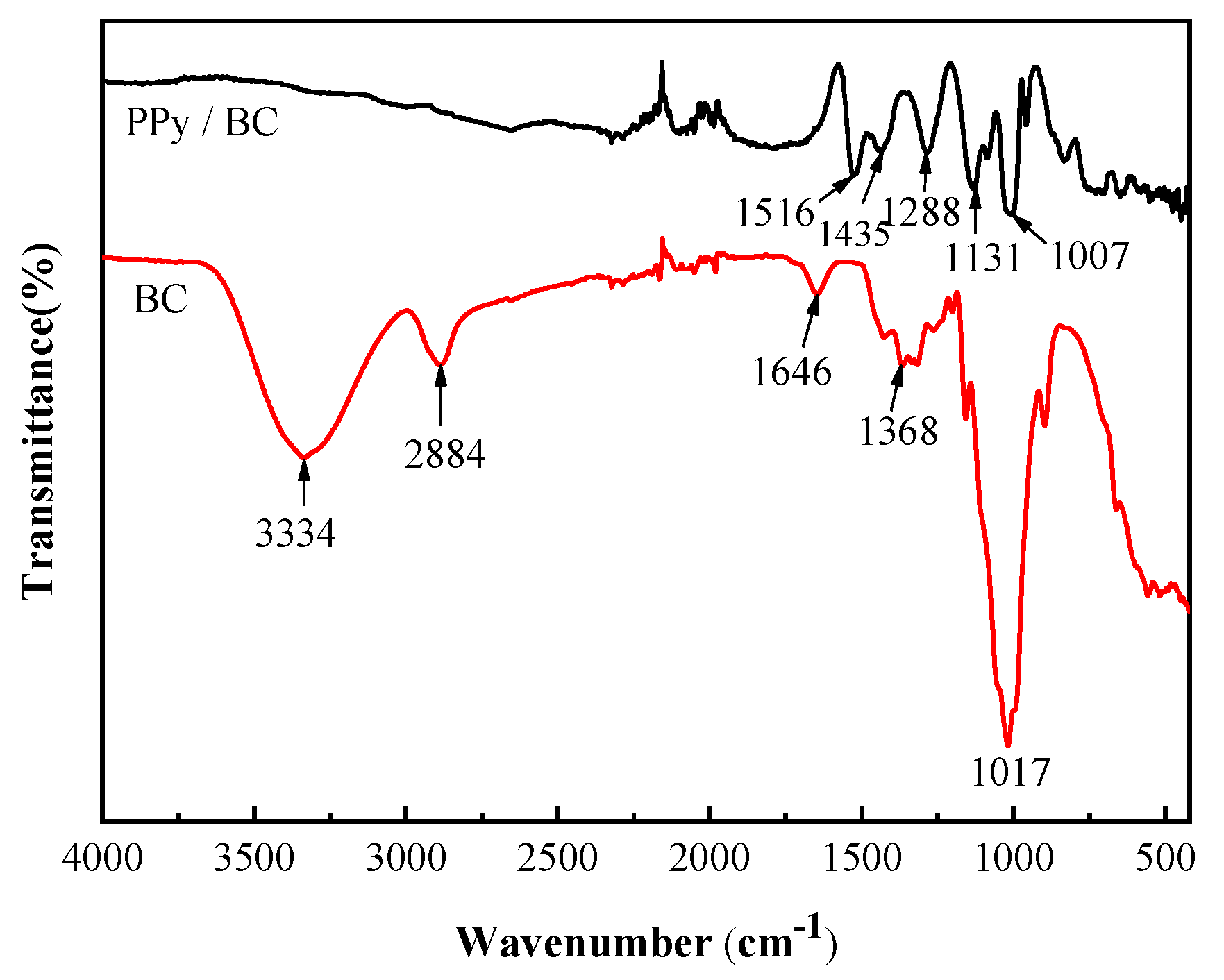
3.3. Fourier Transform Infrared Spectroscopy
3.4. Thermal Stability Analysis
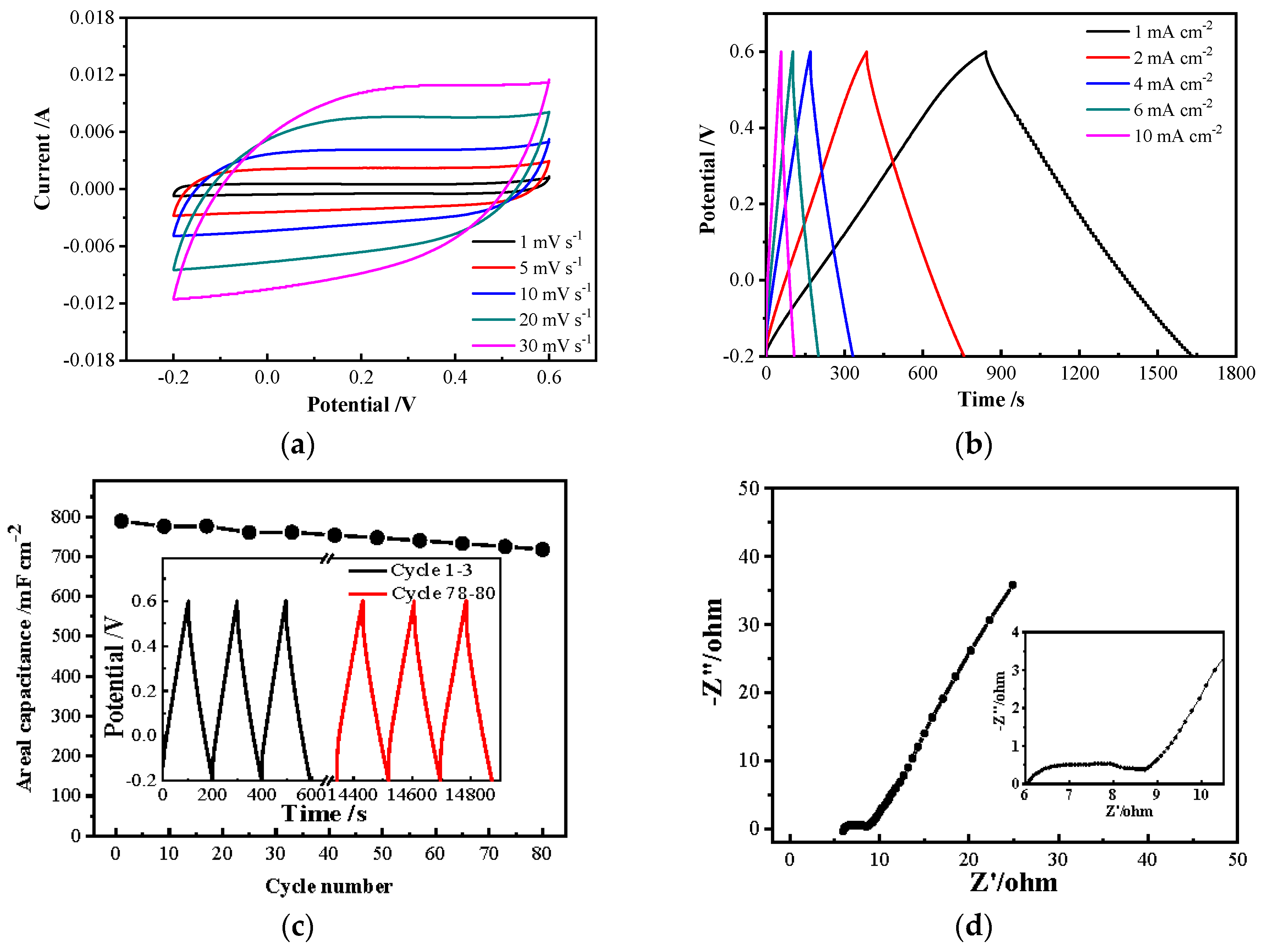
3.5. Electrochemical Performances
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart Electronic Yarns and Wearable Fabrics for Human Biomonitoring made by Carbon Nanotube Coating with Polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.C. Wearable Sensors for Human Activity Monitoring: A Review. IEEE Sens. J. 2015, 15, 1321–1330. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Dhanabalan, B.; Chen, X.; Ponraj, J.S.; Zhang, H. Hybrid carbon nanostructured fibers: Stepping stone for intelligent textile-based electronics. Nanoscale 2019, 11, 3046–3101. [Google Scholar] [CrossRef]
- Liang, H.; Guan, Q.; Zhu, Z.; Song, L.; Yao, H.; Lei, X.; Yu, S. Highly conductive and stretchable conductors fabricated from bacterial cellulose. NPG Asia Mater. 2012, 4, e19. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Ramya, R.; Sivasubramanian, R.; Sangaranarayanan, M.V. Conducting polymers-based electrochemical supercapacitors-Progress and prospects. Electrochim. Acta 2013, 101, 109–129. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, Y.; Fan, J. Novel electronic-ionic hybrid conductive composites for multifunctional flexible bioelectrode based on in situ synthesis of polydopamine on bacterial cellulose. ACS Appl. Mater. Interfaces 2018, 10, 22692–22702. [Google Scholar] [CrossRef]
- Yue, L.; Xie, Y.; Zheng, Y.; He, W.; Guo, S.; Sun, Y.; Zhang, T.; Liu, S. Sulfonated bacterial cellulose/polyaniline composite membrane for use as gel polymer electrolyte. Compos. Sci. Technol. 2017, 145, 122–131. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, G.; He, Y.; Fu, R.; Gu, Y.; Chen, S. Preparation, Characterization, and Electrochromic Properties of Nanocellulose-Based Polyaniline Nanocomposite Films. ACS Appl. Mater. Interfaces 2017, 9, 16426–16434. [Google Scholar] [CrossRef]
- Liew, S.Y.; Thielemans, W.; Walsh, D.A. Electrochemical Capacitance of Nanocomposite Polypyrrole/Cellulose Films. J. Phys. Chem. C 2010, 114, 17926–17933. [Google Scholar] [CrossRef]
- Nystrom, G.; Razaq, A.; Stromme, M.; Nyholm, L.; Mihranyan, A. Ultrafast All-Polymer Paper-Based Batteries. Nano Lett. 2009, 9, 3635–3639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, U.; Maity, S.; Chatterjee, A. Polypyrrole-silk electro-conductive composite fabric by in situ chemical polymerization. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Onar, N.; Akşit, A.C.; Ebeoglugil, M.F.; Birlik, I.; Celik, E.; Ozdemir, I. Structural, electrical, and electromagnetic properties of cotton fabrics coated with polyaniline and polypyrrole. J. Appl. Polym. Sci. 2009, 114, 2003–2010. [Google Scholar] [CrossRef]
