Synthesis of Novel pH-Tunable Thermoresponsive Hydroxyl-Terminated Hyperbranched Polyether
Abstract
:1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Characterization
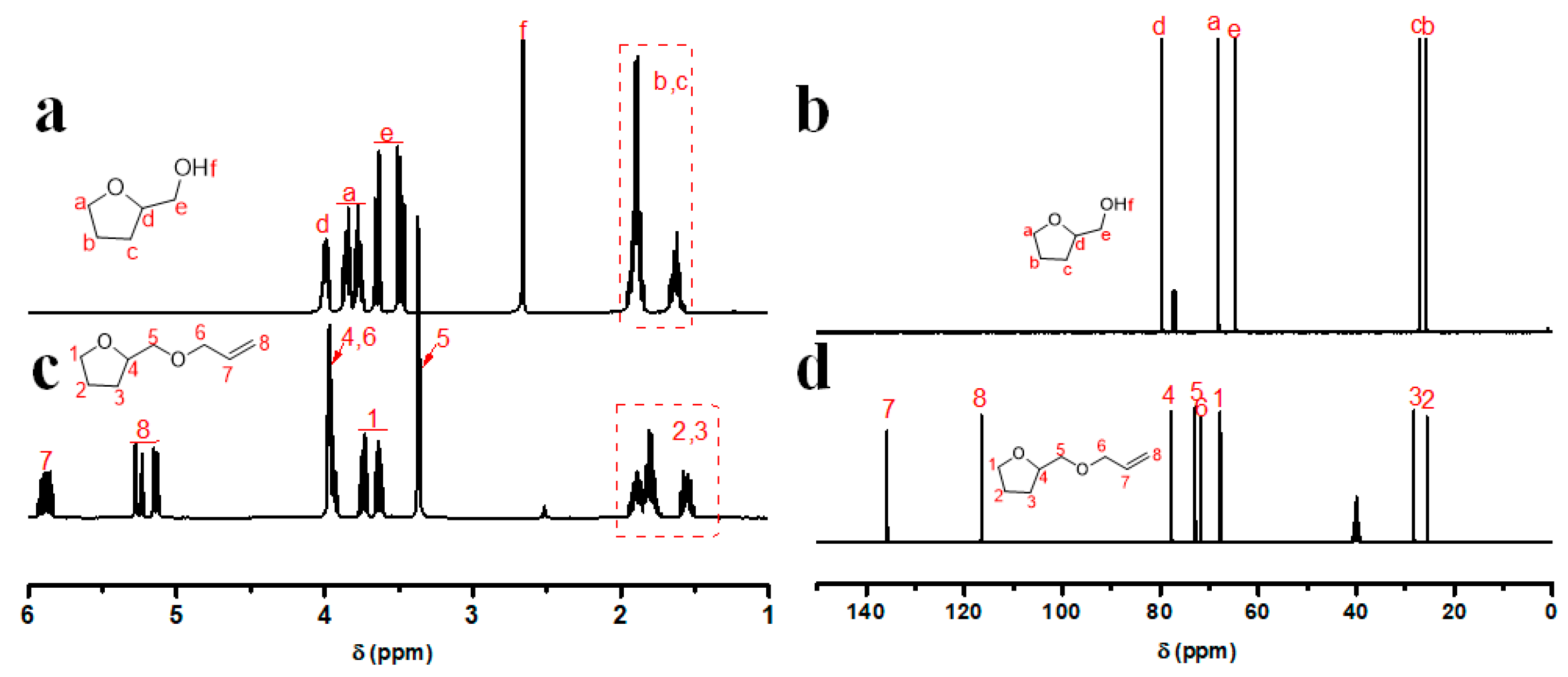
2.3. Synthesis of Monomer AMTHF
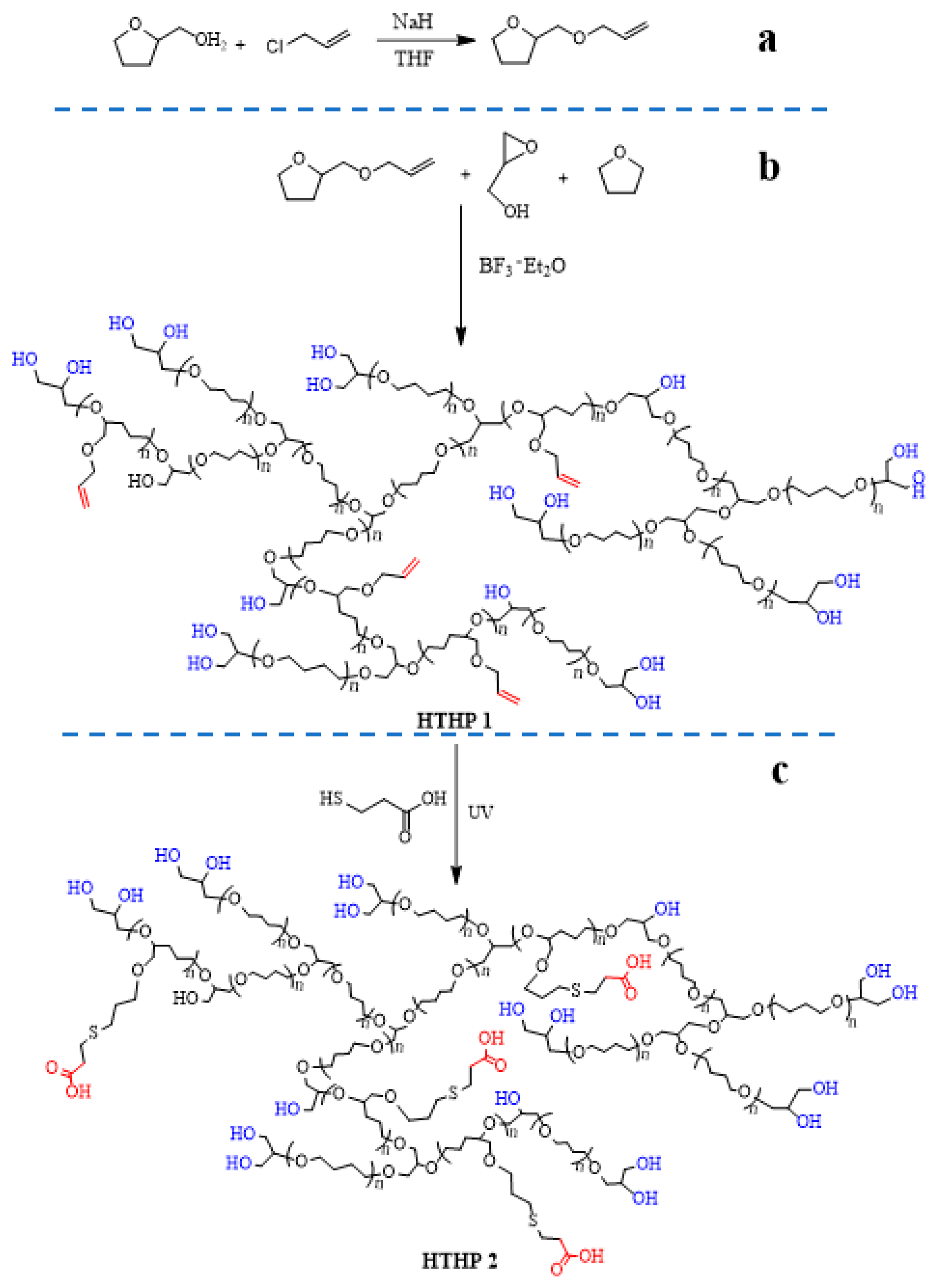
2.4. Synthesis of HTHP 1
2.5. Synthesis of HTHP 2
3. Results and Discussion
3.1. Synthesis and Characterization of Monomer AMTHF
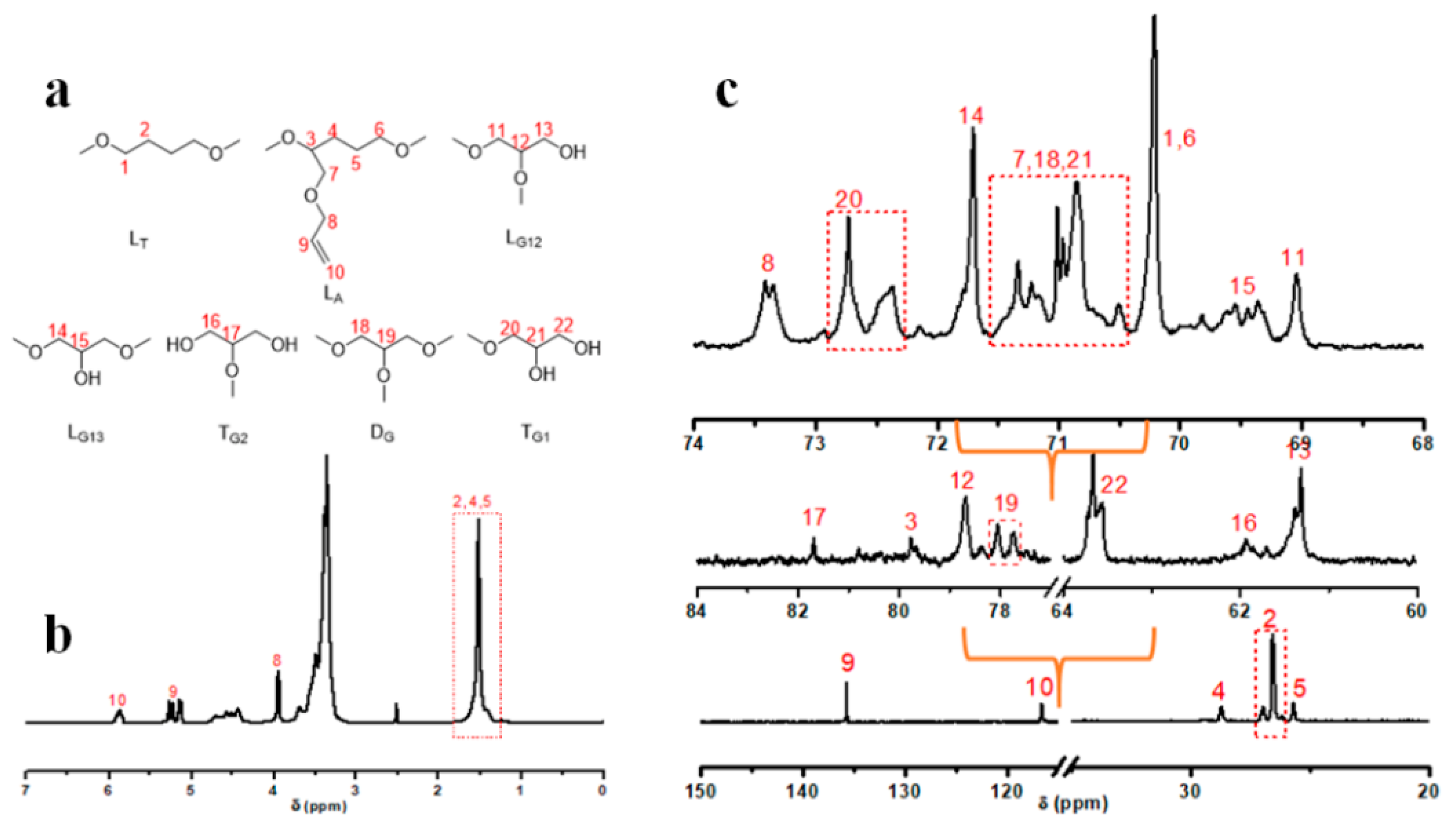
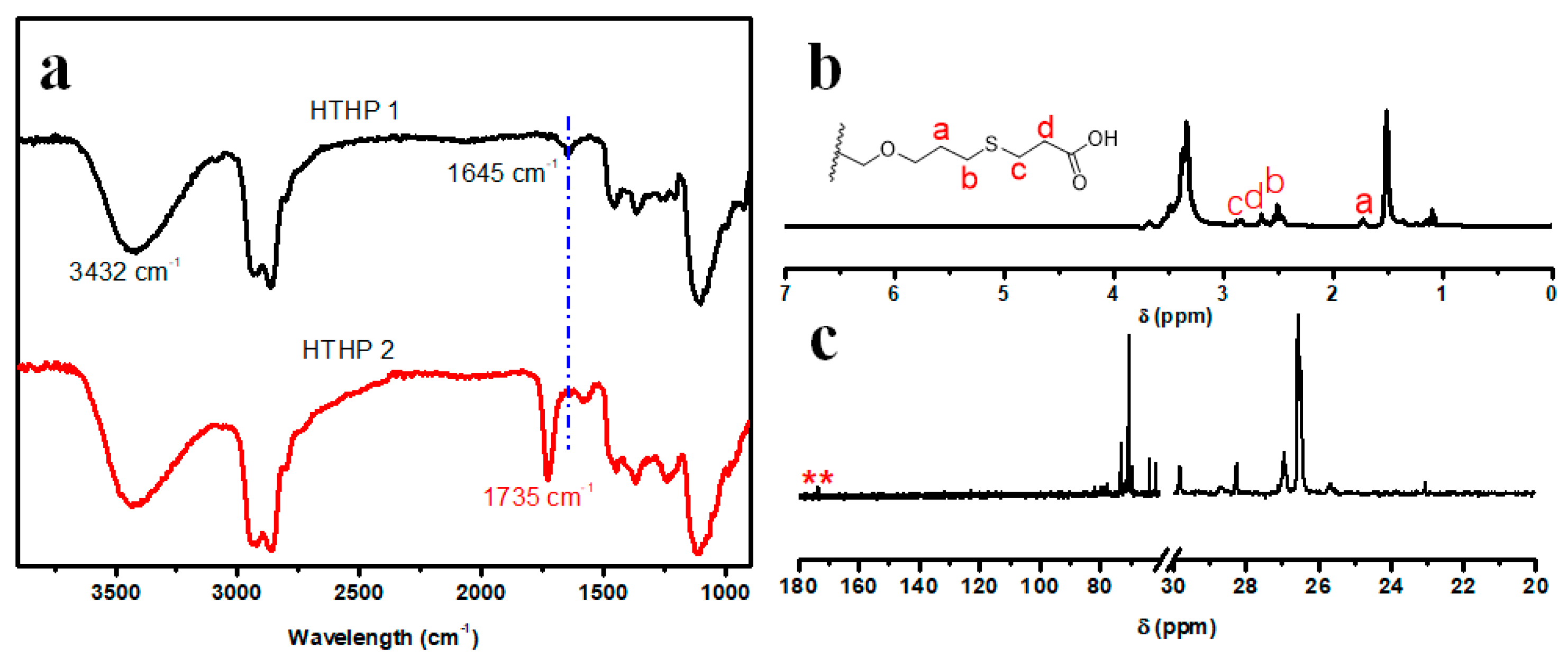
3.2. Synthesis and Characterization of HTHP 1
3.3. Synthesis and Characterization of HTHP 2
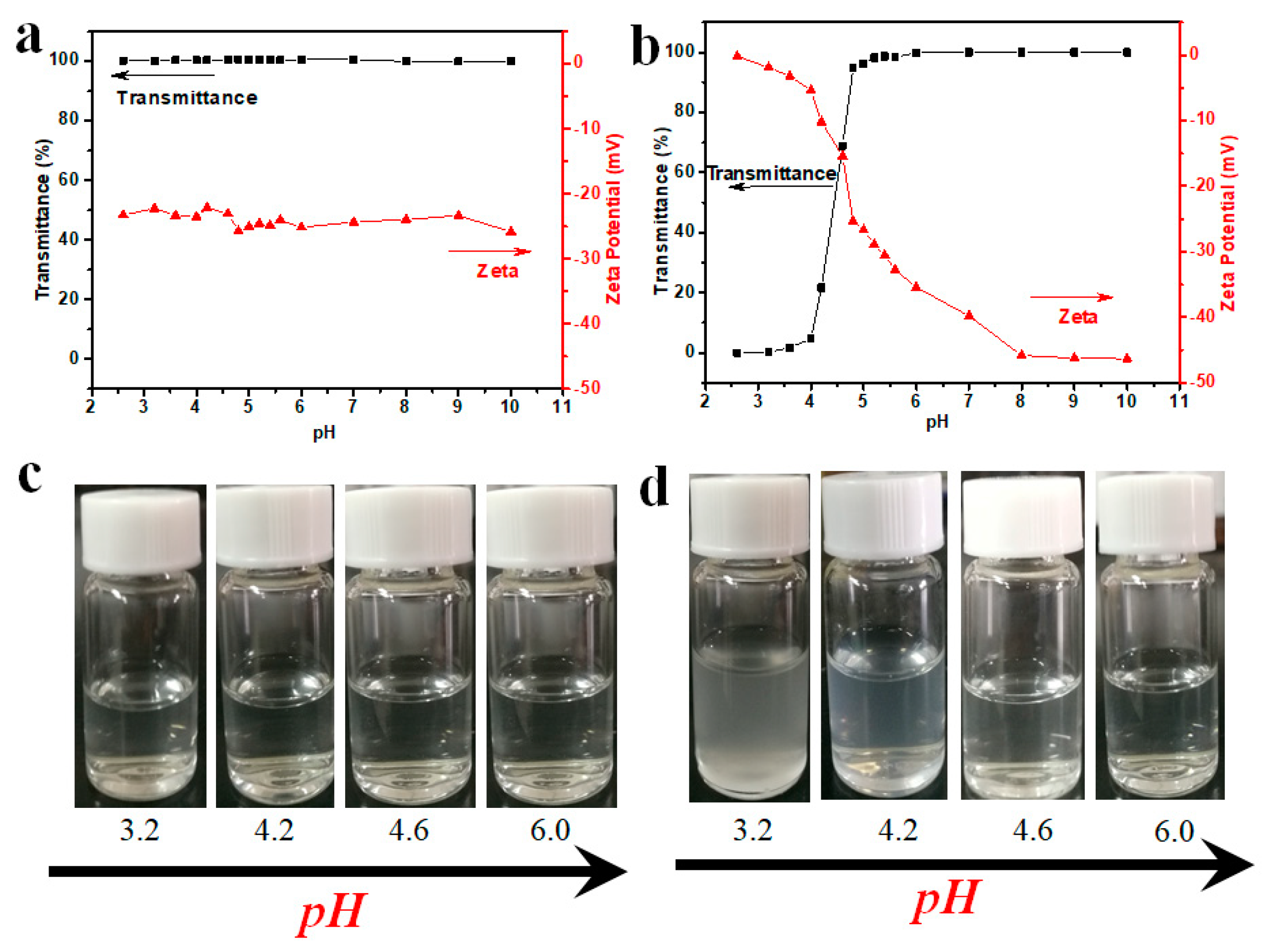
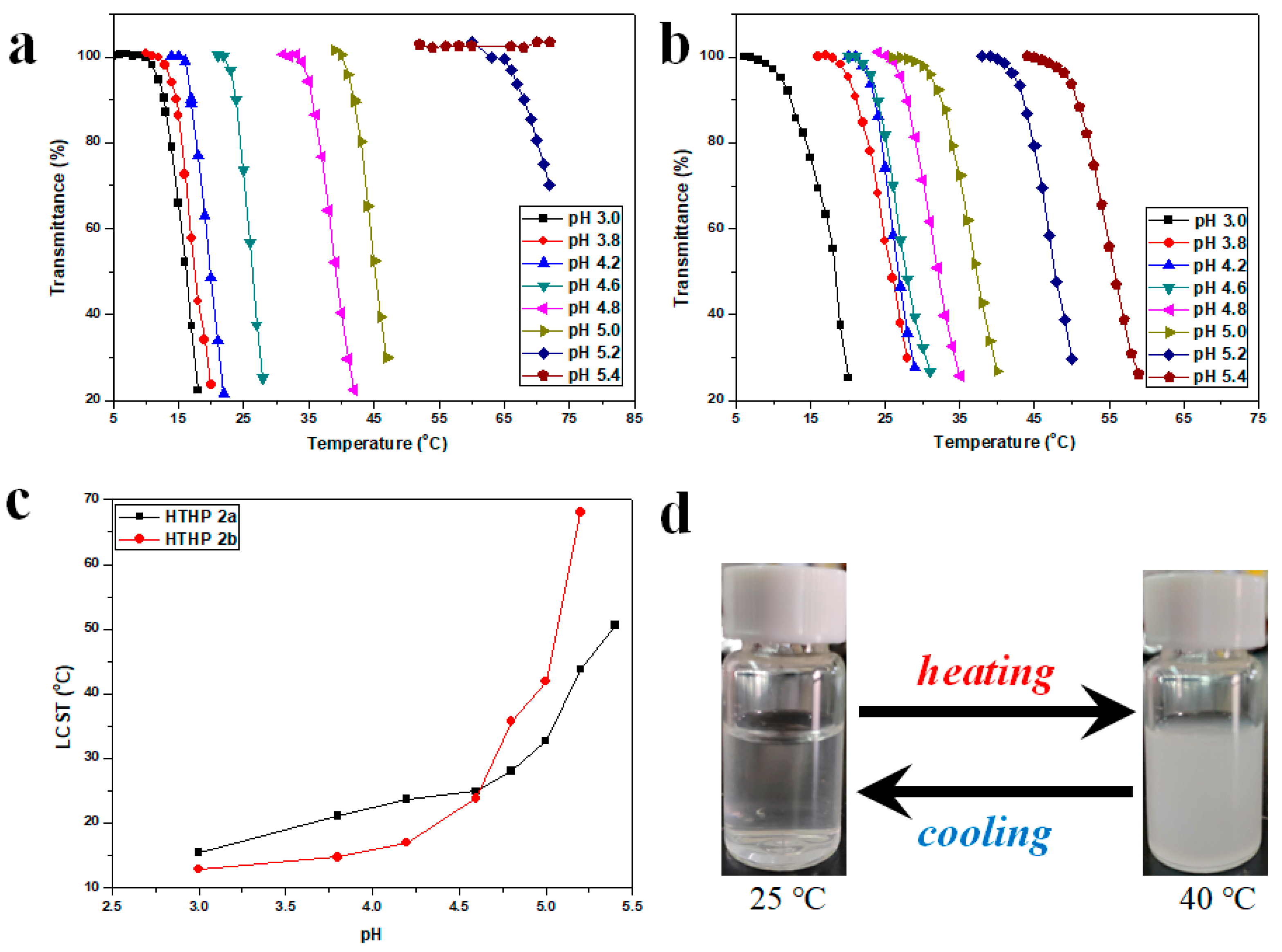
3.4. pH-Responsive Behavior of HTHP 2
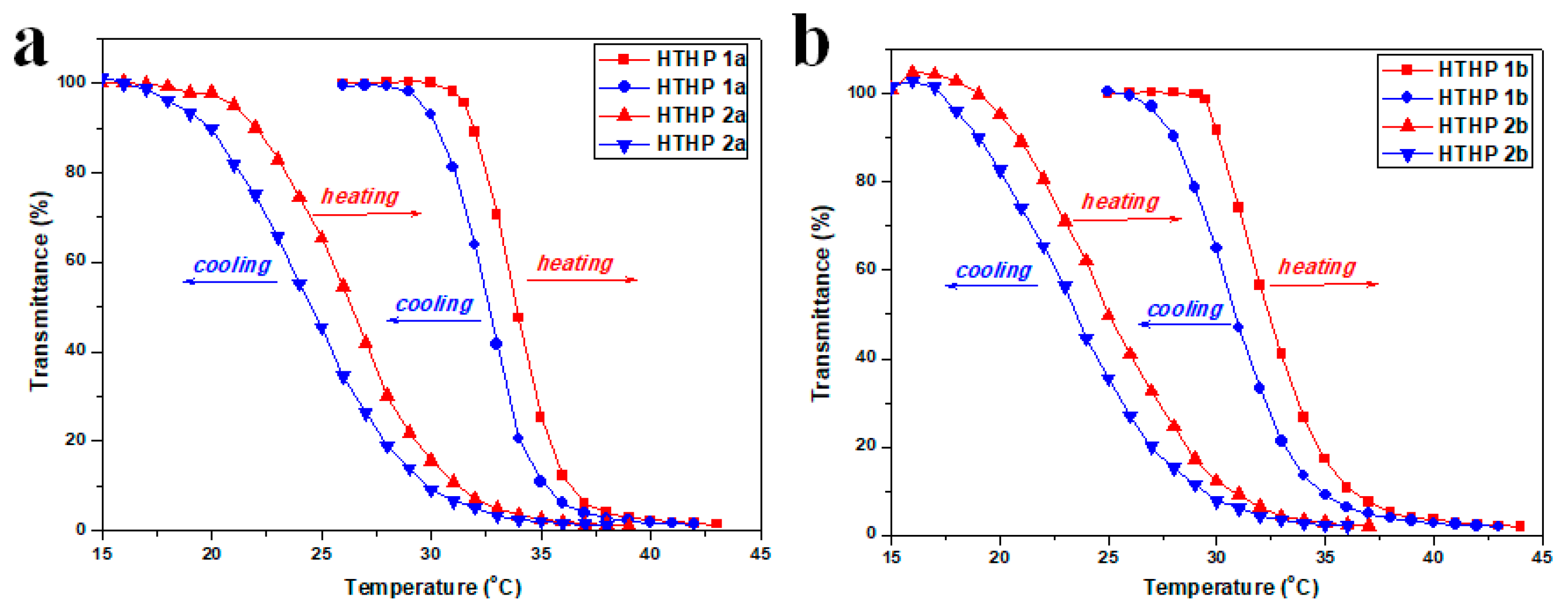
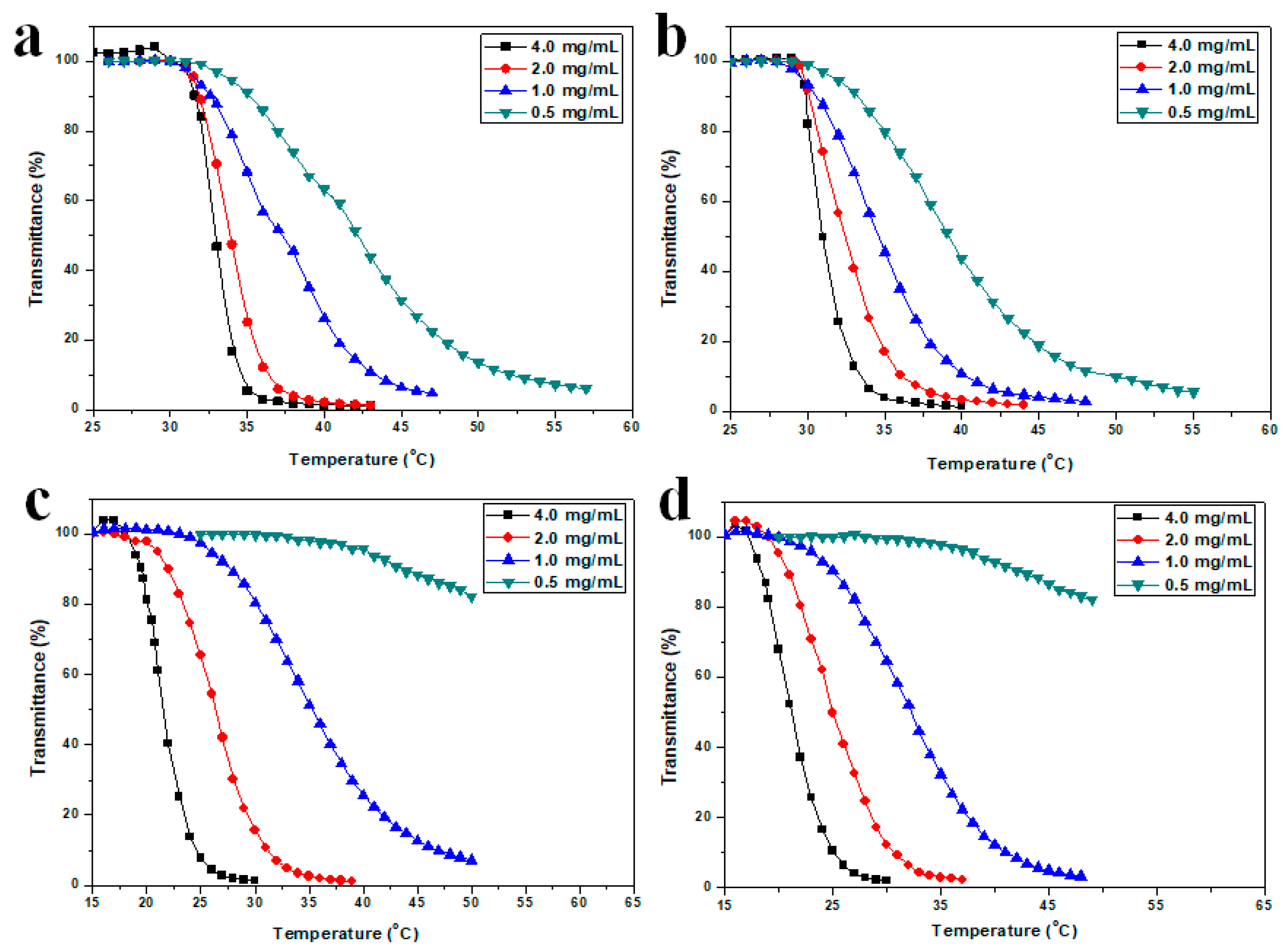
3.5. Thermoresponsive Behavior of HTHP 2
3.6. In Vitro Cytotoxicity of HTHP 1 and HTHP 2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Matsuyama, M.; Yamato, M.; Takeda, N.; Okano, T. Poly(N-isopropylacrylamide)-Grafted Polydimethylsiloxane Substrate for Controlling Cell Adhesion and Detachment by Dual Stimulation of Temperature and Mechanical Stress. Biomacromolecules 2018, 19, 4014–4022. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Kermagoret, A.; Fustin, C.A.; Bourguignon, M.; Detrembleur, C.; Jerome, C.; Debuigne, A. One-pot controlled synthesis of double thermoresponsive N-vinylcaprolactam-based copolymers with tunable LCSTs. Polym. Chem. 2013, 4, 2575–2583. [Google Scholar] [CrossRef]
