Preparation and Characterization of Microcellulose and Nanocellulose Fibers from Artemisia Vulgaris Bast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of Chemical Composition of the Bast
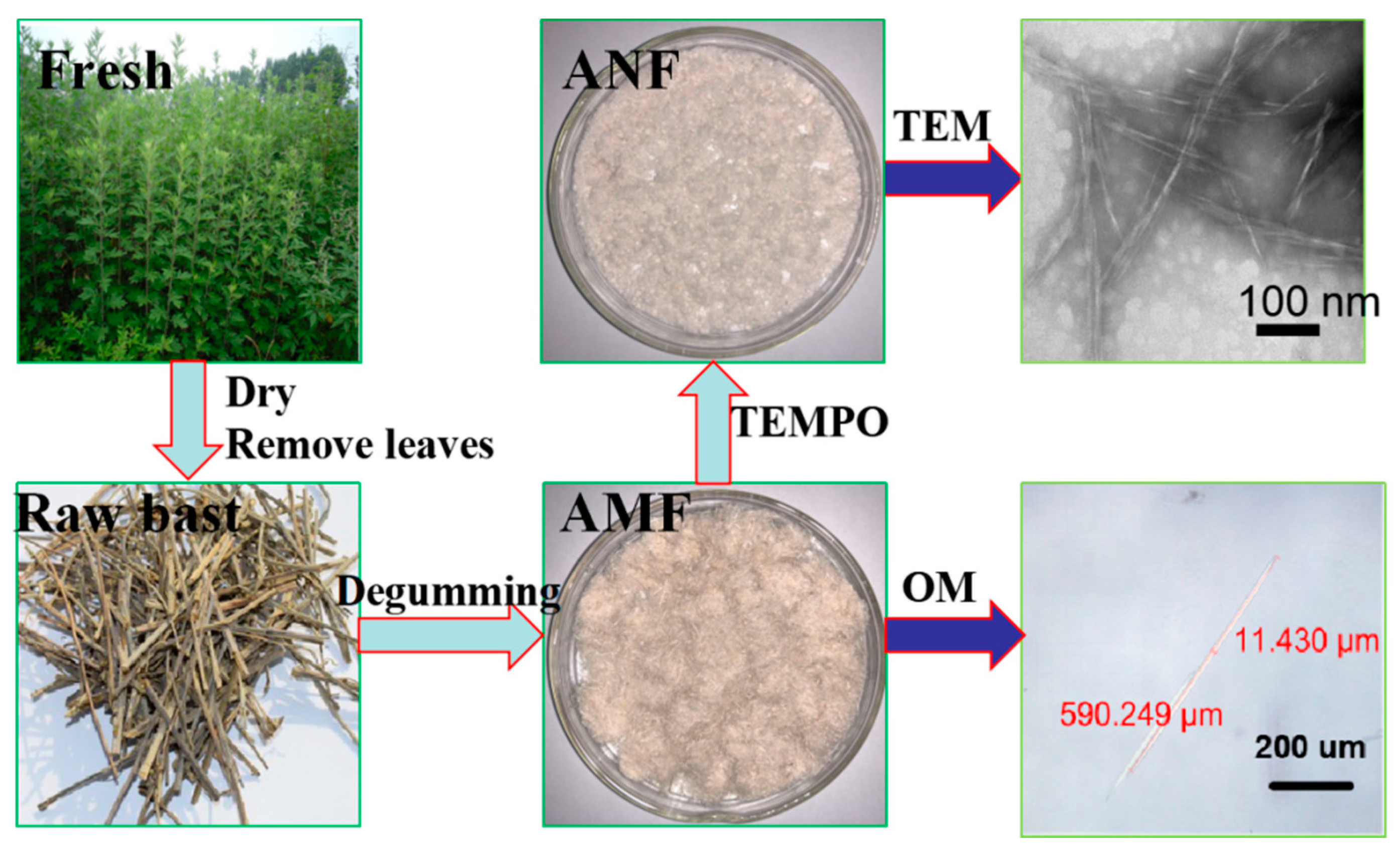
2.2.2. Preparation of Artemisia Vulgaris Bast Cellulose Fiber (Microcellulose Fiber)
2.2.3. Preparation of Artemisia Vulgaris Bast Nanocellulose Fiber
2.2.4. Bacteriostatic Test and Analysis
2.2.5. Optical Microscopy (OM) Analysis
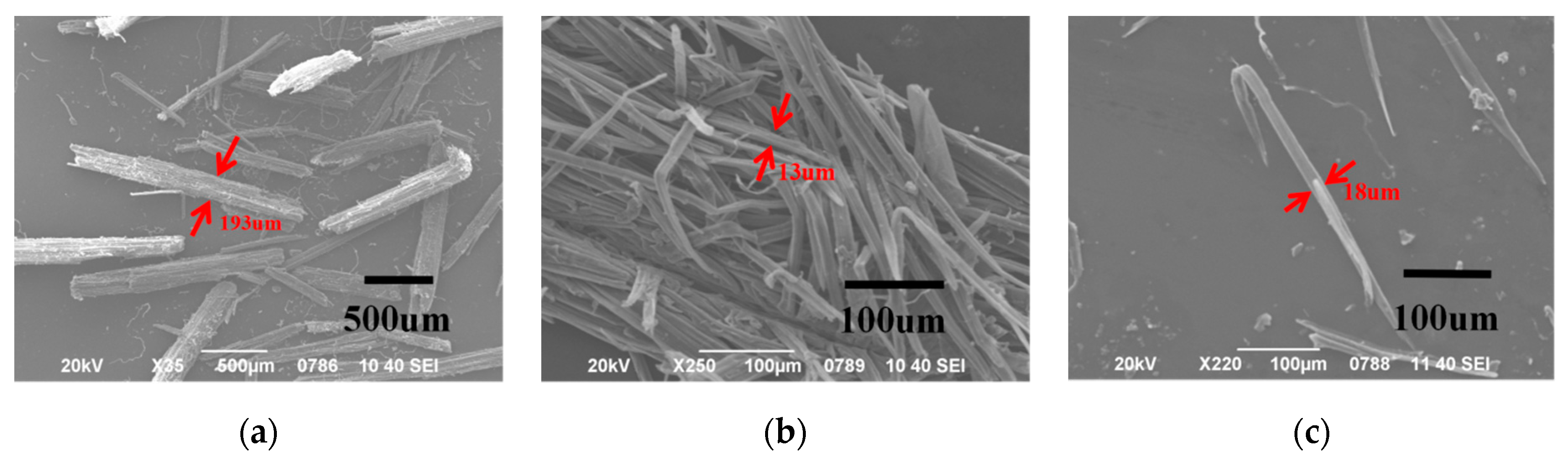
2.2.6. SEM Analysis
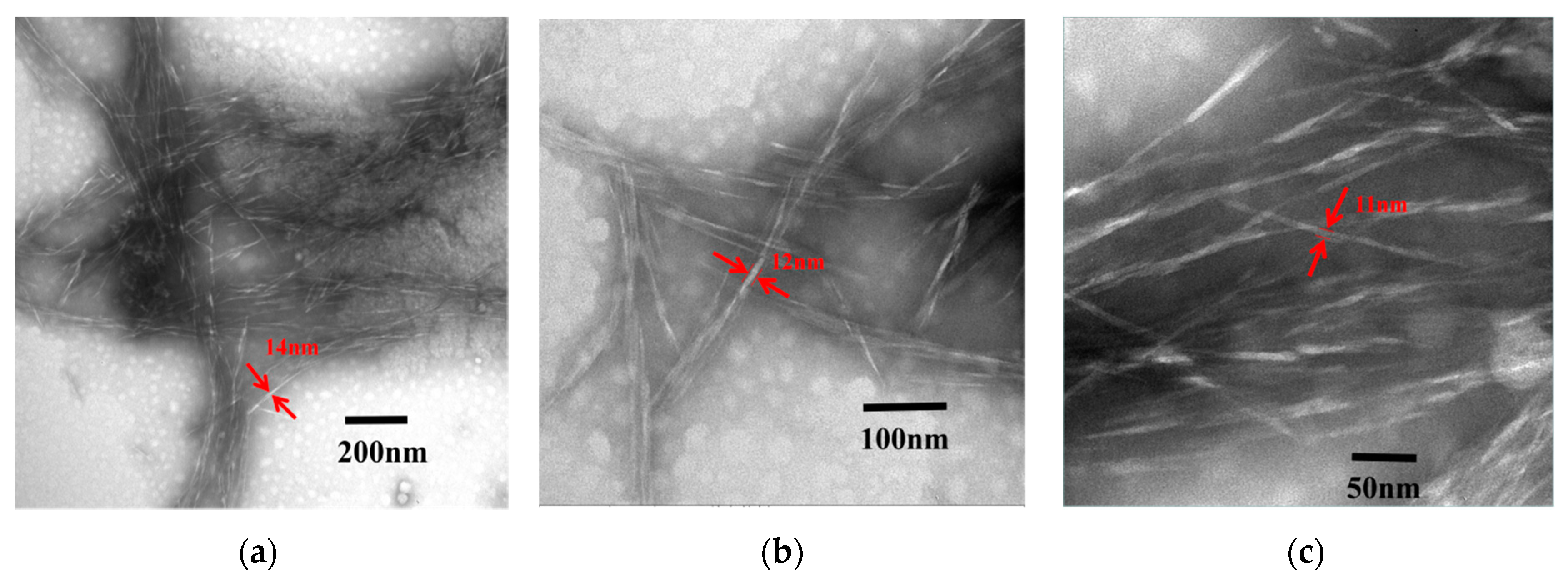
2.2.7. TEM Analysis
2.2.8. FTIR Analysis
2.2.9. XRD Analysis
2.2.10. TG Analysis
3. Results and Discussion
3.1. Chemical Compositions Analysis
3.2. Morphology of the Samples and Powder/Fiber Size Analysis
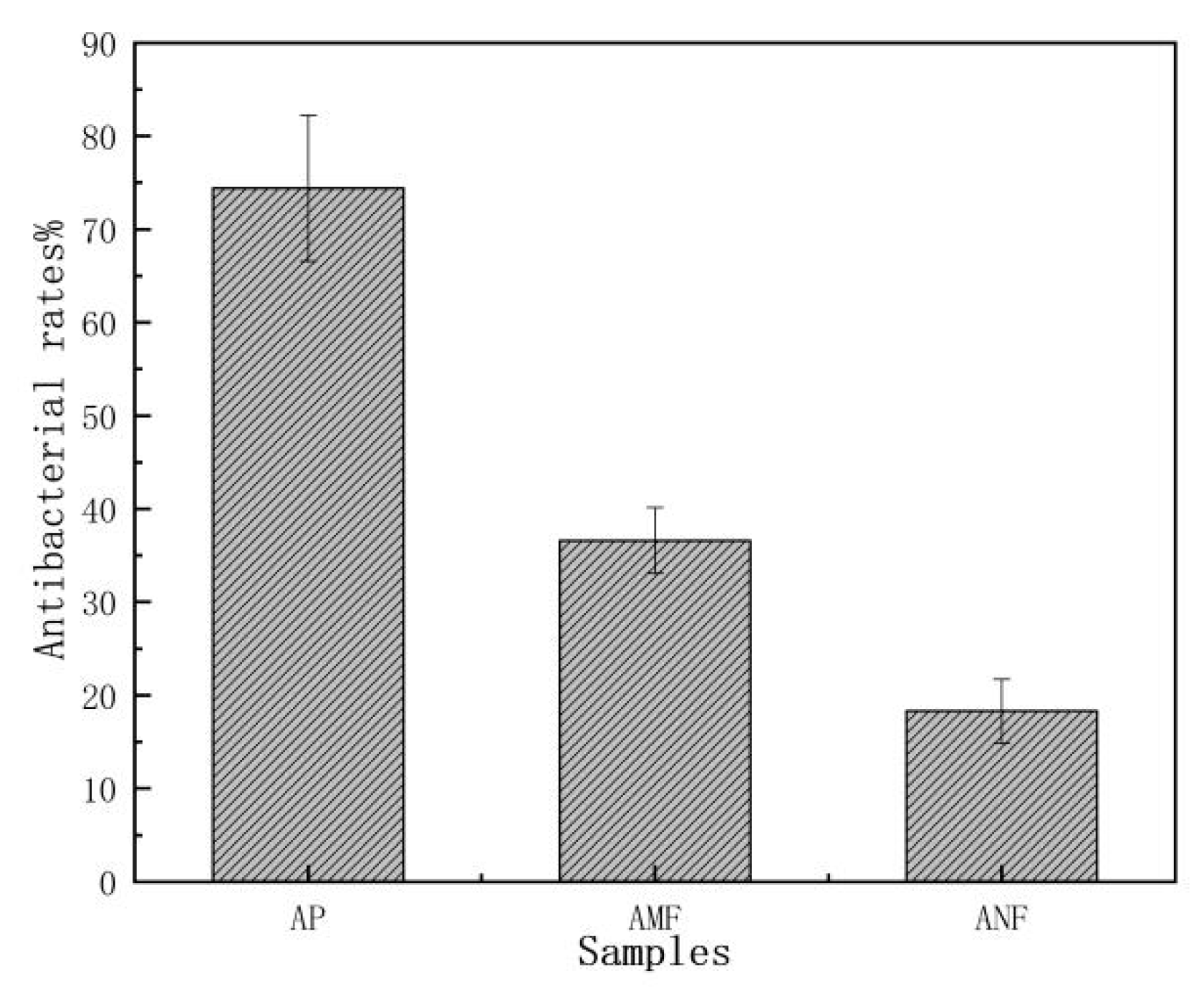
3.3. Antibacterial Properties
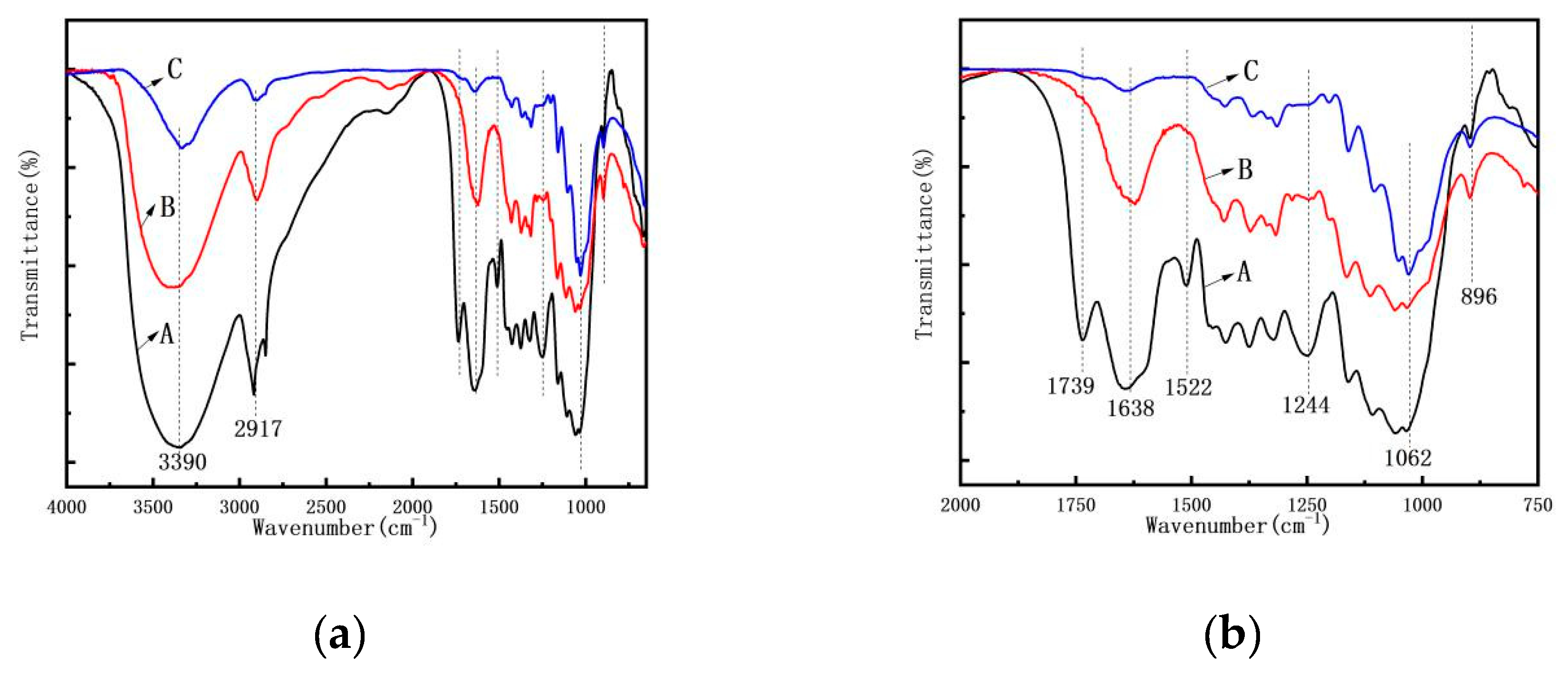
3.4. FTIR Spectroscopy Analysis
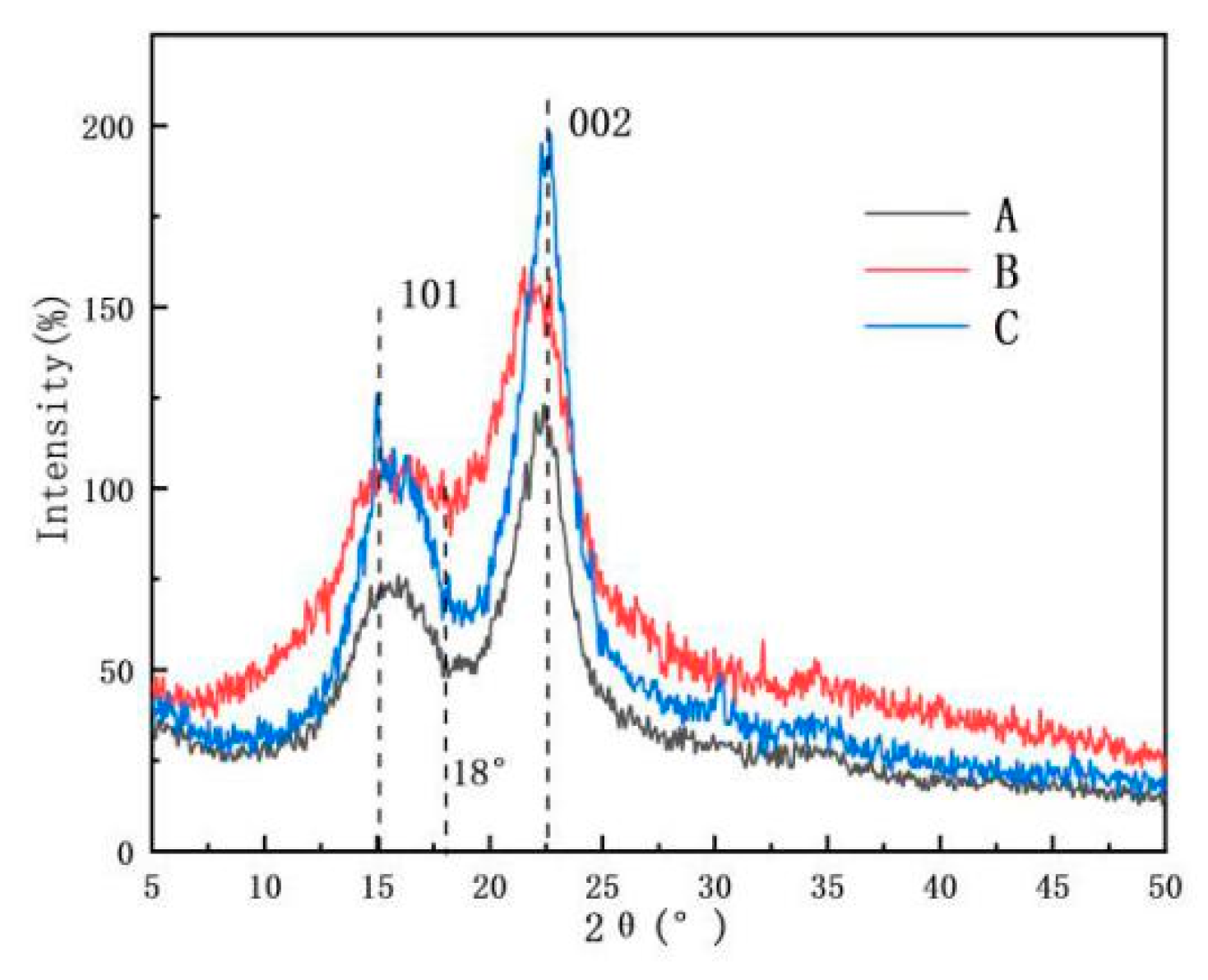
3.5. Crystallinity Index of Samples
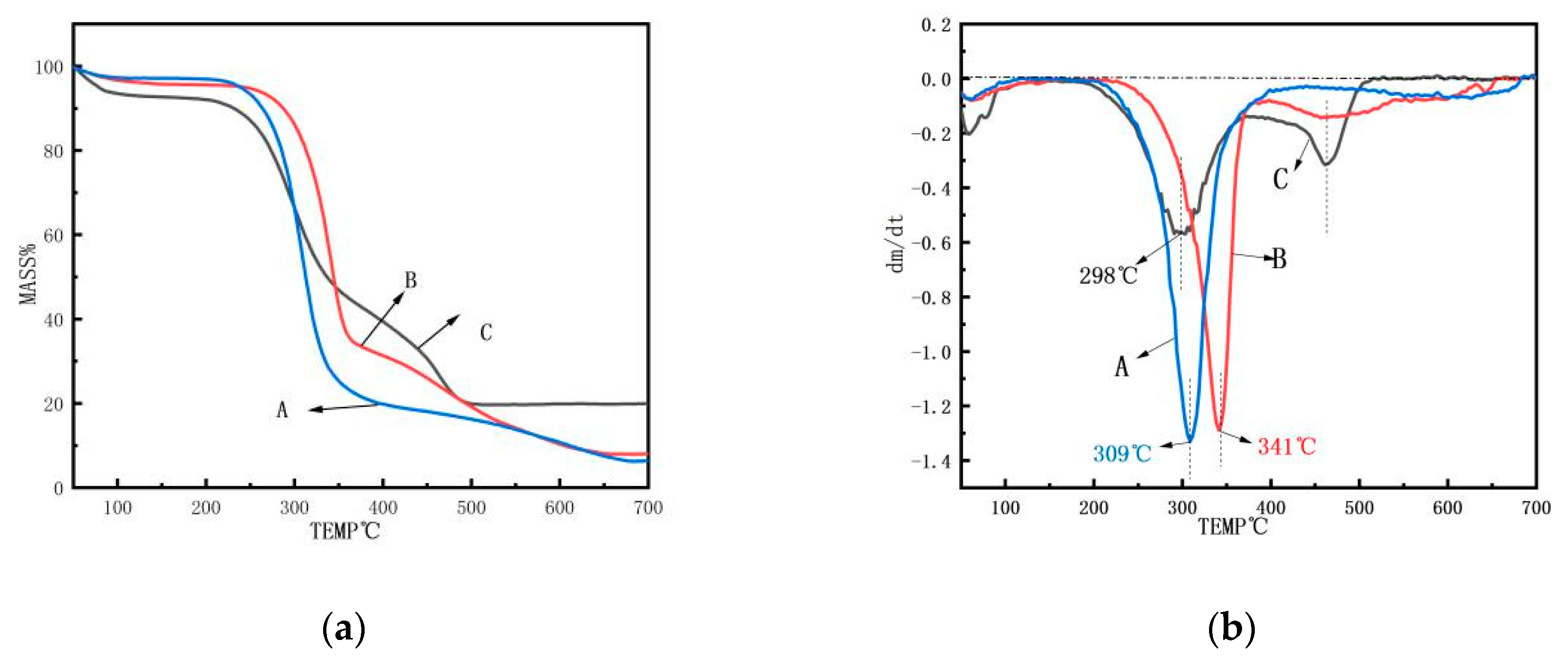
3.6. TG Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibres and their nanocomposites with natural rubber: Preparation and characterisation. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef]
