One-Pot Method of Synthesizing TEMPO-Oxidized Bacterial Cellulose Nanofibers Using Immobilized TEMPO for Skincare Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TEMPO Oxidation of BC
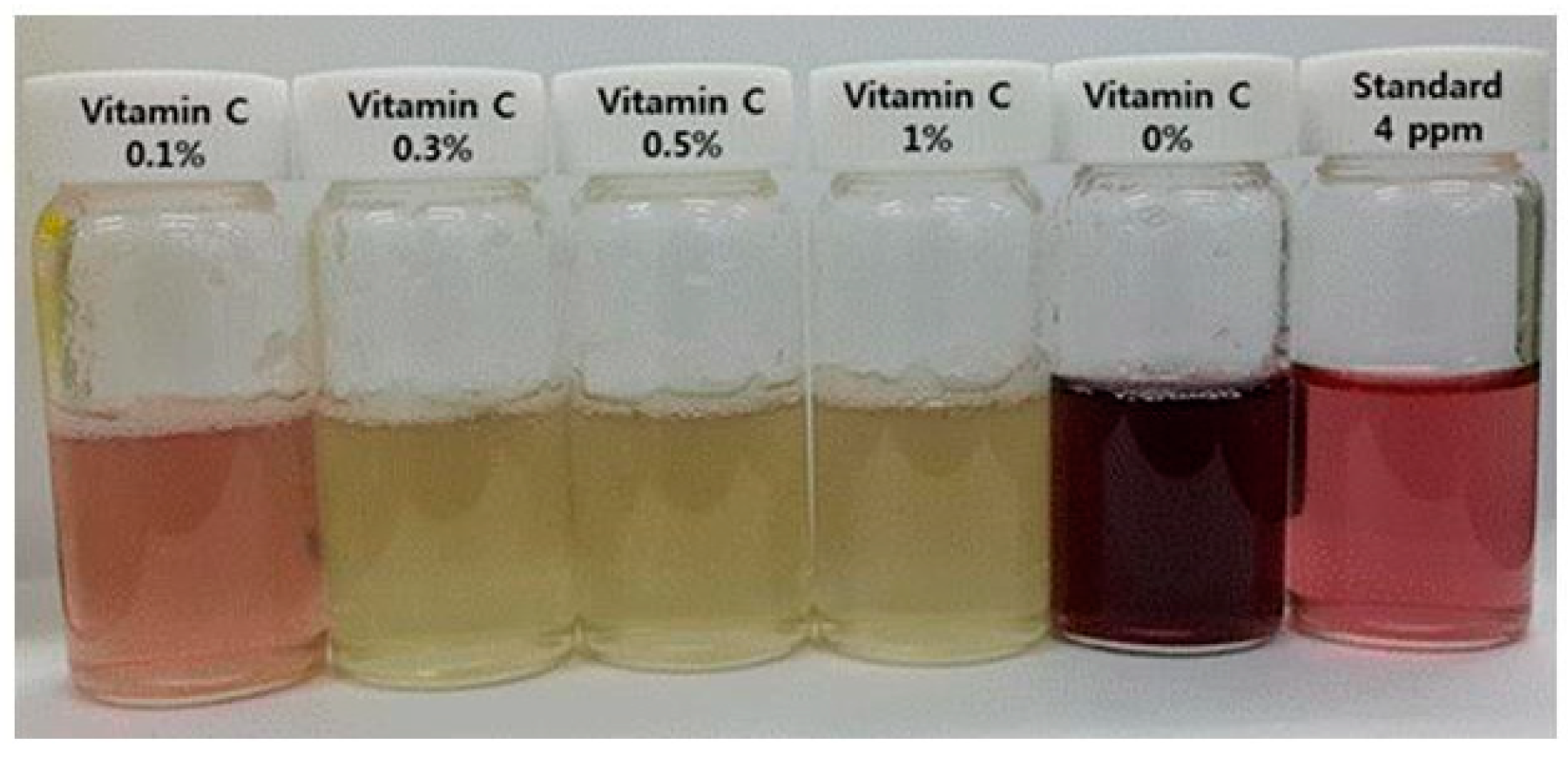
2.3. Detection of the Sodium Hypochlorite Content
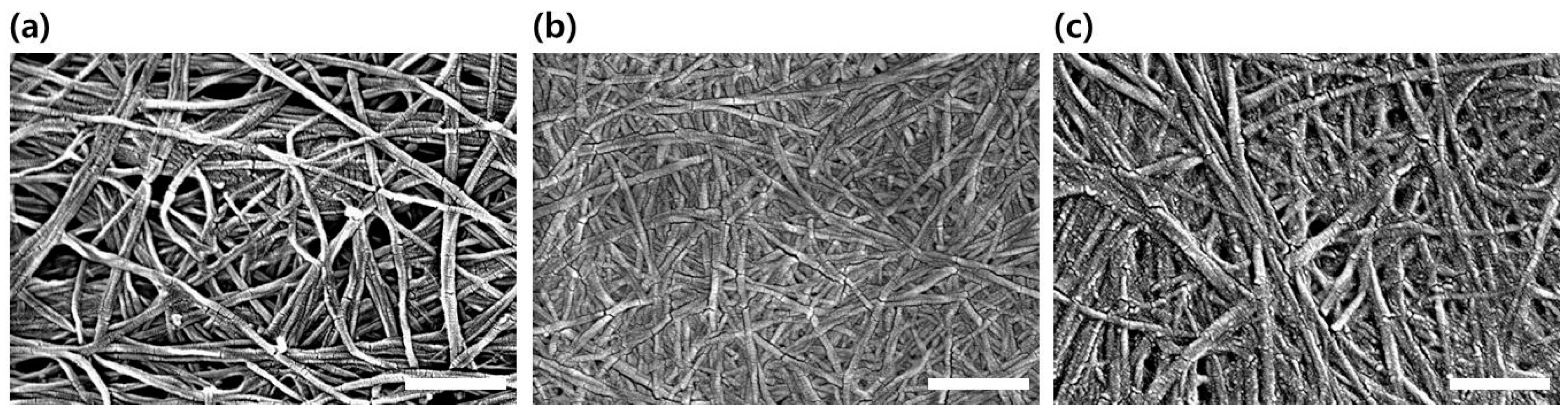
2.4. Characterization by Scanning Electron Microscopy
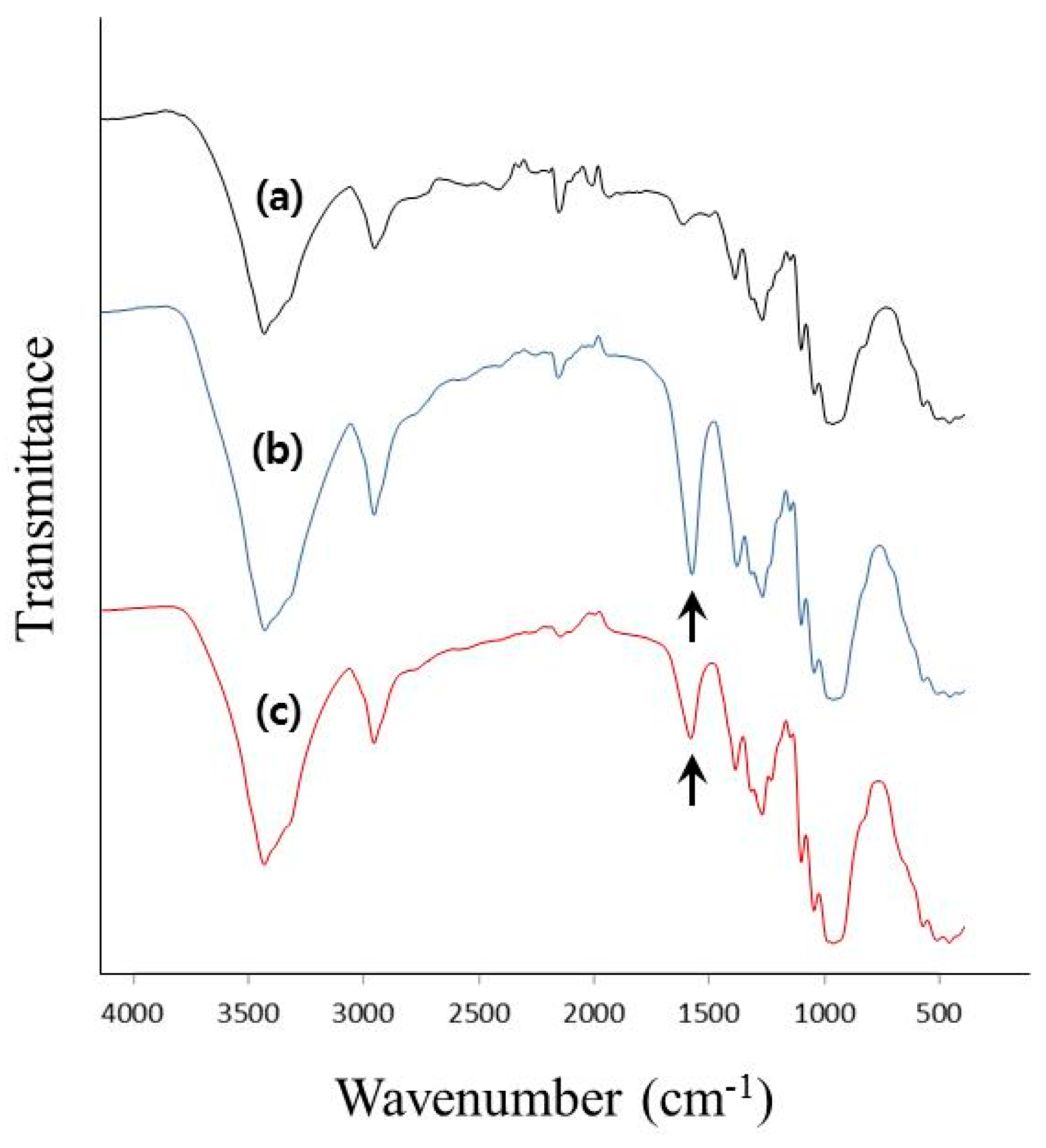
2.5. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
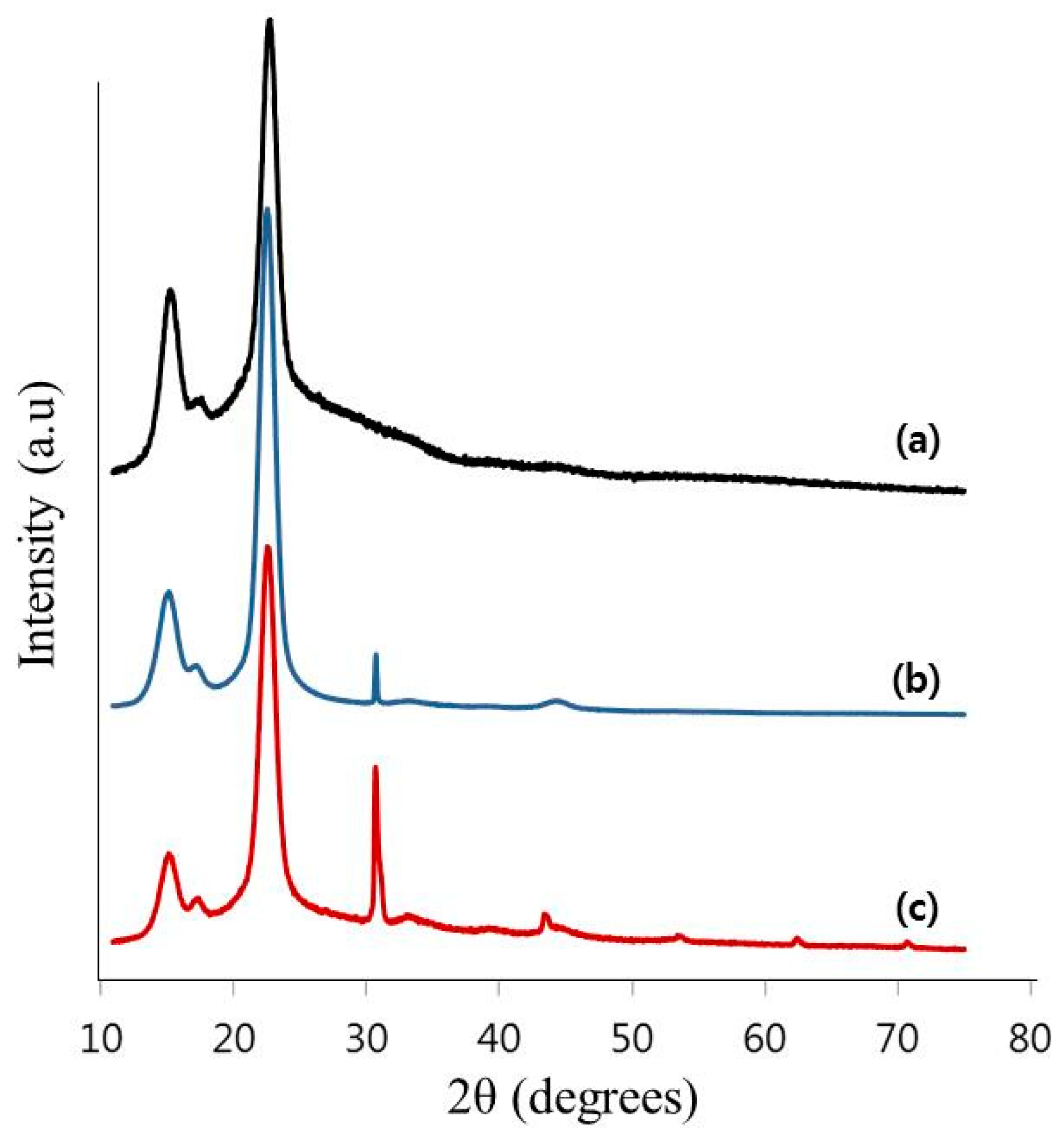
2.6. X-ray Diffractometer (XRD) Analysis
2.7. Contact Angle Measurements
3. Results and Discussion
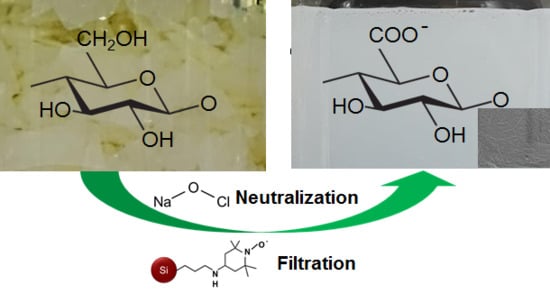
3.1. One-Pot Synthesis of TOCNs
3.2. Characterization of the TOCNs Obtained via One-Pot Synthesis
3.3. Properties of O-TOCNs on the Surface of Skin
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, M.P.; Selvakumar, R.; Kumar, P.S.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef] [Green Version]