- Babu, K.F.; Senthilkumar, R.; Noel, M.; Kulandainathan, M.A. Polypyrrole microstructure deposited by chemical and electrochemical methods on cotton fabrics. Synth. Met. 2009, 159, 1353–1358. [Google Scholar] [CrossRef]
- Molina, J.; Del Río, A.I.; Bonastre, J.; Cases, F. Electrochemical polymerisation of aniline on conducting textiles of polyester covered with polypyrrole/AQSA. Eur. Polym. J. 2009, 45, 1302–1315. [Google Scholar] [CrossRef]
- Kaynak, A.; Najar, S.S.; Foitzik, R.C. Conducting nylon, cotton and wool yarns by continuous vapor polymerization of pyrrole. Synth. Met. 2008, 158, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.; Rambo, C.R.; Recouvreux, D.O.S.; Porto, L.M.; Barra, G.M.O. Chemical in situ polymerization of polypyrrole on bacterial cellulose nanofibers. Synth. Met. 2011, 161, 106–111. [Google Scholar] [CrossRef]
- Lay, M.G.I.T. High electrical and electrochemical properties in bacterial cellulose/polypyrrole membranes. Eur. Polym. J. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M., Jr. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Hosseini, H.; Kokabi, M.; Mousavi, S.M. Conductive bacterial cellulose/multiwall carbon nanotubes nanocomposite aerogel as a potentially flexible lightweight strain sensor. Carbohydr. Polym. 2018, 201, 228–235. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Choi, C.N.; Song, H.J.; Kim, M.J.; Chang, M.H.; Kim, S.J. Properties of bacterial cellulose produced in a pilot-scale spherical type bubble column bioreactor. Korean J. Chem. Eng. 2009, 26, 136–140. [Google Scholar] [CrossRef]
- Li, H.X.; Kim, S.; Lee, Y.; Kee, C.D.; Oh, I.K. Determination of the stoichiometry and critical oxygen tension in the production culture of bacterial cellulose using saccharified food wastes. Korean J. Chem. Eng. 2011, 28, 2306–2311. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, L.; Bai, Z.; Liang, G.; Liu, L.; Fang, D.; Xu, W. Conductive polypyrrole–bacterial cellulose nanocomposite membranes as flexible supercapacitor electrode. Org. Electron. 2013, 14, 3331–3338. [Google Scholar] [CrossRef]
- Huanhuan Wang, L.B.P.Z. Core—Sheath structured bacterial cellulose/polypyrrole nanocomposites with excellent conductivity as supercapacitors. J. Mater. Chem. A 2013, 1, 578–584. [Google Scholar] [CrossRef]