- Gau, E.; Flecken, F.; Ksiazkiewicz, A.N.; Pich, A. Enzymatic synthesis of temperature-responsive poly(N-vinylcaprolactam) microgels with glucose oxidase. Green Chem. 2018, 20, 431–439. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Helmut, S. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef] [Green Version]
- Lorson, T.; Lubtow, M.M.; Wegener, E.; Haider, M.S.; Borova, S.; Nahm, D.; Jordan, R.; Sokolsko-Papkov, M.; Kabanov, A.V.; Luxenhofer, R. Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update. Biomaterials 2018, 178, 204–280. [Google Scholar] [CrossRef] [PubMed]
- Morgese, G.; Gombert, Y.; Ramakrishna, S.N.; Benetti, E.M. Mixing Poly(ethylene glycol) and Poly(2-alkyl-2-oxazoline)s Enhances Hydration and Viscoelasticity of Polymer Brushes and Determines Their Nanotribological and Antifouling Properties. ACS Appl. Mater. Interfaces 2018, 10, 41839–41848. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, H.; Zhu, X.; Yan, D. Backbone-Thermoresponsive Hyperbranched Polyethers. J. Am. Chem. Soc. 2006, 128, 8144–8145. [Google Scholar] [CrossRef]
- Schomer, M.; Seiwert, J.; Frey, H. Hyperbranched Poly(propylene oxide): A Multifunctional Backbone-Thermoresponsive Polyether Polyol Copolymer. ACS Macro Lett. 2012, 1, 888–891. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Y.; Zhu, X.; Yan, D. Synthesis and applications of stimuli-responsive hyperbranched polymers. Prog. Polym. Sci. 2017, 64, 114–153. [Google Scholar] [CrossRef]
- Ward, M.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar]