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential Applications of Nanocellulose-Containing Materials in the Biomedical Field. Materials 2017, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Tabuchi, M.; Watanabe, K.; Yano, H.; Morinaga, Y.; Yoshinaga, F. Degree of Polymerization of Cellulose from Acetobacter xylinum BPR2001 Decreased by Cellulase Produced by the Strain. Biosci. Biotechnol. Biochem. 1997, 61, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Naritomi, T.; Kouda, T.; Yano, H.; Yoshinaga, F. Effect of lactate on bacterial cellulose production from fructose in continuous culture. J. Ferment. Bioeng. 1998, 85, 89–95. [Google Scholar] [CrossRef]
- Lee, J.W.; Deng, F.; Yeomans, W.G.; Allen, A.L.; Gross, R.A.; Kaplan, D.L. Direct Incorporation of Glucosamine and N-Acetylglucosamine into Exopolymers by Gluconacetobacter xylinus (5Acetobacter xylinum) ATCC 10245: Production of Chitosan-Cellulose and Chitin-Cellulose Exopolymers. Appl. Environ. Microbiol. 2001, 67, 3970–3975. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Q.; Cao, J.; Liu, Y.; Li, J.; Zhang, J.; Luo, S.; Yu, H. Revealing the structures of cellulose nanofiber bundles obtained by mechanical nanofibrillation via TEM observation. Carbohydr. Polym. 2015, 117, 950–956. [Google Scholar] [CrossRef]