- Muller, D.; Rambo, C.R.; Porto, L.M.; Schreiner, W.H.; Barra, G.M.O. Structure and properties of polypyrrole/bacterial cellulose nanocomposites. Carbohydr. Polym. 2013, 94, 655–662. [Google Scholar] [CrossRef]
- Tang, L.; Han, J.; Jiang, Z.; Chen, S.; Wang, H. Flexible conductive polypyrrole nanocomposite membranes based on bacterial cellulose with amphiphobicity. Carbohydr. Polym. 2015, 117, 230–235. [Google Scholar] [CrossRef]
- Mengying, L.; Mufang, L.; Yuqin, L.; Kangqi, C.; Ke, L.; Qiongzhen, L.; Yuedan, W.; Zhentan, L.; Xue, L.; Dong, W. In-situ polymerization of PPy/cellulose composite sponge with high elasticity and conductivity for the application of pressure sensor. Compos. Commun. 2017, 6, 68–72. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Advancement in conductive cotton fabrics through in situ polymerization of polypyrrole-nanocellulose composites. Carbohydr. Polym. 2016, 151, 96–102. [Google Scholar] [CrossRef]
- Babu, K.F.; Dhandapani, P.; Maruthamuthu, S.; Kulandainathan, M.A. One pot synthesis of polypyrrole silver nanocomposite on cotton fabrics for multifunctional property. Carbohydr. Polym. 2012, 90, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Jradi, K.; Bideau, B.; Chabot, B.; Daneault, C. Characterization of conductive composite films based on TEMPO-oxidized cellulose nanofibers and polypyrrole. J. Mater. Sci. 2012, 47, 3752–3762. [Google Scholar] [CrossRef]
- Nystrom, G.; Mihranyan, A.; Razaq, A.; Lindstrom, T.; Nyholm, L.; Stromme, M. A Nanocellulose Polypyrrole Composite Based on Microfibrillated Cellulose from Wood. J. Phys. Chem. B 2010, 114, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, H.; Zabihzadeh, S.M.; Ghorbani, M. The application of response surface methodology on the synthesis of conductive polyaniline/cellulosic fiber nanocomposites. Carbohydr. Polym. 2018, 194, 384–394. [Google Scholar] [CrossRef]
- Parra-Campos, A.; Eduardo Ordonez-Santos, L. Natural pigment extraction optimization from coffee exocarp and its use as a natural dye in French meringue. Food Chem. 2019, 285, 59–66. [Google Scholar] [CrossRef]
- Celik, F.; Aslani, M.A.A.; Can, S.S. Study of the Bioaccumulation of UO22+ onto the Green Microalgae Botryococcus braunii Using Response Surface Methodology. Turk. J. Fish. Aquat. Sci. 2019, 19, 593–604. [Google Scholar] [CrossRef]