- Galperin, A.; Long, T.J.; Ratner, B.D. Degradable, Thermo-Sensitive Poly(N-isopropylacrylamide)-Based Scaffolds with Controlled Porosity for Tissue Engineering Applications. Biomacromolecules 2010, 11, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Aijzuddin; Khan, J.; Saraf, S.; Saraf, S. Poly(ethylene glycol)–poly(lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. J. Control. Release 2013, 172, 715–729. [Google Scholar] [CrossRef]
- Lee, J.; Mcgrath, A.J.; Hawker, C.J.; Kim, B.S. pH-Tunable Thermoresponsive PEO-Based Functional Polymers with Pendant Amine Groups. ACS Macro Lett. 2016, 5, 1391–1396. [Google Scholar] [CrossRef]
- Lutz, J.F.; Akdemir, Q.; Hoth, A. Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly(NIPAM) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef]
- Lutz, J.F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)ethyl Methacrylate and Oligo(ethylene glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Lutz, J.F.; Weichenhan, K.; Akdemir, Q.; Hoth, A. About the Phase Transitions in Aqueous Solutions of Thermoresponsive Copolymers and Hydrogels Based on 2-(2-methoxyethoxy)ethyl Methacrylate and Oligo(ethylene glycol) Methacrylate. Macromolecules 2007, 40, 2503–2508. [Google Scholar] [CrossRef]
- Min, S.H.; Kwak, S.K.; Kim, B.S. Atomistic simulation for coil-to-globule transition of poly(2-dimethylaminoethyl methacrylate). Soft Matter 2015, 11, 2423–2433. [Google Scholar] [CrossRef] [Green Version]