- Kondo, T.; Kose, R.; Naito, H.; Kasai, W. Aqueous counter collision using paired water jets as a novel means of preparing bio-nanofibers. Carbohydr. Polymer. 2014, 112, 284–290. [Google Scholar] [CrossRef]
- Spaic, M.; Small, D.P.; Cook, J.R.; Wan, W. Characterization of Anionic and Cationic Functionalized Bacterial Cellulose Nanofibres for Controlled Release Applications. Cellulose 2014, 21, 1529–1540. [Google Scholar] [CrossRef]
- Jun, S.-H.; Lee, S.-H.; Kim, S.; Park, S.-G.; Lee, C.-K.; Kang, N.-K. Physical properties of TEMPO-oxidized bacterial cellulose nanofibers on the skin surface. Cellulose 2017, 24, 5267–5274. [Google Scholar] [CrossRef]
- Biotage® PS-TEMPO. Available online: https://www.biotage.com/product-page/biotage-ps-tempo (accessed on 13 June 2019).
- Gilhespy, M.; Loka, M.; Baucherel, X. Polymer-supported nitroxyl radical catalyst for selective aerobic oxidation of primary alcohols to aldehydes. Chem. Commun. 2005, 8, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Schwartz, A.K.; Garrell, R.L. Simple Preparation and Application of TEMPO-Coated Fe3O4 Superparamagnetic Nanoparticles for Selective Oxidation of Alcohols. Chem. Eur. J. 2010, 16, 12718–12726. [Google Scholar] [CrossRef] [PubMed]
- Michaud, A.; Gingras, G.; Morin, M.; Béland, F.; Ciriminna, R.; Avnir, D.; Pagliaro, M. SiliaCat TEMPO: An Effective and Useful Oxidizing Catalyst. Org. Process Res. Dev. 2007, 11, 766–768. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pandarus, V.; Béland, F.; Pagliaro, M. Sol–gel Entrapped Nitroxyl Radicals: Catalysts of Broad Scope. ChemCatChem 2018, 10, 1731–1738. [Google Scholar] [CrossRef]
- Patankar, S.C.; Renneckar, S. Greener synthesis of nanofibrillated cellulose using magnetically separable TEMPO nanocatalyst. Green Chem. 2017, 19, 4792–4797. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, N.F.; Feitosa, J.P.; Da, G.F.; Morais, J.P.; Andrade, F.K.; de Souza Filho, M.S.M.; Rosa, M.