- Jiang, W.; Song, Y.; Liu, S.; Ben, H.; Zhang, Y.; Zhou, C.; Han, G.; Ragauskas, A.J. A green degumming process of ramie. Ind. Crops Prod. 2018, 120, 131–134. [Google Scholar] [CrossRef]
- Hwang, Y.S. Isolation and identification of mosquito repellents in Artemisia vulgaris. J. Chem. Ecol. 1985, 11, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Repellent and fumigant activity of essential oil from Artemisia vulgaris to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2006, 42, 339–347. [Google Scholar] [CrossRef]
- Ding, W.; Liu, H.; Li, L.S. The main dtratagems and technology for stored product pest control in ancient China. J. Southwest Agric. Univ. 2000, 22, 335–338. [Google Scholar]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mug wort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef]
- Hassan, M.L.; Hassan, E.A.; Oksman, K.N. Effect of pretreatment of bagasse fibers on the properties of chitosan/microfibrillated cellulose nanocomposites. J. Mater. Sci. 2011, 46, 1732–1740. [Google Scholar] [CrossRef]
- Logaranjan, K. Shape- and size-controlled synthesis of silver nanoparticles using aloe vera plant extract and their antimicrobial activity. Nanoscale Res. Lett. 2016, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Theivasanthi, T. Synthesis and characterization of cotton fiber-based nanocellulose. Int. J. Biol. Macromol. 2018, 109, 832–836. [Google Scholar] [CrossRef]
- Oliver-Borrachero, B.; Sánchez-Caballero, S.; Fenollar, O. Natural-fiber-reinforced polymer composites for automotive parts manufacturing. Key Eng. Mater. 2019, 793, 9–16. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Prasad, V.; Suresh Kumar, P. A review of recent developments in natural fiber composites and their mechanical, thermal & machinabilty properties. Adv. Mater. Res. 2018, 1148, 61–71. [Google Scholar]
- Ghasemi, S. Reinforcement of natural fiber yarns by cellulose nanomaterials: A multi-scale study. Ind. Crops Prod. 2018, 111, 471–481. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, W.; Zhang, Y.; Wang, H.; Zou, F.; Yu, K.; Han, G. A novel process of nanocellulose extraction from kenaf bast. Mater. Res. Express 2018, 5, 085032. [Google Scholar] [CrossRef]