- Rwei, S.P.; Chiang, W.Y.; Way, T.F.; Tuan, H.N.A.; Chang, Y.C. Study of theThermo-/pH-Sensitivity of Stereo-Controlled Poly(N-isopropylacrylamide-co-IAM) Copolymers via RAFT Polymerization. Polymers 2018, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, W.; Zhao, X.; Zhao, Y. Synthesis and Thermoresponsive Behaviors of Thermo-, pH-, CO2-, and Oxidation-Responsive Linear and Cyclic Graft Copolymers. Macromolecules 2017, 50, 3411–3423. [Google Scholar] [CrossRef]
- Jung, S.H.; Song, H.Y.; Lee, Y.; Jeong, H.M.; Lee, H. Novel Thermoresponsive Polymers Tunable by pH. Macromolecules 2011, 44, 1628–1634. [Google Scholar] [CrossRef]
- Fournier, D.; Hoogenboom, R.; Thijs, H.M.L.; Paulus, R.M.; Schubert, U.S. Tunable pH- and Temperature-Sensitive Copolymer Libraries by Reversible Addition-Fragmentation Chain Transfer Copolymerizations of Methacrylates. Macromolecules 2007, 40, 915–920. [Google Scholar] [CrossRef]
- Cai, X.; Zhong, L.; Su, Y.; Lin, S.; He, X. Novel pH-tunable thermoresponsive polymers displaying lower and upper critical solution temperatures. Polym. Chem. 2015, 6, 3875–3884. [Google Scholar] [CrossRef]
- Andre, X.; Zhang, M.; Muller, A.H.E. Thermo- and pH-Responsive Micelles of Poly(acrylic acid)-block-Poly(N,N-diethylacrylamide). Macromol. Rapid Commun. 2005, 26, 558–563. [Google Scholar] [CrossRef]
- He, C.; Zhao, C.; Chen, X.; Guo, Z.; Zhuang, X.; Jing, X. Novel pH- and Temperature-Responsive Block Copolymers with Tunable pH-Responsive Range. Macromol. Rapid Commun. 2008, 29, 490–497. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Zhu, H.; Yin, Q.; Liu, H.; Hu, Y. Effect of Composition of PDMAEMA-b-PAA Block Copolymers on Their pH- and Temperature-Responsive Behaviors. Langmuir 2013, 29, 1024–1034. