Polyamine-Based Organo-Clays for Polluted Water Treatment: Effect of Polyamine Structure and Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Organo-Clays Preparation
2.3. Organo-Clays Characterizations
2.4. Metal Ions Uptake and Release Tests
3. Results and Discussion
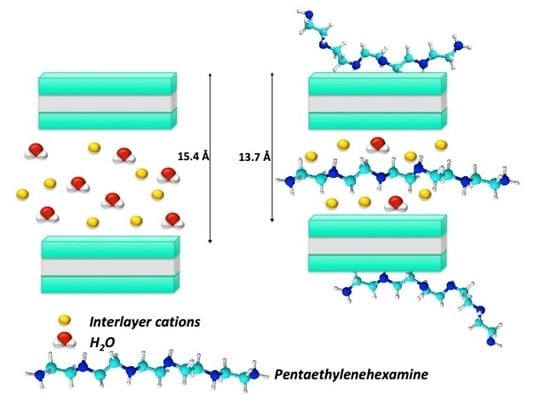
3.1. Characterization of Modified Clays
3.2. Metal Uptake and Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conor, R. Water for a Sustainable World—The United Nations World Water Development Report; Unesco Publishing: Paris, France, 2015. [Google Scholar]
- Sis, H.; Uysal, T. Removal of heavy metal ions from aqueous medium using kuluncak (malatya) vermiculites and effect of precipitation on removal. Appl. Clay Sci. 2014, 95, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Hicher, P.; Muresan, B.; Saiyouri, N.; Hicher, P.-Y. Heavy metal retention properties of kaolin and bentonite in a wide range of concentration and different ph conditions. Appl. Clay Sci. 2016, 119, 365–374. [Google Scholar] [CrossRef]
- Shen, C.; Chen, C.; Wen, T.; Zhao, Z.; Wang, X.; Xu, A. Superior adsorption capacity of g-C3N4 for heavy metal ions from aqueous solutions. J. Colloid Interface Sci. 2015, 456, 7–14. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Wu, W.; Wei, S.; Xu, Y.; Kuang, Y. Studies of heavy metal ion adsorption on chitosan/sulfydryl-functionalized graphene oxide composites. J. Colloid Interface Sci. 2015, 448, 389–397. [Google Scholar] [CrossRef]
- Matusik, J.; Wścisło, A. Enhanced heavy metal adsorption on functionalized nanotubular halloysite interlayer grafted with aminoalcohols. Appl. Clay Sci. 2014, 100, 50–59. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 1–24. [Google Scholar] [CrossRef]
- Song, J.; Kong, H.; Jang, J. Adsorption of heavy metal ions from aqueous solution by polyrhodanine-encapsulated magnetic nanoparticles. J. Colloid Interface Sci. 2011, 359, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Bedelean, H.; Mǎicǎneanu, A.; Burcǎ, S.; Stanca, M. Removal of heavy metal ions from wastewaters using natural clays. Clay Miner. 2010, 44, 487–495. [Google Scholar] [CrossRef]
- Bourliva, A.; Michailidis, K.; Sikalidis, C.; Filippidis, A.; Betsiou, M. Lead removal from aqueous solutions by natural greek bentonites. Clay Miner. 2013, 48, 771–787. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Azizian, S.; Kostoglou, M. Novel approaches in designing natural/synthetic materials for environmental applications. Adv. Mater. Sci. Eng. 2015, 2015, 1. [Google Scholar] [CrossRef]
- Triantafyllou, S.; Christodoulou, E.; Neou-Syngouna, P. Removal of nickel and cobalt from aqueous solutions by Na-activated bentonite. Clays Clay Miner. 1999, 47, 567–572. [Google Scholar] [CrossRef]
- Okada, T.; Ehara, Y.; Ogawa, M. Adsorption of Eu3+ to smectites and fluoro-tetrasilicic mica. Clays Clay Miner. 2007, 55, 348–353. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Gallo Stampino, P.; Pelosato, R.; Mesto, E.; Schingaro, E.; Lacalamita, M. Use of natural clays as sorbent materials for rare earth ions: Materials characterization and set up of the operative parameters. Waste Manag. 2015, 46, 546–556. [Google Scholar] [CrossRef]
- Rathnayake, S.I.; Xi, Y.; Frost, R.L.; Ayoko, G.A. Structural and thermal properties of inorganic–organic montmorillonite: Implications for their potential environmental applications. J. Colloid Interface Sci. 2015, 459, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Yariv, S.; Cross, H. Organo-Clay Complexes and Interactions; Taylor & Francis: Milton Park, UK, 2001. [Google Scholar]