- Jiang, W.; Han, G.; Zhang, Y.; Wang, M. Fast compositional analysis of ramie using near-infrared spectroscopy. Carbohydr. Polym. 2010, 81, 937–941. [Google Scholar] [CrossRef]
- Koga, H.; Saito, T.; Kitaoka, T.; Nogi, M.; Suganuma, K.; Isogai, A. Transparent, Conductive, and Printable Composites Consisting of TEMPO-Oxidized Nanocellulose and Carbon Nanotube. Biomacromolecules 2013, 14, 1160–1165. [Google Scholar] [CrossRef]
- Wang, L.; Han, G.; Zhang, Y. Comparative study of composition, structure and properties of Apocynum venetum fibers under different pretreatments. Carbohydr. Polym. 2007, 69, 391–397. [Google Scholar] [CrossRef]
- Maache, M. Characterization of a novel natural cellulosic fiber from Juncus effusus L. Carbohydr. Polym. 2017, 171, 163–172. [Google Scholar] [CrossRef]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Akil, H.M. Kenaf fiber reinforced composites: A review. Mater. Des. 2011, 32, 4107–4121. [Google Scholar] [CrossRef]
- Serizawa, S.; Inoue, K.; Iji, M. Kenaf-fiber-reinforced poly(lactic acid) used for electronic products. J. Appl. Polym. Sci. 2010, 100, 618–624. [Google Scholar] [CrossRef]
- Nishino, T.; Hirao, K.; Kotera, M.; Nakamae, K.; Inagaki, H. Kenaf reinforced biodegradable composite. Compos. Sci. Technol. 2003, 63, 1281–1286. [Google Scholar] [CrossRef]