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, L.; Ma, R.; Zhang, W.; An, Y.; Zhu, X.X. Synthesis and micellization of thermo- and pH-responsive block copolymer of poly(N-isopropylacrylamide)-block-poly(4-vinylpyridine). Polymer 2007, 48, 1711–1717. [Google Scholar] [CrossRef]
- Vamvakaki, M.; Unali, G.F.; Boucher, B.S.; Robinson, K.L.; Billingham, N.C.; Armes, S.P. Effect of Partial Quaternization on the Aqueous Solution Properties of Tertiary Amine-Based Polymeric Surfactants: Unexpected Separation of Surface Activity and Cloud Point Behavior. Macromolecules 2001, 34, 6839–6841. [Google Scholar] [CrossRef]
- Nakayama, M.; Okano, T. Unique thermoresponsive polymeric micelle behavior via cooperative polymer corona phase transitions. Macromolecules 2008, 41, 504–507. [Google Scholar] [CrossRef]
- Ayres, L.; Koch, K.; Adams, P.H.H.M.; Hest, J.C.M. Stimulus Responsive Behavior of Elastin-Based Side Chain Polymers. Macromolecules 2005, 38, 1699–1704. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Liu, Y.; Li, Z. Thermo- and pH-Responsive Polymer Derived from Methacrylamide and Aspartic Acid. Macromolecules 2010, 43, 8101–8108. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, G.; Du, L.; Zhang, C.; Li, L.; Zhu, J.; Pei, J. Synthesis of hydroxyl-terminated polybutadiene bearing pendant carboxyl groups by combination of anionic polymerization and blue light photocatalytic thiol-ene reaction and its pH-triggered self-assemble behavior. React. Funct. Polym. 2018, 127, 161–167. [Google Scholar] [CrossRef]