- Wu, W.; He, L.; Liang, Y.; Yue, L.; Peng, W.; Jin, G.; Ma, M. Preparation process optimization of pig bone collagen peptide-calcium chelate using response surface methodology and its structural characterization and stability analysis. Food Chem. 2019, 284, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Meini, M.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Monforte, A.R.; Oliveira, C.; Martins, S.I.F.S.; Silva Ferreira, A.C. Response surface methodology: A tool to minimize aldehydes formation and oxygen consumption in wine model system. Food Chem. 2019, 283, 559–565. [Google Scholar] [CrossRef]
- Cervera, A.; Ezra, O.; Kuperman, A.; Peretz, M.M. Modeling and Control of Magnetic Actuation Systems Based on Sensorless Displacement Information. IEEE Trans. Ind. Electron. 2019, 66, 4849–4859. [Google Scholar] [CrossRef]
- Silva Coelho, T.L.; Silva Braga, F.M.; Caldas Silva, N.M.; Dantas, C.; Lopes Junior, C.A.; Alves De Sousa, S.A.; Virira, E.C. Optimization of the protein extraction method of goat meat using factorial design and response surface methodology. Food Chem. 2019, 281, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, D.; Yang, J.; Zhang, B.; Zhang, X.; Yang, G.; Wang, M.; Shen, Y. Freestanding bacterial cellulose–polypyrrole nanofibres paper electrodes for advanced energy storage devices. Nano Energy 2014, 9, 309–317. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, P.; Xie, J.; Yang, Z.; Huang, Y.; Hu, J.; Wan, Y.; Xu, Y. Uniformly Dispersed Freestanding Carbon Nanofiber/Graphene Electrodes Made by a Scalable Biological Method for High-Performance Flexible Supercapacitors. Adv. Funct. Mater. 2018, 28, 1803075. [Google Scholar] [CrossRef]
- Bjorklund, R.B. Kinetics of pyrrole polymerisation in aqueous iron chloride solution. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1987, 83, 1507. [Google Scholar] [CrossRef]
- Patil, B.H.; Bulakhe, R.N.; Lokhande, C.D. Supercapacitive performance of chemically synthesized polypyrrole thin films: Effect of monomer to oxidant ratio. J. Mater. Sci. Mater. Electron. 2014, 25, 2188–2198. [Google Scholar] [CrossRef]
- Sabouraud, G.; Sadki, S.; Brodie, N. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, Z.; Liu, L.; Wang, H. Flexible Electrically Conductive Nanocomposite Membrane Based on Bacterial Cellulose and Polyaniline. J. Phys. Chem. B 2011, 115, 8453–8457. [Google Scholar] [CrossRef] [PubMed]
- Marins, J.A.; Soares, B.G.; Dahmouche, K.; Ribeiro, S.J.L.; Barud, H.; Bonemer, D. Structure and properties of conducting bacterial cellulose-polyaniline nanocomposites. Cellulose 2011, 18, 1285–1294. [Google Scholar] [CrossRef]
- Keyvanloo, K.; Towfighi, J.; Sadrameli, S.M.; Mohamadalizadeh, A. Investigating the effect of key factors, their interactions and optimization of naphtha steam cracking by statistical design of experiments. J. Anal. Appl. Pyrol. 2010, 87, 224–230. [Google Scholar] [CrossRef]