- Zhou, C. Prediction of mixed hardwood lignin and carbohydrate content using ATR-FTIR and FT-NIR. Carbohydr. Polym. 2015, 121, 336–341. [Google Scholar] [CrossRef]
- Silvério, H.A.; Neto, W.P.F.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind. Crops Prod. 2013, 44, 427–436. [Google Scholar] [CrossRef]
- Pandey, B.P.; Thapa, R.; Upreti, A. Chemical composition, antioxidant and antibacterial activities of essential oil and methanol extract of, artemisia vulgaris, and, gaultheria fragrantissima, collected from nepal. Asian Pac. J. Trop. Med. 2017, 10, 952–959. [Google Scholar] [CrossRef]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Chemical composition and antibacterial activity of Iranian lavandula×hybrida. Chem. Biodivers. 2017, 14, e1700064. [Google Scholar] [CrossRef]
- Frydrysiak, E.; Śmigielski, K.; Zabielska, J.; Kunicka-Styczyńska, A.; Frydrysiak, M. Antibacterial activity of essential oils potentially used for natural fiber pantiliner textronic system development. Procedia Eng. 2017, 200, 416–421. [Google Scholar] [CrossRef]
- Abraham, E. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Rwawiire, S.; Tomkova, B. Morphological, thermal, and mechanical characterization of Sansevieria trifasciata fibers. J. Nat. Fibers 2015, 12, 201–210. [Google Scholar] [CrossRef]
- Sun, B.L. Application of NIR spectroscopy to estimate of MFA and fiber length of Neosinocalamus affinis. Spectrosc. Spectr. Anal. 2011, 31, 3251–3255. [Google Scholar]
- Kavkler, K. FTIR spectroscopy of biodegraded historical textiles. Polym. Degrad. Stab. 2011, 96, 574–580. [Google Scholar] [CrossRef]
- Song, Y.; Han, G.; Li, M.; Jiang, W. Performance analysis of kudzu fiber prepared by using combined steam explosion and chemical degumming. J. Nat. Fibers 2017, 14, 759–768. [Google Scholar] [CrossRef]
- Yadav, M.; Chiu, F.C. Cellulose nanocrystals reinforced k-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Hao, M.; Huang, C.; Xue, Z.; Mu, T. Preparation and characterization of regenerated cellulose from ionic liquid using different methods. Carbohydr. Polym. 2015, 117, 99–105. [Google Scholar] [CrossRef]