- Sunder, A.; Mulhaupt, R.; Frey, H. Hyperbranched Polyether Polyols: A Modular Approach to Complex Polymer Architectures. Adv. Mater. 2000, 12, 235–239. [Google Scholar] [CrossRef]
- Sunder, A.; Mulhaupt, R.; Frey, H. Hyperbranched Polyether-Polyols Based on Polyglycerol: Polarity Design by Block Copolymerization with Propylene Oxide. Macromolecules 2000, 33, 309–314. [Google Scholar] [CrossRef]
- Mohammadifar, E.; Bodaghi, A.; Dadkhahtehrani, A.; Kharat, A.N.; Adeli, M.; Haag, R. Green Synthesis of Hyperbranched Polyglycerol at Room Temperature. ACS Macro Lett. 2017, 6, 35–40. [Google Scholar] [CrossRef]
- Christ, E.M.; Muller, S.S.; Berger-Nicoletti, E.; Frey, H. Hydroxyfunctional Oxetane-Inimers with Varied Polarity for the Synthesis of Hyperbranched Polyether Polyols via Cationic ROP. J. Polym. Sci. Pol. Chem. 2014, 52, 2850–2859. [Google Scholar] [CrossRef]
- Christ, E.M.; Hobernik, D.; Bros, M.; Wagner, M.; Frey, H. Cationic Copolymerization of 3,3-Bis(hydroxymethyl)oxetane and Glycidol: Biocompatible Hyperbranched Polyether Polyols with High Content of Primary Hydroxyl Groups. Biomacromolecules 2015, 16, 3297–3307. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D. Hyperbranched polymers: from synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Zhu, X.; Yan, D.; Chen, R. Synthesis and therapeutic applications of biocompatible or biodegradable hyperbranched polymers. Polym. Chem. 2015, 6, 2794–2812. [Google Scholar] [CrossRef] [Green Version]