- Mondal, S.K.; Saha, A.K.; Sinha, A. Removal of ciprofloxacin using modified advanced oxidation processes: Kinetics, pathways and process optimization. J. Clean. Prod. 2018, 171, 1203–1214. [Google Scholar] [CrossRef]
- Khataee, A.R.; Zarei, M.; Moradkhannejhad, L. Application of response surface methodology for optimization of azo dye removal by oxalate catalyzed photo electro-Fenton process using carbon nanotube-PTFE cathode. Desalination 2010, 258, 112–119. [Google Scholar] [CrossRef]
- Kasiri, M.B.; Aleboyeh, H.; Aleboyeh, A. Modeling and Optimization of Heterogeneous Photo-Fenton Process with Response Surface Methodology and Artificial Neural Networks. Environ. Sci. Technol. 2008, 42, 7970–7975. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, M.; Fazeli, M. Improved biodegradability of hardly-decomposable wastewaters from petrochemical industry through photo-Fenton method and determination of optimum operational conditions by response surface methodology. J. Biol. Eng. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, Q.; Gao, D.; Shi, H. Degradation of an Anthraquinone Dye by Ozone/Fenton: Response Surface Approach and Degradation Pathway. Ozone Sci. Eng. 2017, 39, 219–232. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Das, P. Optimization of crystal violet dye removal using novel soil-silver nanocomposite as nanoadsorbent using response surface methodology. J. Environ. Chem. Eng. 2014, 2, 708–714. [Google Scholar] [CrossRef]
- Amini, M.; Younesi, H.; Bahramifar, N.; Lorestani, A.A.Z.; Ghorbani, F.; Daneshi, A.; Sharifzadeh, M. Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. J. Hazard. Mater. 2008, 154, 694–702. [Google Scholar] [CrossRef]
- Hamsaveni, D.R.; Prapulla, S.G.; Divakar, S. Response surface methodological approach for the synthesis of isobutyl isobutyrate. Process Biochem. 2001, 36, 1103–1109. [Google Scholar] [CrossRef]
- Ding, C.; Qian, X.; Yu, G.; An, X. Dopant effect and characterization of polypyrrole-cellulose composites prepared by in situ polymerization process. Cellulose 2010, 17, 1067–1077. [Google Scholar] [CrossRef]
- Razak, S.I.A.; Rahman, W.A.W.A.; Sharif, N.F.A.; Yahya, M.Y. Simultaneous numerical optimization of the mechanical and electrical properties of polyaniline coated kenaf fiber using response surface methodology: Nanostructured polyaniline on natural fiber. Compos. Interface 2012, 19, 411–424. [Google Scholar] [CrossRef]