- Magniez, K. Overcoming interfacial affinity issues in natural fiber reinforced polylactide biocomposites by surface adsorption of amphiphilic block copolymers. ACS Appl. Mater. Interfaces 2013, 5, 276–283. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Huang, Y.; Huang, H. Utilization of pineapple peel for production of nanocellulose and film application. Cellulose 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Shahabi-Ghahafarrokhi, I.; Khodaiyan, F.; Mousavi, M. Preparation and characterization of nanocellulose from beer industrial residues using acid hydrolysis/ultrasound. Fibers Polym. 2015, 16, 529–536. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Okita, Y.; Isogai, A. Thermal stabilization of tempo-oxidized cellulose. Polym. Degrad. Stab. 2010, 95, 1502–1508. [Google Scholar] [CrossRef]

| Species | Hemicellulose | Lignin | Cellulose | Lipid | Water Soluble Matter | Pectin | Gum Content |
|---|---|---|---|---|---|---|---|
| Artemisia vulgaris bast | 29.10% ± 0.97% | 28.25% ± 0.45% | 40.92% ± 0.70% | 1.05% ± 0.04% | 13.6% ± 2.41% | 3.2% ± 0.34% | 35.90% ± 1.16% |
| Kenaf bast [20,21,22] | 15.75% ± 1.07% | 15.65% ± 1.53% | 56.81% ± 2.95% | 8.42% ± 0.08% | 6.28% ± 0.23% | 4.43% ± 0.39% | 28.46% ± 0.95% |
| Wood [23] | 35.97% ± 3.88% | 26.24% ± 2.41% | 43.71% ± 2.10% | 2.11% ± 1.42% | - | - | - |