- Yates, N.G.; Hayes, W. Synthesis and applications of hyperbranched polymers. Eur. Polym. J. 2004, 40, 1257–1281. [Google Scholar] [CrossRef]
- Kojima, C.; Yoshimura, K.; Harada, A.; Sakanishi, Y.; Komo, K.S. Synthesis and Characterization of Hyperbranched Poly(glycidol) Modified with pH- and Temperature-Sensitive Groups. Bioconjugate Chem. 2009, 20, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Wang, L.; Ma, L.; Yu, H.; Liu, Q.; Xiao, A. Electrochemical Behaviors and Anion Recognition of Ferrocene Modified Hyperbranched Polyether. Macromolecules 2009, 42, 4500–4510. [Google Scholar] [CrossRef]
- Alkan, A.; Klein, R.; Shylin, S.I.; Kemmer-Jonas, U.; Frey, H.; Wurm, F.R. Water-soluble and redox-responsive hyperbranched polyether copolymers based on ferrocenyl glycidyl ether. Polym. Chem. 2015, 6, 7112–7118. [Google Scholar] [CrossRef] [Green Version]
- Tokar, R.; Kubisa, P.; Penczek, S.; Dworak, A. Cationic polymerization of glycidol: coexistence of the activated monomer and active chain end mechanism. Macromolecules 1994, 27, 320–322. [Google Scholar] [CrossRef]
- Bednarek, M.; Kubisa, P. Cationic copolymerization of tetrahydrofuran with ethylene oxide in the presence of diols: Composition, microstructure, and properties of copolymers. J. Polym. Sci. Part A 1999, 37, 3455–3463. [Google Scholar] [CrossRef]
- Fan, W.; Fan, X.; Tian, W.; Zhang, X.; Wang, G.; Zhang, W.; Bai, Y.; Zhu, X. Phase transition dynamics and mechanism for backbone-thermoresponsive hyperbranched polyethers. Polym. Chem. 2014, 5, 4022–4031. [Google Scholar] [CrossRef]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Temperature influence of nonionic polyethylene oxide and anionic polyacrylamide on flocculation and dewatering behavior of kaolinite dispersions. J. Colloid Interface Sci. 2004, 1, 145–156. [Google Scholar] [CrossRef]