- Dick, S.; Weiss, A. Reactions of smectites with binuclear oxobridged iron complexes of n-alkyl-n,nbis(2-pyridylmethyl)amines. Clay Miner. 1998, 33, 35–42. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, Z.; Huang, W. Water-adsorption properties of xiazijie montmorillonite intercalated with polyamine. Clay Miner. 2015, 50, 537–548. [Google Scholar] [CrossRef]
- Malakul, P.; Srinivasan, K.R.; Wang, H.Y. Metal adsorption and desorption characteristics of surfactant-modified clay complexes. Ind. Eng. Chem. Res. 1998, 37, 4296–4301. [Google Scholar] [CrossRef]
- Deng, Y.; Dixon, J.B.; White, G.N. Intercalation and surface modification of smectite by two non-ionic surfactants. Clays Clay Miner. 2003, 51, 150–161. [Google Scholar] [CrossRef]
- Iannicelli Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Gallo Stampino, P.; Pelosato, R.; Bengo, I.; Masi, M. Polymers modified clays for separating rare earths from WEEE. Environ. Eng. Manag. J. 2013, 12 (Suppl. 11), 23–26. [Google Scholar]
- Zampori, L.; Stampino, P.G.; Cristiani, C.; Dotelli, G.; Cazzola, P. Synthesis of organoclays using non-ionic surfactants: Effect of time, temperature and concentration. Appl. Clay Sci. 2010, 48, 97–102. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmad, A.; Umar, K.; Nabi, S.A. Synthesis, characterization and biological applications of nanocomposites for the removal of heavy metals and dyes. Ind. Eng. Chem. Res. 2015, 54, 76–82. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Jalajamony, S.; Sreekumari, S.S. Adsorption of heavy metal ions from aqueous solutions by amine and carboxylate functionalised bentonites. Appl. Clay Sci. 2012, 65–66, 67–71. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Gallo Stampino, P. Recovery of valuable metals from electronic scraps by clays and organo-clays: Study on bi-ionic model solutions. Waste Manag. 2017, 60, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Basolo, F. Inorganic Syntheses; McGraw-Hill: New York, NY, USA, 1976. [Google Scholar]
- Iannicelli-Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Stampino, P.G.; Pelosato, R. Recovery of rare earths and precious metals from waste electrical and electronic equipment by acid leaching and immobilized chelating agents. Chem. Eng. Trans. 2015, 43, 2413–2418. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Chemical Oxygen Demand (Dichromate Oxygen Demand) of Water; ASTM: West Conshohocken, PA, USA, 2006; Volume D1252–06. [Google Scholar]
- Iannicelli-Zubiani, E.M.; Gallo Stampino, P.; Cristiani, C.; Dotelli, G. Enhanced lanthanum adsorption by amine modified activated carbon. Chem. Eng. J. 2018, 341, 75–82. [Google Scholar] [CrossRef]
- Blachier, C.; Michot, L.; Bihannic, I.; Barres, O.; Jacquet, A.; Mosquet, M. Adsorption of polyamine on clay minerals. J. Colloid Interface Sci. 2009, 336, 599–606. [Google Scholar] [CrossRef]
- Finocchio, E.; Cristiani, C.; Dotelli, G.; Stampino, P.G.; Zampori, L. Thermal evolution of peg-based and brij-based hybrid organo-inorganic materials. FT-IR studies. Vib. Spectrosc. 2014, 71, 47–56. [Google Scholar] [CrossRef]
- Deng, Y.; Dixon, J.; White, G.N. Bonding mechanisms and conformation of poly(ethylene oxide)-based surfactants in interlayer of smectite. Colloid Polym. Sci. 2006, 284, 347–356. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Huang, T.-K.; Wang, Y.-C.; Alamani, B.G.; Lin, J.-J. Intercalation strategies in clay/polymer hybrids. Prog. Polym. Sci. 2013, 39, 443–485. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Duan, Y.; Liu, Y.; Du, S. Modification of montmorillonite with poly(oxypropylene) amine hydrochlorides: Basal spacing, amount intercalated and thermal stability. Clays Clay Miner. 2011, 59, 507–517. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline studies of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Scholtzová, E.; Madejová, J.; Tunega, D. Structural properties of montmorillonite intercalated with tetraalkylammonium cations—Computational and experimental study. Vib. Spectrosc. 2014, 74, 120–126. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R.; Hong, L.; Liu, T. Functionalization of adsorbent with different aliphatic polyamines for heavy metal ion removal: Characteristics and performance. J. Colloid Interface Sci. 2010, 345, 454–460. [Google Scholar] [CrossRef]