| Species | Length | Diameter | Aspect Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Avg. | Max. | Min. | SD | Avg. | Max. | Min. | SD | ||
| AMF | 850.6 μm | 1212 μm | 400.2 μm | 119.1 | 14.3 μm | 15.8 μm | 12.3 μm | 1.9 | 59.5 |
| ANF | 260.5 nm | 307.7 nm | 207.7 nm | 33.6 | 11.4 nm | 15.7 nm | 7.7 nm | 1.9 | 22.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, K.; Song, Y.; Liu, S.; Han, G.; Ben, H.; Ragauskas, A.J.; Jiang, W. Preparation and Characterization of Microcellulose and Nanocellulose Fibers from Artemisia Vulgaris Bast. Polymers 2019, 11, 907. https://doi.org/10.3390/polym11050907
Nie K, Song Y, Liu S, Han G, Ben H, Ragauskas AJ, Jiang W. Preparation and Characterization of Microcellulose and Nanocellulose Fibers from Artemisia Vulgaris Bast. Polymers. 2019; 11(5):907. https://doi.org/10.3390/polym11050907
Chicago/Turabian StyleNie, Kai, Yan Song, Shaoyang Liu, Guangting Han, Haoxi Ben, Arthur J. Ragauskas, and Wei Jiang. 2019. "Preparation and Characterization of Microcellulose and Nanocellulose Fibers from Artemisia Vulgaris Bast" Polymers 11, no. 5: 907. https://doi.org/10.3390/polym11050907