- Chen, S.; Wang, K.; Zhang, W. A new thermoresponsive polymer of poly(N-acryloylsarcosine methyl ester) with a tunable LCST. Polym. Chem. 2017, 8, 3090–3101. [Google Scholar] [CrossRef]
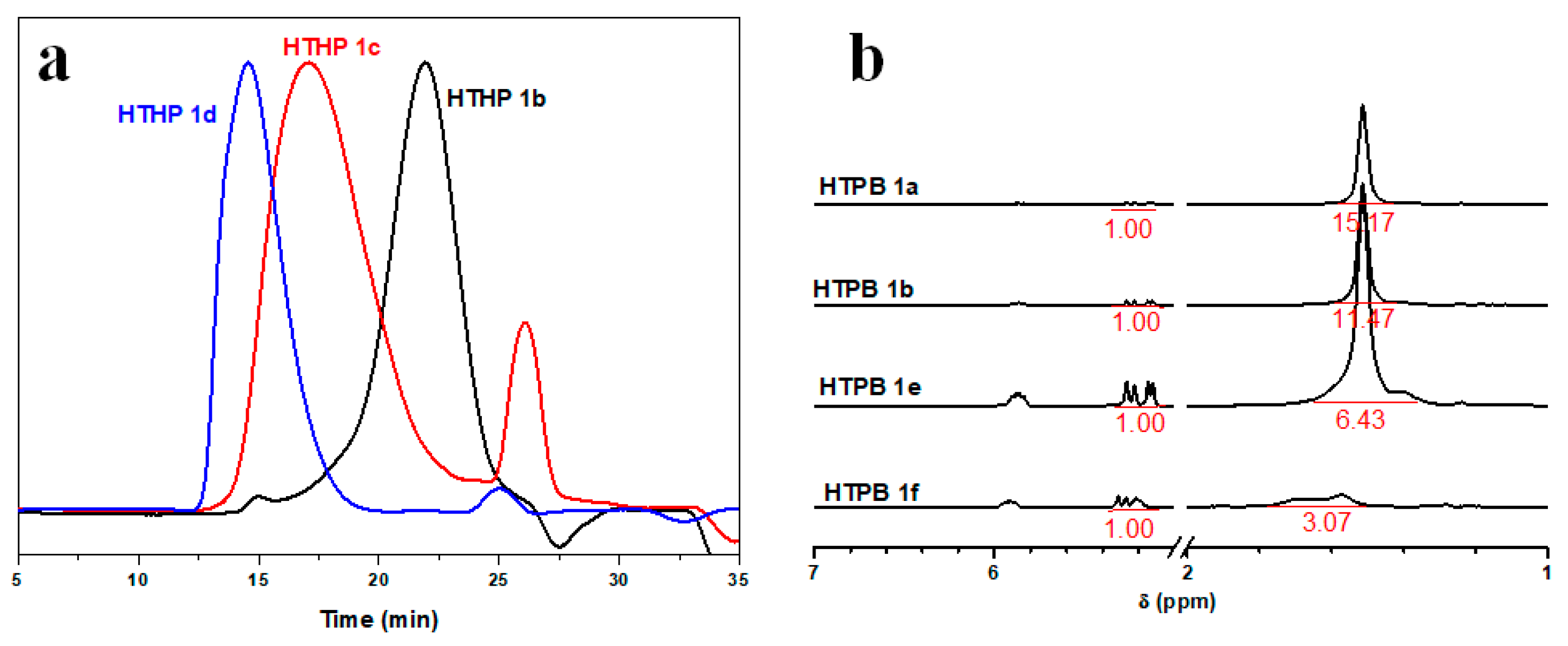

| Sample | Feed Ratio THF:AMPTHF:glycidol (mL:mL:mL) | Mna (g/mol) | Mwa (g/mol) | Mw/Mna |
|---|---|---|---|---|
| a | 2.0:0.5:0.6 | 7600 | 10,700 | 1.41 |
| b | 2.0:1.0:0.6 | 8400 | 11,100 | 1.32 |
| c | 2.0:1.0:0.8 | 10,500 | 15,100 | 1.44 |
| d | 2.0:1.0:1.0 | 12,400 | 18,800 | 1.51 |
| e | 1.5:1.5:0.6 | 6000 | 8800 | 1.46 |
| f | 1.0:2.0:0.6 | 5400 | 7700 | 1.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Duan, X.; Bai, T.; Zhang, X.; Wang, T.; Cao, T.; Fan, X. Synthesis of Novel pH-Tunable Thermoresponsive Hydroxyl-Terminated Hyperbranched Polyether. Polymers 2019, 11, 895. https://doi.org/10.3390/polym11050895
Zhu X, Duan X, Bai T, Zhang X, Wang T, Cao T, Fan X. Synthesis of Novel pH-Tunable Thermoresponsive Hydroxyl-Terminated Hyperbranched Polyether. Polymers. 2019; 11(5):895. https://doi.org/10.3390/polym11050895
Chicago/Turabian StyleZhu, Xiuzhong, Xiao Duan, Ting Bai, Xuan Zhang, Tong Wang, Tao Cao, and Xiaodong Fan. 2019. "Synthesis of Novel pH-Tunable Thermoresponsive Hydroxyl-Terminated Hyperbranched Polyether" Polymers 11, no. 5: 895. https://doi.org/10.3390/polym11050895