- Pentrák, M.; Bizovská, V.; Madejová, J. Near-IR study of water adsorption on acid-treated montmorillonite. Vib. Spectrosc. 2012, 63, 360–366. [Google Scholar] [CrossRef]
- Finocchio, E.; Baccini, I.; Cristiani, C.; Dotelli, G.; Gallo Stampino, P.; Zampori, L. Hybrid organo–inorganic clay with nonionic interlayers. Mid- and Near-IR spectroscopic studies. J. Phys. Chem. A 2011, 115, 7484–7493. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Ko, C.H.; Fan, C.; Chiang, P.N.; Wang, M.K.; Lin, K.C. P-nitrophenol, phenol and aniline sorption by organo-clays. J. Hazard. Mater. 2007, 149, 275–282. [Google Scholar] [CrossRef]
- Laaz, I.; Stébé, M.-J.; Benhamou, A.; Zoubir, D.; Blin, J.-L. Influence of porosity and surface modification on the adsorption of both cationic and anionic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 30–40. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Okeola, F.; Odebunmi, E. Freundlich and Langmuir isotherms parameters for adsorption of methylene blue by activated carbon derived from agrowastes. Adv. Nat. Appl. Sci. 2010, 4, 281–288. [Google Scholar]
- Iftekhar, S.; Ramasamy, D.L.; Srivastava, V.; Asif, M.B.; Sillanpää, M. Understanding the factors affecting the adsorption of lanthanum using different adsorbents: A critical review. Chemosphere 2018, 204, 413–430. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Gallo Stampino, P.; Pelosato, R.; Finocchio, E. Effect of pH in the synthesis of organo-clays for rare earths removal. Environ. Eng. Manag. J. 2017, 16, 1719–1727. [Google Scholar] [CrossRef]










| Structure | Number of Amino Groups | Label |
|---|---|---|
 | 6 | L6 |
 | 10 | L10 |
 | 14 | B14 |
| Sample (Label) | Amine Solution Initial Concentration (mM) | Initial Contacted Amine C0 (mmol/gclay) |
|---|---|---|
| L6-25 | 25 | 0.5 |
| L6-70 | 70 | 1.5 |
| L6-135 | 135 | 3.1 |
| L6-175 | 175 | 4.0 |
| L10-15 | 15 | 0.3 |
| L10-25 | 25 | 0.6 |
| L10-35 | 35 | 0.8 |
| L10-60 | 60 | 1.4 |
| L10-90 | 90 | 2.1 |
| B14-15 | 15 | 0.3 |
| B14-25 | 25 | 0.6 |
| B14-40 | 40 | 0.9 |
| B14-90 | 90 | 2.0 |
| Model | qmax(mmol/g) | KL(L/mmol) | R2 |
| Langmuir | 0.47 | 0.08 | 0.71 |
| n | KF (mmol/(g·(mmol/L)1/n) | R2 | |
| Freundlich | 3.03 | 0.10 | 0.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristiani, C.; Iannicelli-Zubiani, E.M.; Dotelli, G.; Finocchio, E.; Gallo Stampino, P.; Licchelli, M. Polyamine-Based Organo-Clays for Polluted Water Treatment: Effect of Polyamine Structure and Content. Polymers 2019, 11, 897. https://doi.org/10.3390/polym11050897
Cristiani C, Iannicelli-Zubiani EM, Dotelli G, Finocchio E, Gallo Stampino P, Licchelli M. Polyamine-Based Organo-Clays for Polluted Water Treatment: Effect of Polyamine Structure and Content. Polymers. 2019; 11(5):897. https://doi.org/10.3390/polym11050897
Chicago/Turabian StyleCristiani, Cinzia, Elena Maria Iannicelli-Zubiani, Giovanni Dotelli, Elisabetta Finocchio, Paola Gallo Stampino, and Maurizio Licchelli. 2019. "Polyamine-Based Organo-Clays for Polluted Water Treatment: Effect of Polyamine Structure and Content" Polymers 11, no. 5: 897. https://doi.org/10.3390/polym11050897