- Maity, S.; Chatterjee, A. Preparation and characterization of electro-conductive rotor yarn by in situ chemical polymerization of pyrrole. Fiber. Polym. 2013, 14, 1407–1413. [Google Scholar] [CrossRef]
- Maiti, S.; Das, D.; Sen, K. Studies on Electro-Conductive Yarns Prepared by In Situ Chemical and Electrochemical Polymerization of Pyrrole. J. Appl. Polym. Sci. 2012, 123, 455–462. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Shi, Z.; Qin, H.; Wang, L.; Pei, X.; Jin, J. Spontaneous Growth of Free-Standing Polypyrrole Films at an Air/Ionic Liquid Interface. Langmuir 2010, 26, 14405–14408. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yao, B.; Hu, B.; Huo, K.; Chen, W.; Zhou, J. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci. 2013, 6, 470–476. [Google Scholar] [CrossRef]
- Bose, S.; Kim, N.H.; Kuila, T.; Lau, K.; Lee, J.H. Electrochemical performance of a graphene-polypyrrole nanocomposite as a supercapacitor electrode. Nanotechnology 2011, 22. [Google Scholar] [CrossRef]
- Cucchi, I.; Boschi, A.; Arosio, C.; Bertini, F.; Freddi, G.; Catellani, M. Bio-based conductive composites: Preparation and properties of polypyrrole (PPy)-coated silk fabrics. Synth. Met. 2009, 159, 246–253. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Yuan, L.; Xiao, X.; Ding, T.; Zhong, J.; Zhang, X.; Shen, Y.; Hu, B.; Huang, Y.; Zhou, J.; Wang, Z.L. Paper-Based Supercapacitors for Self-Powered Nanosystems. Angew. Chem. Int. Ed. 2012, 51, 4934–4938. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and Polypyrrole Pseudocapacitor Electrodes with Excellent Cycling Stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Song, M.K.; Park, Y.J.; Kim, J.M.; Liu, M.; Wang, Z.L. Fiber Supercapacitors Made of Nanowire-Fiber Hybrid Structures for Wearable/Flexible Energy Storage. Angew. Chem. Int. Ed. 2011, 50, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- In, H.J.; Kumar, S.; Shao-Horn, Y.; Barbastathis, G. Origami fabrication of nanostructured, three-dimensional devices: Electrochemical capacitors with carbon electrodes. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Stilwell, D.E.; Su-Moon, P. Electrochemistry of conductive polymers. II. Electrochemical studies on growth properties of polyaniline. J. Electrochem. Soc. 1988, 135, 2254–2262. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Chen, L.; Hu, C.; Fan, S. Highly Flexible and All-Solid-State Paper like Polymer Supercapacitors. Nano Lett. 2010, 10, 4025–4031. [Google Scholar] [CrossRef] [PubMed]