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Tsuji, M.; Morimoto, M.; Saimoto, H.; Yano, H. Synthesis of silver nanoparticles templated by TEMPO-mediated oxidized bacterial cellulose nanofibers. Biomacromolecules 2009, 10, 2714–2717. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Zheng, Y.; Lin, Q.; Song, W.; Qiao, K.; Liu, S. Selective oxidation of bacterial cellulose by NO2–HNO3. RSC Adv. 2014, 4, 1630–1639. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, S.-H.; Park, S.-G.; Kang, N.-G. One-Pot Method of Synthesizing TEMPO-Oxidized Bacterial Cellulose Nanofibers Using Immobilized TEMPO for Skincare Applications. Polymers 2019, 11, 1044. https://doi.org/10.3390/polym11061044
Jun S-H, Park S-G, Kang N-G. One-Pot Method of Synthesizing TEMPO-Oxidized Bacterial Cellulose Nanofibers Using Immobilized TEMPO for Skincare Applications. Polymers. 2019; 11(6):1044. https://doi.org/10.3390/polym11061044
Chicago/Turabian StyleJun, Seung-Hyun, Sun-Gyoo Park, and Nae-Gyu Kang. 2019. "One-Pot Method of Synthesizing TEMPO-Oxidized Bacterial Cellulose Nanofibers Using Immobilized TEMPO for Skincare Applications" Polymers 11, no. 6: 1044. https://doi.org/10.3390/polym11061044