| Factors | Labels | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Pyrrole amount/mL | X1 | 0.1 | 0.3 | 0.5 |
| Molar ratio of HCl to pyrrole | X2 | 5 | 20 | 35 |
| Polymerization time/min | X3 | 60 | 180 | 300 |
| Run | X1 | X2 | X3 | Y Conductivity/(S cm−1) | Error/(S cm−1) | |
|---|---|---|---|---|---|---|
| Experimental | Predicted | |||||
| 1 | 0 | 1 | −1 | 4.78 | 5.17 | −0.39 |
| 2 | 0 | 0 | 0 | 6.93 | 7.14 | −0.21 |
| 3 | 0 | 0 | 0 | 7.03 | 7.14 | −0.11 |
| 4 | 1 | −1 | 0 | 3.08 | 3.20 | −0.12 |
| 5 | −1 | 0 | −1 | 3.54 | 3.27 | 0.27 |
| 6 | 0 | −1 | −1 | 5.48 | 5.16 | 0.32 |
| 7 | −1 | 0 | 1 | 4.47 | 4.27 | 0.20 |
| 8 | 1 | 1 | 0 | 4.71 | 4.12 | 0.59 |
| 9 | 0 | −1 | 1 | 4.41 | 4.02 | 0.39 |
| 10 | 0 | 0 | 0 | 7.08 | 7.14 | −0.06 |
| 11 | −1 | −1 | 0 | 2.52 | 3.11 | −0.59 |
| 12 | 0 | 0 | 0 | 6.87 | 7.14 | −0.27 |
| 13 | 0 | 0 | 0 | 7.81 | 7.14 | 0.67 |
| 14 | 1 | 0 | 1 | 2.86 | 3.13 | −0.27 |
| 15 | 1 | 0 | −1 | 5.35 | 5.55 | −0.20 |
| 16 | −1 | 1 | 0 | 3.18 | 3.06 | 0.12 |
| 17 | 0 | 1 | 1 | 4.56 | 4.88 | −0.32 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 42.68 | 9 | 4.74 | 16.23 | 0.0007 | ** |
| 0.66 | 1 | 0.66 | 2.24 | 0.1779 | ||
| 0.38 | 1 | 0.38 | 1.3 | 0.2925 | ||
| 1.02 | 1 | 1.02 | 3.47 | 0.1046 | ||
| 0.24 | 1 | 0.24 | 0.81 | 0.3994 | ||
| 2.92 | 1 | 2.92 | 10.01 | 0.0159 | * | |
| 0.18 | 1 | 0.18 | 0.62 | 0.4575 | ||
| 21.54 | 1 | 21.54 | 73.73 | <0.0001 | ** | |
| 9.59 | 1 | 9.59 | 32.83 | 0.0007 | ** | |
| 2.88 | 1 | 2.88 | 9.86 | 0.0164 | * | |
| Residual | 2.05 | 7 | 0.29 | |||
| Lack of Fit | 1.46 | 3 | 0.49 | 3.36 | 0.1363 | |
| Pure Error | 0.58 | 4 | 0.15 | |||
| Cor Total | 44.73 | 16 | ||||
| = 0.9543 = 0.8955 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, F.; Dong, L.; Li, Z.; Chen, L.; He, X.; Gong, J.; Zhang, J.; Li, Q. Design and Optimization of Flexible Polypyrrole/Bacterial Cellulose Conductive Nanocomposites Using Response Surface Methodology. Polymers 2019, 11, 960. https://doi.org/10.3390/polym11060960
Chen Y, Wang F, Dong L, Li Z, Chen L, He X, Gong J, Zhang J, Li Q. Design and Optimization of Flexible Polypyrrole/Bacterial Cellulose Conductive Nanocomposites Using Response Surface Methodology. Polymers. 2019; 11(6):960. https://doi.org/10.3390/polym11060960
Chicago/Turabian StyleChen, Yasong, Fuying Wang, Lipan Dong, Zheng Li, Li Chen, Xinhai He, Jixian Gong, Jianfei Zhang, and Qiujin Li. 2019. "Design and Optimization of Flexible Polypyrrole/Bacterial Cellulose Conductive Nanocomposites Using Response Surface Methodology" Polymers 11, no. 6: 960. https://doi.org/10.3390/polym11060960





